Impact statement
The role of coastal ecosystems in sequestering atmospheric carbon has been demonstrated and efforts are underway to leverage this service for climate change mitigation. Focussing largely on mangroves and saltmarshes, this paper applies concepts of uniformitarianism to consider how the present behaviour of blue carbon ecosystems (BCEs), and their response to varying sea level and climate change in the past, provides knowledge of BCE futures. There is evidence BCEs are being modified by climate change, and losses are compounded by ongoing clearance and degradation. Consequently, atmospheric warming, sea-level rise and human modifications have important implications for greenhouse gas fluxes from BCEs. Observations of the response of BCEs to sea-level rise indicate some capacity to adapt by accumulating mineral and organic material and increasing substrate elevations. Contributions of organic matter to substrates as sea level rises, coupled with landward retreat of BCEs to maintain their intertidal position, bolsters capacity to sequester atmospheric carbon within substrates and biomass. However, evidence of the response of mangroves and saltmarshes to sea-level rise over the Holocene indicate a threshold for adaptation that is exceeded when sea-level rises at ~5–7 mm yr.−1. This rate of sea-level rise is anticipated to be surpassed by the end of the 21st century unless deep cuts to atmospheric carbon concentrations occur rapidly. Accordingly, the fate of BCEs remains uncertain. However, it is probable mangrove forests and saltmarshes high in the tidal frame will transition towards lower tidal positions as sea level rises and may survive for some time, offering some confidence when trading carbon with a 25-year permanence timeframe. Landward retreat will be critical for blue carbon additionality, and this will require concerted management to minimise coastal squeeze and preserve BCE services. This uncertainty should be accommodated when considering the permanence of blue carbon in financial markets.
Introduction
Blue carbon is a collective term referring to the carbon associated with marine and coastal ecosystems, and includes all fluxes and stores that are biologically driven (Bindoff et al., Reference Bindoff, Cheung, Kairo, Arístegui, Guinder, Hallberg, Pörtner, Roberts and Masson-Delmotte2019). Similar to carbon sequestered in terrestrial forests, blue carbon has piqued the interest of practitioners seeking to mitigate climate change by enhancing carbon storage within natural ecosystems and improving the provision of ecosystem services (Macreadie et al., Reference Macreadie, Costa, Atwood, Friess, Kelleway, Kennedy, Lovelock, Serrano and Duarte2021). This interest is based on the high carbon storage potential of many blue carbon ecosystems (BCEs), a potential that is reported to be much higher on a unit area basis than other ecosystem-based climate solutions (Donato et al., Reference Donato, Kauffman, Murdiyarso, Kurnianto, Stidham and Kanninen2011; Pendleton et al., Reference Pendleton, Donato, Murray, Crooks, Jenkins, Sifleet, Craft, Fourqurean, Kauffman and Marbà2012; Duarte et al., Reference Duarte, Losada, Hendriks, Mazarrasa and Marba2013). BCEs are typically vegetated with mangroves, saltmarshes (also termed tidal marshes) and seagrasses, and to a lesser extent macroalgae, cyanobacteria and supratidal forests (Duarte et al., Reference Duarte, Losada, Hendriks, Mazarrasa and Marba2013; Raven, Reference Raven2018; Bindoff et al., Reference Bindoff, Cheung, Kairo, Arístegui, Guinder, Hallberg, Pörtner, Roberts and Masson-Delmotte2019; Lovelock and Duarte, Reference Lovelock and Duarte2019) (Figure 1). Carbon is drawn from the atmosphere via photosynthesis and stored within living biomass at a concentration of 40–50% of the mass, a value that is reasonably consistent among plants (Ma et al., Reference Ma, He, Tian, Zou, Yan, Yang, Zhou, Huang, Shen and Fang2018). Blue carbon is partitioned into above- and below-ground biomass and the soil carbon pool. Above-ground biomass is typically estimated from allometric equations, initially derived from destructive measurements that relate vegetation structure to mass (Thursby et al., Reference Thursby, Chintala, Stetson, Wigand and Champlin2002; Komiyama et al., Reference Komiyama, Ong and Poungparn2008; Radabaugh et al., Reference Radabaugh, Powell, Bociu, Clark and Moyer2017), or by applying remote sensing techniques to extrapolate spatial relationships (Pham et al., Reference Pham, Xia, Ha, Bui, Le and Tekeuchi2019; Sani et al., Reference Sani, Hashim and Hossain2019). The below-ground component is somewhat more difficult to quantify as substrates contain both living biomass and dead organic material that has accumulated over decades to thousands of years, as evident from radiocarbon dating of BCEs (Horton et al., Reference Horton, Shennan, Bradley, Cahill, Kirwan, Kopp and Shaw2018; Saintilan et al., Reference Saintilan, Khan, Ashe, Kelleway, Rogers, Woodroffe and Horton2020; Sefton et al., Reference Sefton, Woodroffe, Ascough, Friess and Sidik2021).
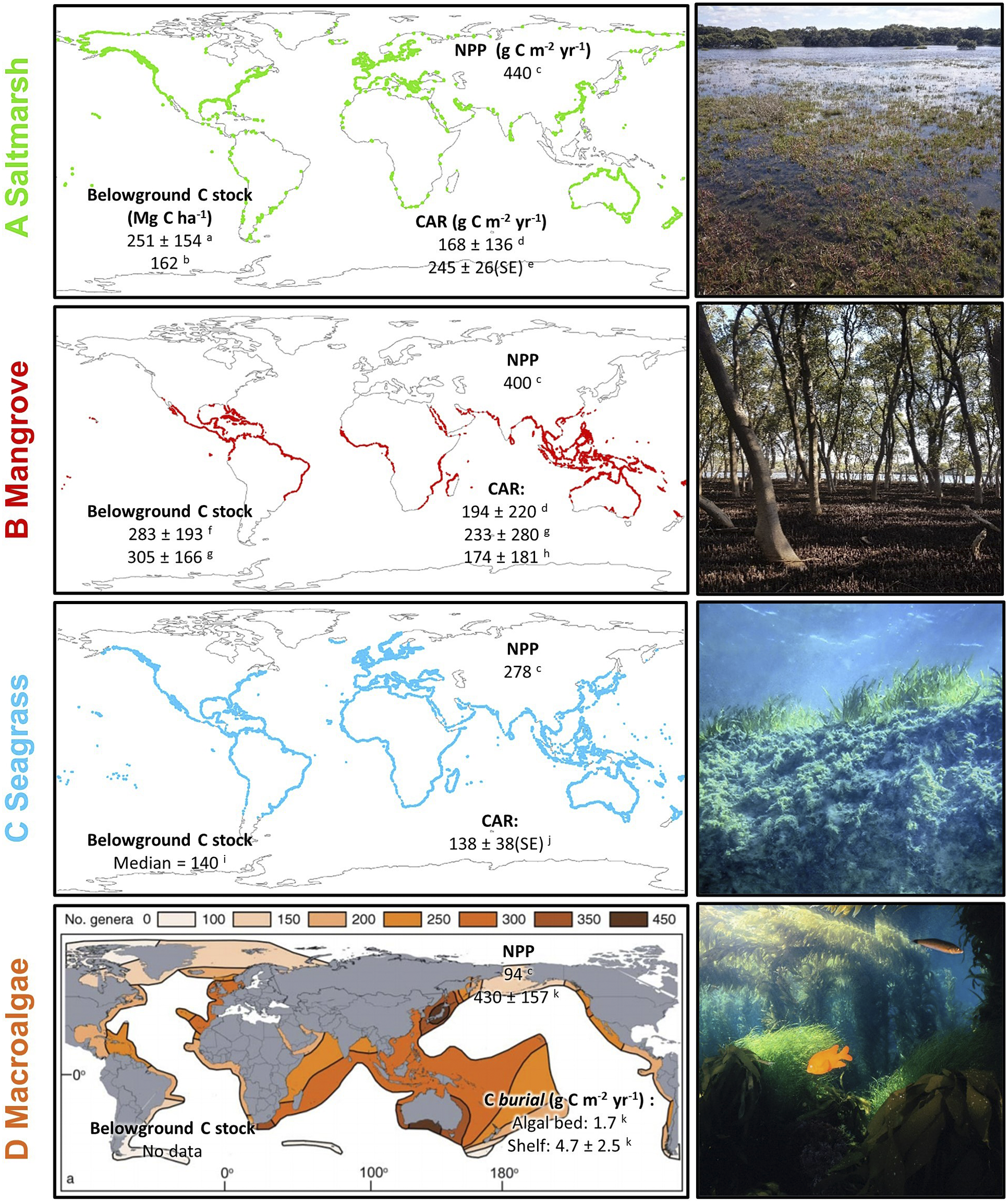
Figure 1. Global mapped distribution and existing estimates of carbon cycling parameters of (A) saltmarsh and (B) mangrove; (C) modelled distribution of seagrass; and (D) genus richness of benthic marine macroalgae. All values are global mean values ±1 standard deviation (where available) unless otherwise specified. Belowground carbon stocks are estimated to 1 m depth. CAR = (surface) carbon accumulation rate; NPP = net ecosystem primary productivity; SE = 1 standard error. Note that for macroalgae, CAR is replaced by estimates of carbon burial in situ (i.e. in algal beds) and exported particulate organic carbon buried in shelf sediments. Map data sources: saltmarsh (Mcowen et al., Reference Mcowen, Weatherdon, Bochove, Sullivan, Blyth, Zockler, Stanwell-Smith, Kingston, Martin, Spalding and Fletcher2017); mangrove (Bunting et al., Reference Bunting, Rosenqvist, Lucas, Rebelo, Hilarides, Thomas, Hardy, Itoh, Shimada and Finlayson2018); seagrass (Jayathilake and Costello, Reference Jayathilake and Costello2018); macroalgae (Kerswell, Reference Kerswell2006). Carbon data sources: a (Rogers et al., Reference Rogers, Kelleway, Saintilan, Megonigal, Adams, Holmquist, Lu, Schile-Beers, Zawadzki, Mazumder and Woodroffe2019a); b (Pendleton et al. Reference Pendleton, Donato, Murray, Crooks, Jenkins, Sifleet, Craft, Fourqurean, Kauffman and Marbà2012a); c (Duarte and Cebrian, Reference Duarte and Cebrian1996); d (Wang et al., Reference Wang, Sanders, Santos, Tang, Schuerch, Kirwan, Kopp, Zhu, Li and Yuan2021); e (Ouyang and Lee, Reference Ouyang and Lee2014); f (Atwood et al., Reference Atwood, Connolly, Almahasheer, Carnell, Duarte, Ewers Lewis, Irigoien, Kelleway, Lavery, Macreadie, Serrano, Sanders, Santos, Steven and Lovelock2017); g; h (Alongi, Reference Alongi2012); i; j (McLeod et al., Reference McLeod, Chmura, Bouillon, Salm, Björk, Duarte, Lovelock, Schlesinger and Silliman2011); k (Krause-Jensen and Duarte, Reference Krause-Jensen and Duarte2016).
The enhanced capacity for storage is dependent upon rates of carbon addition exceeding loss of carbon via the decomposition of organic material, and there is increasing agreement that this should also exceed in situ carbonate production (Saderne et al., Reference Saderne, Geraldi, Macreadie, Maher, Middelburg, Serrano, Almahasheer, Arias-Ortiz, Cusack and BDJNc2019). High net primary production from in situ vegetation underpins the supply of organic matter to substrates, mostly from root material (Saintilan et al., Reference Saintilan, Rogers, Mazumder and Woodroffe2013; Xiong et al., Reference Xiong, Liao and Wang2018). However, organic matter transported on tides can also become trapped and sequestered into substrates, and there is an increasing need to discriminate the varying role of autochthonous and allochthonous sources (Saintilan et al., Reference Saintilan, Rogers, Mazumder and Woodroffe2013; Canuel and Hardison, Reference Canuel and Hardison2016; Van de Broek et al., Reference Van de Broek, Vandendriessche, Poppelmonde, Merckx, Temmerman and Govers2018; Macreadie et al., Reference Macreadie, Anton, Raven, Beaumont, Connolly, Friess, Kelleway, Kennedy, Kuwae, Lavery, Lovelock, Smale, Apostolaki, Atwood, Baldock, Bianchi, Chmura, Eyre, Fourqurean, Hall-Spencer, Huxham, Hendriks, Krause-Jensen, Laffoley, Luisetti, Marbà, Masque, KJ, Megonigal, Murdiyarso, Russell, Santos, Serrano, Silliman, Watanabe and Duarte2019). Periodic inundation by saline tidal waters creates anaerobic conditions in saturated substrates that supress microbial activity and slow the decomposition of sequestered organic material (Duarte et al., Reference Duarte, Losada, Hendriks, Mazarrasa and Marba2013; Spivak et al., Reference Spivak, Sanderman, Bowen, Canuel and Hopkinson2019). Addition of mineral sediments supplied by tides serves to trap sequestered organic material (Spivak et al., Reference Spivak, Sanderman, Bowen, Canuel and Hopkinson2019) and saline substrates hamper methanogenic processes (Poffenbarger et al., Reference Poffenbarger, Needelman and Megonigal2011). While greenhouse gas emissions are not fully supressed (Rosentreter et al., Reference Rosentreter, Maher, Erler, Murray and Eyre2018), the general outcome is physicochemical conditions that favour slow decomposition of organic material, long-term preservation of a portion of fixed carbon within substrates, and limited release of powerful greenhouse gases (e.g. methane and nitrous oxide) to the atmosphere (McKee et al., Reference McKee, Cahoon and Feller2007; McLeod et al., Reference McLeod, Chmura, Bouillon, Salm, Björk, Duarte, Lovelock, Schlesinger and Silliman2011; Kroeger et al., Reference Kroeger, Crooks, Moseman-Valtierra and Tang2017). Storage may be further enhanced when coastal processes operate to ensure that space within substrates for carbon storage continues to be available, and this appears to be strongly influenced by rates of sediment supply, sedimentation, and coastal evolution in the context of changing sea levels (Rogers et al., Reference Rogers, Kelleway, Saintilan, Megonigal, Adams, Holmquist, Lu, Schile-Beers, Zawadzki, Mazumder and Woodroffe2019a). Together these conditions mean that BCEs can store orders of magnitude more carbon within their substrates than other terrestrial ecosystems, estimated to be in the order of 0.4–6.5 Pg. C in the upper 1 m of saltmarsh substrates globally, 9.4–10.4 Pg. C for mangrove forest substrates and 4.2–8.4 Pg. C for seagrass substrates (Duarte et al., Reference Duarte, Losada, Hendriks, Mazarrasa and Marba2013).
Estimates of carbon storage in BCEs are typically determined based on their current distribution. At the coarsest level, a central measure (e.g. mean, median) of carbon concentration is multiplied by BCE extent to estimate carbon storage in various components (above- and below-ground biomass, soil carbon pool) (Howard et al., Reference Howard, Hoyt, Isensee, Pidgeon and Telszewski2014). As can be seen in Figure 1 such projections to a global scale will be subject to large uncertainties associated with the high variability, across multiple spatial scales, in estimates within each ecosystem type; spatial biases in data availability also influence confidence in global projections.
In spite of this increasing recognition of spatial and temporal variation in carbon storage, it is probable that additional soil organic carbon originating from BCEs is preserved within coastal floodplains and on continental shelfs where conditions are now no longer favourable for BCEs, but where long-term preservation may have occurred over millennia as coastal landscapes evolved (Hanebuth et al., Reference Hanebuth, Stattegger and Grootes2000; Grindrod, Reference Grindrod, Kershaw, David, Tapper, Penny and Brown2002; Rogers et al., Reference Rogers, Macreadie, Kelleway and Saintilan2019b). Additionally, there is also an imprint of direct human impacts on BCEs, with losses largely due to land cover change and gains largely due to restoration activities leading to a net decline in tidal wetland extent (inclusive of tidal flats, mangrove forests and tidal marshes) of ~4,000 km2 between 1999 and 2019 (Murray et al., Reference Murray, Worthington, Bunting, Duce, Hagger, Lovelock, Lucas, Saunders, Sheaves and Spalding2022). There is evidence that this rate of loss is diminishing (Friess et al., Reference Friess, Yando, Abuchahla, Adams, Cannicci, Canty, Cavanaugh, Connolly, Cormier and Dahdouh-Guebas2020; Campbell et al., Reference Campbell, Fatoyinbo, Goldberg and Lagomasino2022) and substantial gains to tidal wetland extent, in the order of 9,700 km2, have been related to the success of restoration activities and natural expansion (Friess et al., Reference Friess, Rogers, Lovelock, Krauss, Hamilton, Lee, Lucas, Primavera, Rajkaran and Shi2019; Murray et al., Reference Murray, Worthington, Bunting, Duce, Hagger, Lovelock, Lucas, Saunders, Sheaves and Spalding2022). Accordingly, the current distribution of BCEs indicates where current storage and additionality occurs but does not indicate storage that occurred prior to changes in land cover at millennia timescales or over the record of Earth observations. This has important implications for the future of blue carbon, and it is probable that the geographic distribution of BCEs will continue to change as sea level rises, coasts evolve, and humans living in the coastal zone adapt to a new configuration of the coast and BCE distribution.
To characterise the future of blue carbon as a natural climate solution, we apply concepts of uniformitarianism to consider how the contemporary distribution and behaviour of blue carbon can inform our understanding of the long-term evolution of coastal carbon storage. In this regard, we consider processes that influence blue carbon over the observational record (i.e. the present) are the same as those that have operated for millennia (i.e. the past), and, by extrapolation, consider blue carbon futures. We specifically consider the evidence preserved in stratigraphic records of the evolution of BCEs and present the case that blue carbon storage is related to coastal evolution and influenced by sediment supply and Holocene sea-level change. We also recognise that coastal evolution and the influence of humans on coastal landscapes means that the response of BCEs in the past is not a direct analogue for their future adaptation to anticipated sea-level rise and climate change (Woodroffe and Murray-Wallace, Reference Woodroffe and Murray-Wallace2012). Nevertheless, present-day coastal landscapes are an archive of information that can be used to parameterise models projecting the response of BCEs to environmental change; thereby providing the critical information needed to inform coastal zone planning and decision-making, improve the resilience of BCEs and ensure their long-term application as a nature-based solution that contributes to climate mitigation efforts.
The PRESENT: Blue carbon in coastal landscapes
Mangroves and saltmarsh typically occupy the upper half of the intertidal zone, with mangrove forests dominating intertidal shorelines of the tropics and saltmarshes dominating intertidal shorelines of temperate zones (Figure 2). There is considerable overlap in the latitudinal distribution of mangroves and saltmarshes (Figure 1), with mangroves generally limited to ocean temperatures that exceed 20 °C during the coldest month (West, Reference West1956; Quisthoudt et al., Reference Quisthoudt, Schmitz, Randin, Dahdouh-Guebas, Robert and Koedam2012; Osland et al., Reference Osland, Enwright, Day, Gabler, Stagg and Grace2016), while saltmarsh distribution is influenced by substrate salinity (Bertness et al., Reference Bertness, Gough and Shumway1992; Silvestri et al., Reference Silvestri, Defina and Marani2005). Where salinity is very high and salts concentrate hypersaline flats/sandflats or sabkha predominate, often with cyanobacterial mats, and saltmarsh vegetation is sparse; in the tropics where rainfall is high, mangroves predominate in the upper half of the intertidal zone (Rogers and Woodroffe, Reference Rogers, Woodroffe, Masselink and Gehrels2014). Most seagrass species occupy fully inundated substrates (i.e. subtidal elevations) where water clarity is a significant control on productivity (Madsen et al., Reference Madsen, Chambers, James, Koch and Westlake2001). Where hydrodynamic conditions allow, a diversity of seagrass genera may also occupy the lower intertidal niche (i.e. below MSL) (Björk et al., Reference Björk, Uku, Weil and Beer1999). Depth-constrained species of seagrass and macroalgae can accumulate organic material within substrates as they adjust to changing water levels (e.g. Zostera spp., Halophila spp. and Phyllospadix spp.) (Koch, Reference Koch2001; Madsen et al., Reference Madsen, Chambers, James, Koch and Westlake2001). While seagrass meadows may be an exceptional carbon source for sequestration elsewhere, their capacity for in situ blue carbon storage is largely limited to that stored within the living biomass and some detritus. Increased sequestration is therefore largely dependent upon an increase in the lateral extent of seagrass meadows (Greiner et al., Reference Greiner, McGlathery, Gunnell and McKee2013) and should be balanced against the additional CO2 that is released by carbonate sediment production (Howard et al., Reference Howard, Creed, Aguiar and Fourqurean2018). While net ecosystem primary production may be high for macroalgae, with potential importance for the export of carbon to other environments, in situ burial of carbon is limited (Figure 1; Krause-Jensen and Duarte, Reference Krause-Jensen and Duarte2016). For these reasons, this review focuses on mangrove and saltmarsh blue carbon futures.
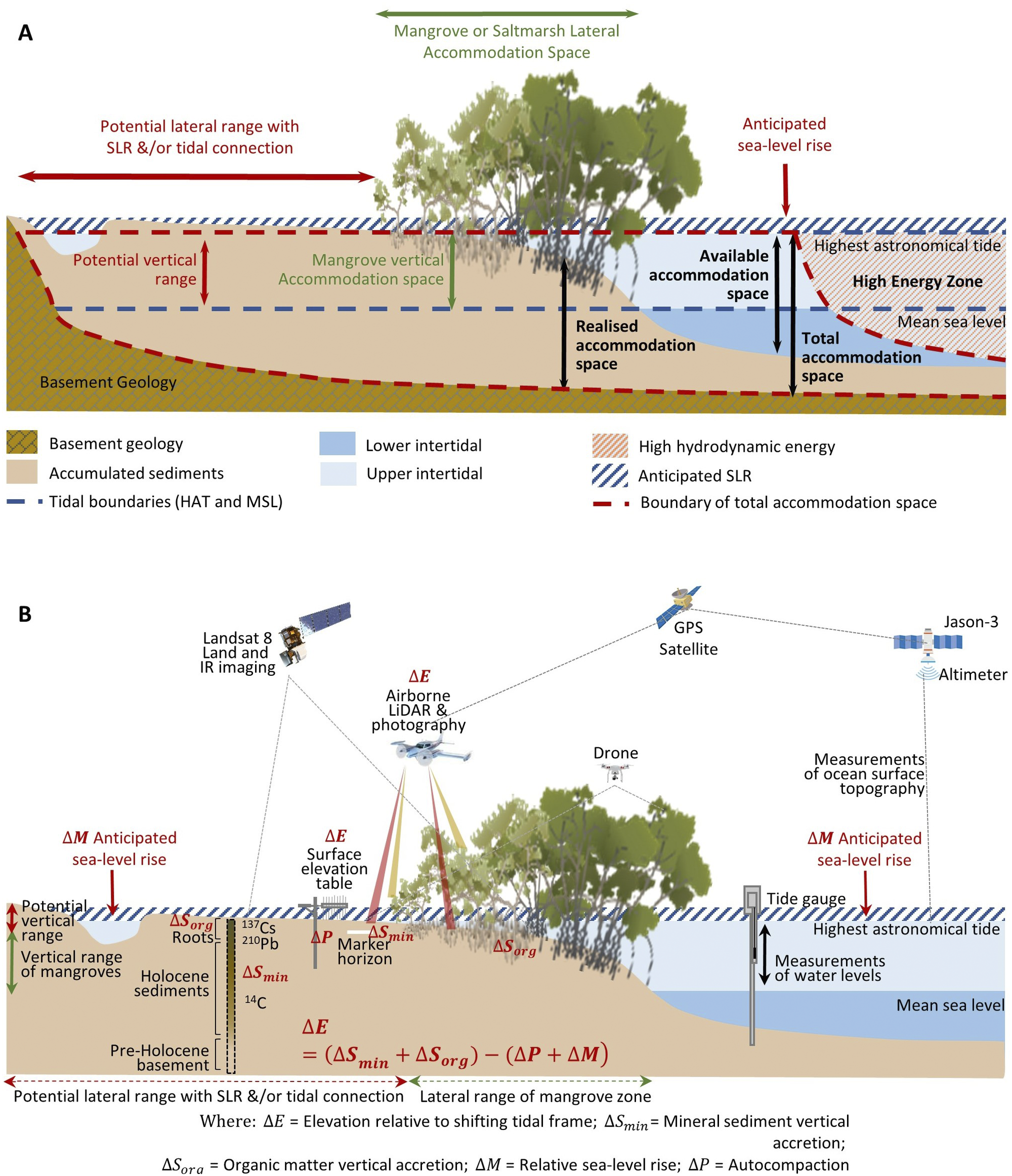
Figure 2. Profiles of BCE landscapes indicating (A) accommodation space, delimited by the highest astronomical tide, basement or bedrock geology, and hydrodynamic conditions favourable for mineral and organic matter accumulation (modified from Rogers (Reference Rogers2021)); and (B) the range of techniques that can be used to observe and measure changes in substrate volume, mineral and organic matter accumulation, and position within the tidal frame, with specific focus on changing tidal position with sea-level rise, as per Allen (Reference Allen2000).
Geomorphological settings occupied by BCEs are delimited to areas where low-energy intertidal substrates support the establishment and maintenance of salt-tolerant vegetation. Globally, deltas are hotspots for BCEs due to high rates of sediment supply promoting the development of broad intertidal environments where resource availability is high (Rovai et al., Reference Rovai, Twilley, Castañeda-Moya, Riul, Cifuentes-Jara, Manrow-Villalobos, Horta, Simonassi, Fonseca and Pagliosa2018; Worthington et al., Reference Worthington, Zu Ermgassen, Friess, Krauss, Lovelock, Thorley, Tingey, Woodroffe, Bunting and Cormier2020; Murray et al., Reference Murray, Worthington, Bunting, Duce, Hagger, Lovelock, Lucas, Saunders, Sheaves and Spalding2022). Along tide-dominated coasts, favourable conditions may arise on the open coast where tidally borne sediments can accumulate, or within the intertidal zone of tide-dominated estuaries. Along wave-dominated coasts, barriers at estuary entrances dampen wave energy and terrigenous sediments supplied from catchments via distributaries contribute to intertidal development of fluvial deltas, and to a lesser extent marine sediment delivered by tides contribute to the development of flood-tide deltas that support BCEs. These fluvial and floodtide deltas associated with wave-dominated estuaries are also hotspots for blue carbon due to the development of low-gradient intertidal substrates (Kelleway et al., Reference Kelleway, Saintilan, Macreadie and Ralph2016a).
Global and continental scale analyses of above-ground biomass of mangroves and saltmarshes variably highlight the role of climatic factors that vary with latitude, proposing that optimal temperature and higher rainfall favours productivity and carbon addition to plants within BCEs (Kirwan and Mudd, Reference Kirwan and Mudd2012; Rovai et al., Reference Rovai, Riul, Twilley, Castañeda‐Moya, Rivera‐Monroy, Williams, Simard, Cifuentes‐Jara, Lewis and Crooks2016; Sanders et al., Reference Sanders, Maher, Tait, Williams, Holloway, Sippo and Santos2016). For example, analyses of mangrove heights and biomass using the global shuttle radar topography mission altimetry dataset highlight the role of precipitation, temperature and cyclone frequency, explaining 74% of global trends in mangrove canopy height (Simard et al., Reference Simard, Fatoyinbo, Smetanka, Rivera-Monroy, Castañeda-Moya, Thomas and Van der Stocken2019). Yet, these are also factors that modify substrate salinity and may promote decomposition of organic material (Chmura et al., Reference Chmura, Anisfeld, Cahoon and Lynch2003; Kirwan et al., Reference Kirwan, Guntenspergen and Langley2014; Mueller et al., Reference Mueller, Schile-Beers, Mozdzer, Chmura, Dinter, Kuzyakov, de Groot, Esselink, Smit and D’Alpaos2018). The outcome may be that once living standing stock reaches a threshold biomass, additions to the living biomass are offset by losses to the standing stock (Chmura et al., Reference Chmura, Anisfeld, Cahoon and Lynch2003).
Observations of mangrove above-ground biomass addition in forestry plots indicate that individual tree growth will asymptote at a threshold height and biomass addition is largely limited to small increments to woody components (i.e. thickening of trunks and stems) (Jin-Eong et al., Reference Jin-Eong, Khoon and Clough1995; Osland et al., Reference Osland, Feher, Spivak, Nestlerode, Almario, Cormier, From, Krauss, Russell and Alvarez2020; Alongi, Reference Alongi2020b). It is this asymptotic nature of above-ground biomass addition over time that has led to many forestry-based carbon offsetting schemes having a minimum commitment period of at least 20 years before harvesting can occur (Galik et al., Reference Galik, Baker, Daigneault and Latta2022) and this has translated into voluntary methods for blue carbon offsetting (Lovelock et al., Reference Lovelock, Adame, Bradley, Dittmann, Hagger, Hickey, Hutley, Jones, Kelleway and Lavery2022a). This aligns with the period over which biomass addition accelerates as plants establish and the rate of carbon sequestration is high. When mangrove forests and saltmarshes have reached their threshold capacity for standing above-ground biomass, then increases in the standing living stock are largely achieved by lateral increases in extent as increases in plant density in mature forests will be resource limited. Increases in below-ground biomass are presumed to be limited by vertical space within substrates for net biomass additionality and addition of biomass will increasingly be offset by decomposition of below-ground biomass as substrates asymptote towards higher elevations; that is unless relative sea-level rise creates more vertical space for below-ground storage. Critically, lateral increases in extent are constrained by the availability of land where conditions are favourable within the intertidal and supratidal zone. In addition, although intense cyclones and storms are reported to have a return interval of approximately 20 years (Elsner et al., Reference Elsner, Jagger and Tsonis2006), aligning with the commitment period for restoration projects before harvesting can occur (Galik et al., Reference Galik, Baker, Daigneault and Latta2022), the feasibility of restoration projects in regions with a propensity for cyclone activity may decrease should projected increases in the frequency and intensity of major storms eventuate (IPCC, Reference Masson-Delmotte, Zhai and Pirani2021).
Partitioning of mangrove biomass between above-ground and below-ground differs from terrestrial forests in that a relatively high proportion is allocated to below-ground root systems (Saintilan, Reference Saintilan1997; Lichacz et al., Reference Lichacz, Hardiman and Buckney2009), a possible adaptation to saline conditions (Ball, Reference Ball1988). However, below-ground biomass is somewhat difficult to determine because decomposition is limited by saline and anaerobic conditions of tidally inundated substrates, and differentiating living and dead components of below-ground biomass is difficult (Adame et al., Reference Adame, Cherian, Reef and Stewart-Koster2017). The proportion of mass allocated to above and below-ground components is influenced by environmental conditions, most notable is variation arising from soil water salinity, observed in both laboratory (Ball, Reference Ball1988; Ball, Reference Ball2002) and field studies (Saintilan, Reference Saintilan1997), but also soil water nutrient conditions (Darby and Turner, Reference Darby and Turner2008) and atmospheric CO2 concentrations (Langley et al., Reference Langley, McKee, Cahoon, Cherry and Megonigal2009). Observations of below-ground root addition have been undertaken using root ingrowth bags and ‘marsh organ’ experiments, indicating rapid root development (Muhammad-Nor et al., Reference Muhammad-Nor, Huxham, Salmon, Duddy, Mazars-Simon, Mencuccini, Meir and Jackson2019; Kihara et al., Reference Kihara, Dannoura and Ohashi2022), an observation supported by repeat measures of root biomass (Lamont et al., Reference Lamont, Saintilan, Kelleway, Mazumder and Zawadzki2020).
While the addition of carbon to living biomass makes an important contribution to carbon drawdown from the atmosphere, the substrates of BCEs are typically the largest carbon pool within BCEs with soil organic carbon to a depth of 1 m estimated to comprise 77% of the total global mangrove stock and 95% of the total global saltmarsh stock (Alongi, Reference Alongi2020a). Constraining controls on global below-ground carbon storage has been more elusive, and variably related to edaphic conditions associated with climate and substrate salinity (Chmura et al., Reference Chmura, Anisfeld, Cahoon and Lynch2003; Kirwan and Mudd, Reference Kirwan and Mudd2012; Sanders et al., Reference Sanders, Maher, Tait, Williams, Holloway, Sippo and Santos2016; Rovai et al., Reference Rovai, Twilley, Castañeda-Moya, Riul, Cifuentes-Jara, Manrow-Villalobos, Horta, Simonassi, Fonseca and Pagliosa2018; Sanderman et al., Reference Sanderman, Hengl, Fiske, Solvik, Adame, Benson, Bukoski, Carnell, Cifuentes-Jara and Donato2018). It took some time for the role of sea-level rise to be linked to soil organic carbon accumulation (Wang et al., Reference Wang, Lu, Sanders and Tang2019; Rogers et al., Reference Rogers, Kelleway, Saintilan, Megonigal, Adams, Holmquist, Lu, Schile-Beers, Zawadzki, Mazumder and Woodroffe2019a); this is surprising given the geographic position of intertidal BCEs near mean sea level and the well-established influence of sea-level rise on coastal geomorphology. Increasing mangrove extent with relative sea-level rise and warmer temperatures over the past few decades has been observed and is particularly notable in Australia, Brazil, the Gulf and Atlantic US Coastline, Mexico, and South Africa where mangroves have expanded landward to higher elevations and/or to more poleward positions (Saintilan et al., Reference Saintilan, Wilson, Rogers, Rajkaran and Krauss2014; Godoy and de Lacerda, Reference Godoy and de Lacerda2015; Ximenes et al., Reference Ximenes, Maeda, Arcoverde and Dahdouh-Guebas2016; Osland et al., Reference Osland, Feher, Griffith, Cavanaugh, Enwright, Day, Stagg, Krauss, Howard, Grace and Rogers2017). Increases in soil organic carbon storage have occurred in consort (Kelleway et al., Reference Kelleway, Saintilan, Macreadie, Skilbeck, Zawadzki and Ralph2016b; Simpson et al., Reference Simpson, Stein, Osborne and Feller2019), implying that links between soil organic carbon storage and sea level are partly mediated by vegetation change.
Increases in organic carbon accumulation have been measured in wetlands subject to increased rates of sea-level rise in southwest Florida (Breithaupt et al., Reference Breithaupt, Smoak, Bianchi, Vaughn, Sanders, Radabaugh, Osland, Feher, Lynch and Cahoon2020), and eastern Australia (Marx et al., Reference Marx, Knight, Dwyer, Child, Hotchkis and Zawadzki2020). Extreme rapid subsidence beneath a coastal wetland in Australia, in the order of 1 m, has served as a natural laboratory for observing the influence of relative sea-level rise on carbon storage and addition to mangrove and saltmarsh ecosystems (Rogers et al., Reference Rogers, Kelleway, Saintilan, Megonigal, Adams, Holmquist, Lu, Schile-Beers, Zawadzki, Mazumder and Woodroffe2019a). Here soil organic carbon addition accelerated following an increase in relative sea level. Following subsidence, conditions in the former higher elevation saltmarsh became favourable for mangroves, which rapidly established a deeper root network, supplementing the soil organic carbon pool. The submerged mangrove forest was inundated more frequently, providing more opportunities for carbon-rich tidally borne sediments to accumulate and increase the rate of carbon addition.
The great mangrove forests and saltmarsh plains prior to widespread human-driven change to the coastal zone have been reduced to remnants following decades of clearance for aquaculture, agriculture, coastal developments, and tidal obstructions (Gedan et al., Reference Gedan, Silliman and Bertness2009; Friess et al., Reference Friess, Rogers, Lovelock, Krauss, Hamilton, Lee, Lucas, Primavera, Rajkaran and Shi2019; Goldberg et al., Reference Goldberg, Lagomasino, Thomas and Fatoyinbo2020). This is compounded by conversion to open water arising from land subsidence following groundwater and hydrocarbon extraction and/or associated with diminishing sediment supply arising from damming and diversions. Losses in recent decades of mangrove forests (0.7–3.0% yr.−1), saltmarshes (1.0–2.0% yr.−1) and seagrass meadows (0.4–2.6% yr.−1) have contributed an estimated 0.15–1.02 Pg of CO2 emission per year (Pendleton et al., Reference Pendleton, Donato, Murray, Crooks, Jenkins, Sifleet, Craft, Fourqurean, Kauffman and Marbà2012) (by comparison, 3–19% of emissions from deforestation). These losses reverse decades of carbon sequestration within biomass and millennia of sequestration from substrates. In particular methane flux, a greenhouse gas with ~28 times higher global warming potential than carbon dioxide (Foster et al., Reference Forster, Storelvmo, Armour, Collins, Dufresne, Frame, Lunt, Mauritsen, Palmer, Watanabe, Wild, Zhang, Masson-Delmotte, Zhai, Pirani, Connors, Péan, Berger, Caud, Chen, Goldfarb, Gomis, Huang, Leitzell, Lonnoy, Matthews, Maycock, Waterfield, Yelekçi, Yu and Zhou2021), is markedly higher following substrate disturbance. A global review of methane emissions arising from conversion of mangroves, saltmarshes, seagrasses and tidal flats for coastal aquaculture estimated a rise in methane emissions per area 7–430 times higher than emissions from non-converted coastal habitats (Rosentreter et al., Reference Rosentreter, Borges, Deemer, Holgerson, Liu, Song, Melack, Raymond, Duarte and Allen2021b). Fortunately, there is evidence that this trend of declining BCE extent is slowing (Pendleton et al., Reference Pendleton, Donato, Murray, Crooks, Jenkins, Sifleet, Craft, Fourqurean, Kauffman and Marbà2012; Friess et al., Reference Friess, Rogers, Lovelock, Krauss, Hamilton, Lee, Lucas, Primavera, Rajkaran and Shi2019; Friess et al., Reference Friess, Yando, Abuchahla, Adams, Cannicci, Canty, Cavanaugh, Connolly, Cormier and Dahdouh-Guebas2020), and losses are being offset by the creation of new wetlands (Murray et al., Reference Murray, Worthington, Bunting, Duce, Hagger, Lovelock, Lucas, Saunders, Sheaves and Spalding2022).
Conceptualising blue carbon accommodation space
Accommodation is a term used to define the three-dimensional space available for mineral sediments and soil organic matter (Jervey, Reference Jervey, Wilgus, Hastings and Posamentier1988) (Figure 2A). The maximum elevation of tidal inundation delimits both the landward extent that tidally borne mineral and organic matter can accumulate, and delimits the zone supporting living mangrove and saltmarsh vegetation and in situ soil organic matter contributions (Rogers, Reference Rogers2021). In situ contributions are also delimited at the seaward margin and along tidal creeks by the low-energy hydrodynamic conditions required for vegetation establishment and ongoing survival; in these locations, it is only detrital material that can accumulate within sediments. Initially, bedrock or basement geology delimits the zone in which sediments can accumulate, but as accommodation becomes increasingly infilled, or ‘realised’, via the accumulation of mineral and organic material, substrate elevations increase and become progressively terrestrialised and exposed to the oxidising conditions underpinning aerobic processes of soil organic matter decomposition. In these circumstances, an increase in available accommodation, either via autocompaction of sediments that have accumulated within the ‘realised’ accommodation, subsidence of the basement, or sea-level rise, is required to reinstate tidal inundation, preserve the niche of BCEs, minimise decomposition of organic material by processes of oxidation or methanogenesis and provide new space for additional soil organic matter.
Observations of mangrove and saltmarsh substrate elevation changes using techniques such as surface elevation tables, marker horizons and radiometric dating (Figure 2B) confirm that sedimentation and surface elevation gain are proportional to position in the tidal frame, reflecting the influence of accommodation on accumulation of mineral and organic material in substrates (Webb et al., Reference Webb, Friess, Krauss, Cahoon, Guntenspergen and Phelps2013; Raw et al., Reference Raw, Riddin, Wasserman, Lehman, Bornman and Adams2020; Cahoon et al., Reference Cahoon, McKee and Morris2021; Saintilan et al., Reference Saintilan, Kovalenko, Guntenspergen, Rogers, Lynch, Cahoon, Lovelock, Friess, Ashe and Krauss2022). The effect of sea-level rise on the position in the tidal frame of BCEs has been conceptualised by Allen (Reference Allen2000) to account for the addition of mineral and organic material, autocompaction and relative sea-level rise. Providing accommodation is available, below-ground biomass from established vegetation increases substrate volume and the mass of the soil organic carbon pool; above-ground biomass baffles tidal energy, improving hydrodynamic conditions for the deposition of suspended sediments. As substrate elevations increase, the space available for below-ground organic matter additions and mineral sediment addition diminishes, and organic matter decomposition may increase due to an associated reduction in inundation depth, duration and/or frequency (often termed hydroperiod). In combination, these factors generate a self-organising negative feedback that favours the stabilisation of substrate elevations. As a small increment in sea level increases accommodation, the associated increase in tidal inundation serves to enhance conditions favouring the accumulation of mineral and organic sediments, thereby offsetting the small increment in sea level, and maintaining the intertidal position of the substrate; addition of organic material is a vital component of this negative feedback. The coupling between inundation and organic matter addition that contributes to this negative feedback was initially conceptualised for marshes of the SE coast of the USA (Morris et al., Reference Morris, Sundareshwar, Nietch, Kjerfve and Cahoon2002) and has formed the basis for models projecting the organic response of substrates to sea-level rise (Mudd et al., Reference Mudd, Howell and Morris2009; Mack et al., Reference Mack, Lane, Deng, Morris and Bauer2023). Field studies have also confirmed linkages between coastal wetland evolution, accommodation space and carbon concentrations in mangrove and saltmarsh substrates of SE Australia (Owers et al., Reference Owers, Woodroffe, Mazumder and Rogers2022) (Figure 3).
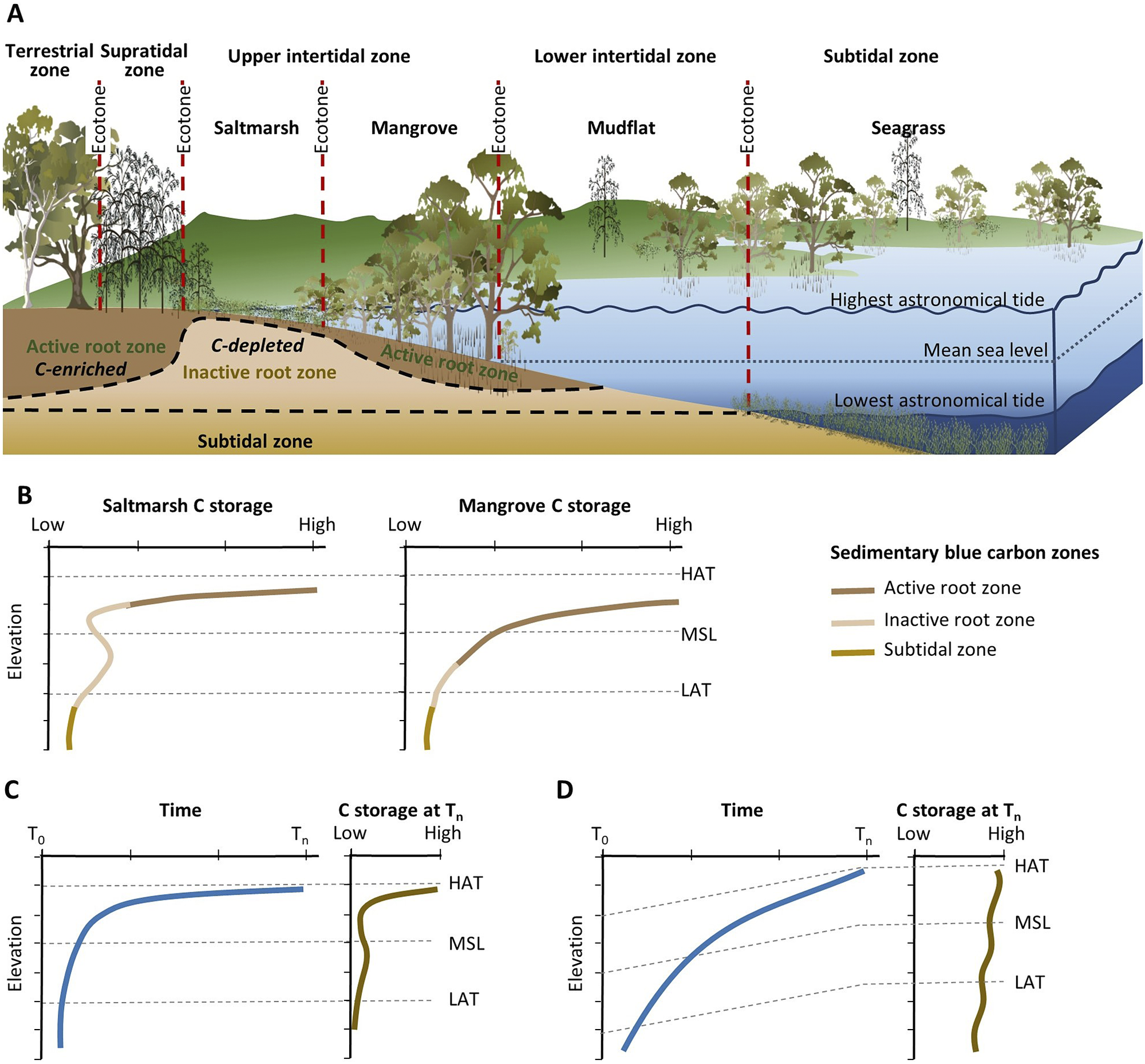
Figure 3. (A) Conceptual model of lateral zonation of BCEs of southeastern Australia with respect to tidal parameters, and varying distribution of soil organic carbon within the active root zone, inactive root zone and subtidal zone; and (B) associated generalised variation in carbon storage within BCEs (modified from Owers et al. (Reference Owers, Woodroffe, Mazumder and Rogers2022)). (C and D) Relationships between carbon storage and sea-level change (from T 0 to T n) under conditions of (C) relatively stable sea level since the mid-Holocene and (D) rising sea level since the mid-Holocene (modified from Allen (Reference Allen2000)).
The PAST: Sea-level rise, and blue carbon accommodation
For extended periods over Earth’s history, when the coincidence of rising sea level, favourable coastal geomorphology and suitable tidal range has been conducive to extensive coastal wetland development, blue carbon has been an important and arguably dominant control on global trends in atmospheric CO2. During the Oligo-Miocene, the combined influence of sea-level rise, high tidal range and a resultant extensive mangrove development in the South China Sea trapped up to 2000 Pg of organic carbon, equivalent to up to 60 p.p.m. of atmospheric CO2 per Myr (Collins et al., Reference Collins, Avdis, Allison, Johnson, Hill, Piggott, Hassan and Damit2017). The development of these forests could have been a major contributor to the reduction in atmospheric CO2 concentrations from circa 800 to 300 p.p.m. since the Late Oligocene (34-0 Ma) (Collins et al., Reference Collins, Avdis, Allison, Johnson, Hill, Piggott, Hassan and Damit2017).
At the peak of the last glacial maximum, sea level was 130–120 m lower than present; this low stand and the present high stand are indicative end points of global eustatic sea-level cycles (Murray-Wallace and Woodroffe, Reference Murray-Wallace and Woodroffe2014). The response of mangrove forests and saltmarshes to sea-level rise since the last glacial maximum has been likened to the behaviour of coral reefs at the same period (Neumann and MacIntyre, Reference Neumann, Macintyre, Gabrie, Toffart and Salvat1985; Reed, Reference Reed1990; Woodroffe and Davies, Reference Woodroffe, Davies, Perillo, Wolanski, Cahoon and Brinson2009), where, depending upon the rate of sediment supply relative to the rate of sea-level rise, mangrove forests and saltmarshes may be ‘drowned’, ‘backstep’, ‘catch-up’, ‘keep-up’, ‘prograde’ or ‘emerge’. When rates of sea-level rise exceeded 1 m per century during the late-Pleistocene and early-Holocene, the capacity of mangrove forests to accumulate mineral and organic material appears to have been exhausted, and evidence of ‘drowned’ mangrove peats overtopped by marine transgressive sand sheets have been preserved on the Sahul Shelf (northwest Western Australia (Nicholas et al., Reference Nicholas, Nichol, Howard, Picard, Dulfer, Radke, Carroll, Tran and Siwabessy2014) and the Sunda Shelf (on the western rim of the South China Sea) at water depths of up to 100 m (Hanebuth et al., Reference Hanebuth, Stattegger and Grootes2000). Extensive mangrove forest development at the time appears to have been terminated by Meltwater Pulse 1A, during which rates of relative sea-level rise increased to >20 mm yr.−1 (Lambeck et al., Reference Lambeck, Rouby, Purcell, Sun and Sambridge2014).
Deceleration in the rate of sea-level rise in the early-Holocene was broadly marked by mangrove development in the millennia prior to sea-level stabilisation near present levels. In settings of high sediment yield, including the Ganges-Brahmaputra delta, India (Hait and Behling, Reference Hait and Behling2009) and the Queensland continental shelf, Australia (Grindrod et al., Reference Grindrod, Moss and Kaars1999), mangrove forests adjusted in situ to sea-level rise from the early-Holocene (~9,000 BP), but were subsequently ‘drowned’ and then ‘backstepped’. By ~7,500 BP, relative sea-level rise had decelerated to less than 6–7 mm yr.−1, and ‘catch-up’, or mangrove landward transgression, followed by ‘progradation’ occurred where sediment supply was high, resulting in extensive mangrove forests in tropical minerogenic settings. In many places, these forests were considerably greater in extent than contemporary mangrove forests, including Australia (Woodroffe et al., Reference Woodroffe, Thom and Chappell1985), the Mekong and Red River deltas of Vietnam (Tran and Ngo, Reference Tran, Ngo and Nguyen2000; Li et al., Reference Li, Saito, Mao, Tamura, Song, Zhang, Lu, Sieng and Li2012), and the Great Songkla Lakes, Thailand (Horton et al., Reference Horton, Gibbard, Mine, Morley, Purintavaragul and Stargardt2005). High rates of organic matter accumulation in this globally synchronous phase of blue carbon development sequestered an estimated 20–60 Pg. C, contributing to a 5 p.p.m. decline in atmospheric CO2 concentrations in the early Holocene (Saintilan et al., Reference Saintilan, Khan, Ashe, Kelleway, Rogers, Woodroffe and Horton2020). An early-mid Holocene decline in methane, primarily in the Southern Hemisphere, according to ice-core data (Beck et al., Reference Beck, Bock, Schmitt, Seth, Blunier and Fischer2018), commenced over the same period, with reductions in Southern Hemisphere emissions estimates of ~19 Tg CH4 yr.−1 (Beck et al., Reference Beck, Bock, Schmitt, Seth, Blunier and Fischer2018). The δ13C signals in methane in the Southern Hemisphere for the period show a 1.5 p.p.t. depletion (Beck et al., Reference Beck, Bock, Schmitt, Seth, Blunier and Fischer2018) consistent with a replacement of vegetation utilising the C4 photosynthetic pathway (tropical grasslands and saltmarsh adapted to low atmospheric carbon dioxide) with mangroves utilising the C3 pathway (Sowers, Reference Sowers2010).
By the mid-Holocene, eustatic sea level stabilised within approximately 2 m of its current elevation (Clark et al., Reference Clark, Farrell and Peltier1978; Khan et al., Reference Khan, Ashe, Shaw, Vacchi, Walker, Peltier, Kopp and Horton2015). However, the initiation of mangrove and saltmarsh transgressive phases was globally variable due to the influence of glacio-isostatic adjustment on varying rates of mid- to late-Holocene relative sea-level rise (Ribeiro et al., Reference Ribeiro, Batista, Cohen, França, Pessenda, Fontes, Alves and Bendassolli2018), and the modulating effect of other climatic variables (e.g. droughts and/or frequent storms) on conditions conducive to intertidal vegetation expansion or decline (Sherrod and McMillan, Reference Sherrod and McMillan1985; Jones et al., Reference Jones, Wingard, Stackhouse, Keller, Willard, Marot, Landacre and Bernhardt2019). Global scale variation in relative sea-level trends, largely arising from glacio-isostatic adjustment, had a profound influence on blue carbon accumulation throughout the mid- to late-Holocene and storage since this time (Rogers et al., Reference Rogers, Kelleway, Saintilan, Megonigal, Adams, Holmquist, Lu, Schile-Beers, Zawadzki, Mazumder and Woodroffe2019a; Reference Rogers, Macreadie, Kelleway and Saintilan2019b). Principally related to distance from regions of maximal ice sheet extent during the last glacial period, relative sea-level rise modifies the accommodation available for blue carbon. The delineation of zones across oceans globally where post-glacial sea-level trends are relatively similar (Figure 4A and 4B, Clark et al., Reference Clark, Farrell and Peltier1978) therefore provides an indication of the accommodation available for blue carbon storage over the past few millennia. While more is known of post-glacial sea-level change since these zones were initially demarcated, their broad correspondence with what we now know of relative sea-level trends (Khan et al., Reference Khan, Ashe, Shaw, Vacchi, Walker, Peltier, Kopp and Horton2015) provides some confidence in the geographic position of zones (noting that boundaries between zones are diffuse and not definite). Clark et al. (Reference Clark, Farrell and Peltier1978) delineated five zones across oceans globally (a sixth zone was associated with continental coastlines) that can be broadly grouped into three regions: (i) near-field locations are proximal to ice sheets of the last glacial maximum and typically exhibit continuous patterns of relative sea-level fall (i.e. Clark et al., Reference Clark, Farrell and Peltier1978, zone I); (ii) intermediate locations exhibit complex sea-level trends; however, relative sea-level rise over the past few millennia is typical; and (iii) far-field locations are distal from ice sheets and eustatic sea-level trends dominate processes of glacio-isostatic adjustment. For brevity, we focus on end members: far-field locations (Zones IV and V) where the relative sea level has been relatively stable for millennia (or may have fallen); intermediate locations where relative sea level has been rising over the mid- to late-Holocene (Zones II and III); and near-field locations (Zone I) where relative sea level has been falling (Figure 4A).
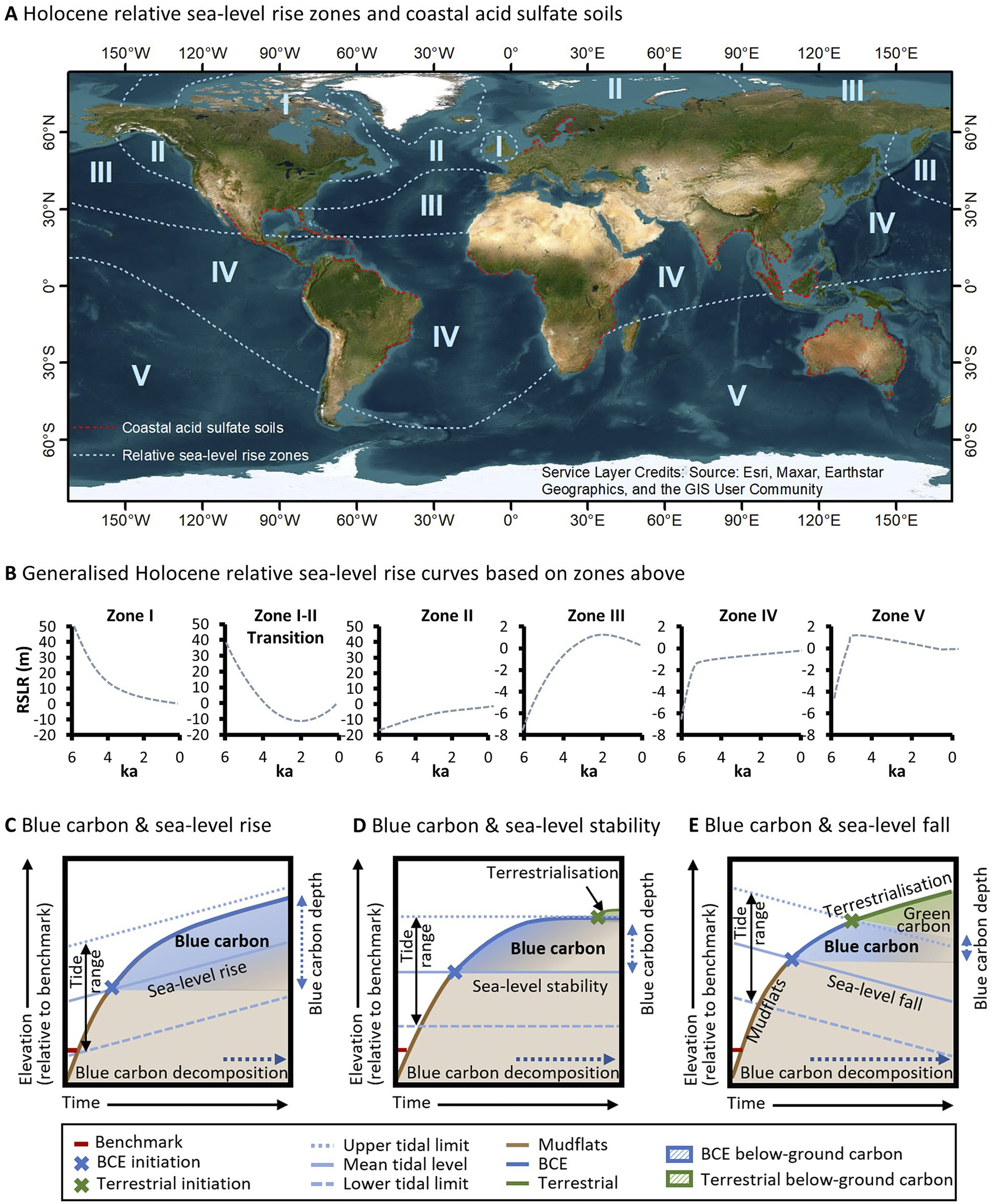
Figure 4. Relative sea-level change is a significant control on processes of carbon accumulation and decomposition, and varies globally according to the generalised Holocene relative sea-level zones (A) (Clark et al., Reference Clark, Farrell and Peltier1978) and generalised Holocene relative sea-level curves across these zones (B). Note the distribution of coastal acid sulphate soils in (Michael, Reference Michael2013) (A), which corresponds broadly with regions where sea-level conditions facilitated widescale mangrove and saltmarsh development throughout the late-Holocene. When sea levels are rising (C), sedimentary carbon continues to accumulate within available accommodation and pathways of decomposition are dampened under increasingly anaerobic conditions. Where sea level has been relatively stable (D), blue carbon additionality is limited by the upper limit of tidal inundation and substrates become increasingly mineral dominated and support terrestrial vegetation as accommodation diminishes. Under conditions of falling sea levels (E), substrates become increasingly terrestrialised (i.e. with terrestrial vegetation) and conditions favour aerobic decomposition and methanogenesis of blue carbon.
Far-field locations (Zones IV and V), distal from regions of maximal ice sheet extent, exhibit patterns of mid-Holocene infill in mangrove (Woodroffe et al., Reference Woodroffe, Mulrennan and Chappell1993; Cohen et al., Reference Cohen, Filho, Lara, Behling and Angulo2005; Hashimoto et al., Reference Hashimoto, Saintilan and Haberle2006; Proske and Haberle, Reference Proske and Haberle2012; França et al., Reference França, Cohen, Pessenda, Rossetti, Lorente, Junior, Guimarães, Friaes and Macario2013; Boski et al., Reference Boski, Bezerra, de Fátima, Souza, Maia and Lima-Filho2015; Punwong et al., Reference Punwong, Selby and Marchant2018) and saltmarsh settings, with coastal barriers typically enclosing bays along the more southern wave-dominated coastlines (Compton, Reference Compton2001; Vilanova et al., Reference Vilanova, Prieto and Espinosa2006; Fornari et al., Reference Fornari, Giannini and Junior2012; Kennedy et al., Reference Kennedy, Wong and Jacobsen2021). Ongoing coastal and estuarine sedimentation, and a fall in sea level to present levels where a high stand occurred in the late Holocene, caused coastal floodplains to increasingly ‘prograde’ or become ‘emergent’, with accommodation being limited, and BCEs restricted to the fringes of estuarine shorelines (Woodroffe and Davies, Reference Woodroffe, Davies, Perillo, Wolanski, Cahoon and Brinson2009). BCEs were replaced by floodplain terrestrial forests and freshwater wetlands, a shift that may have contributed to gradual increases in atmospheric methane concentrations in the late-Holocene after declining in the early- to mid-Holocene (Beck et al., Reference Beck, Bock, Schmitt, Seth, Blunier and Fischer2018). Preservation of soil organic carbon in far-field locations is limited by decomposition as coastal floodplains become increasingly terrestrialised and support grasses and sedges, else soil organic matter may be vulnerable to metabolisation and formation of pyrites when sulphate and sulphate reducing bacteria are present. This sets up the conditions for generation of acid sulphate soils when coastal floodplains are drained, and oxidisation occurs. The relationship between former distribution of saltmarshes and mangrove forests, relative sea-level stability and coastal acid sulphate soil development is well established (Pons et al., Reference Pons, Van Breemen and Driessen1982; Van Breemen, Reference Van Breemen1982) and reflected in the greater extent of actual and potential coastal acid sulphate soils in SE Asia, Africa, Australia and South America (Michael, Reference Michael2013) and their virtual absence from intermediate-field locations (see Figure 4A).
Intermediate field locations also exhibit broad agreement in geomorphological evolution over the Holocene (Woodroffe, Reference Woodroffe1981; Digerfeldt and Hendry, Reference Digerfeldt and Hendry1987; Parkinson, Reference Parkinson1989; Parkinson et al., Reference Parkinson, Ron and White1994; McKee, Reference McKee2011). In Florida and the northern Gulf of Mexico, the early Holocene was marked by rates of relative sea-level rise that were too high for broadscale mangrove development (<~7,500 BP) (Sherrod and McMillan, Reference Sherrod and McMillan1985; Parkinson, Reference Parkinson1989). Evident from interbedded peats and marls, the onset of the transgressive ‘catch-up’ phase occurred from about 3,500 BP (Scholl, Reference Scholl1964; Parkinson et al., Reference Parkinson, Ron and White1994; Jones et al., Reference Jones, Wingard, Stackhouse, Keller, Willard, Marot, Landacre and Bernhardt2019), although the occurrence of continuous vertical peat growth, typical of ‘keep-up’ behaviour, is modulated in some locations by other climatic factors, including a period of cooling, that may not have been conducive to widespread mangrove expansion and vertical growth (Sherrod and McMillan, Reference Sherrod and McMillan1985; Jones et al., Reference Jones, Wingard, Stackhouse, Keller, Willard, Marot, Landacre and Bernhardt2019). Where conditions were favourable, vertical growth of mangrove peats is near continuous. In particular, the cenotes of the Yucatan Peninsula are reported to have among the highest mangrove carbon stocks globally, and their accumulation has been related to ongoing relative sea-level rise over the late-Holocene (Adame et al., Reference Adame, Santini, Torres-Talamante and Rogers2021). The later onset of transgressive phases of mangrove development in intermediate field locations reflect stronger rates of relative sea-level rise throughout the mid-Holocene, ongoing sea-level rise in the late-Holocene, the influence of other climatic variables, as well as limited capacity for vertically adjustment to sea-level rise due to low rates of sediment supply in some carbonate dominated settings. Accordingly, ongoing sea-level rise and limited mineral sediment supply since the late-Holocene may explain the preservation of carbon-rich mangrove peats in this region (McKee, Reference McKee2011). Similar preservation of saltmarsh peats and associated foraminifera is evident at intermediate-field sites with a history of increasing accommodation with sea-level rise throughout the mid- to late-Holocene (Redfield, Reference Redfield1972; Orson et al., Reference Orson, Warren and Niering1998; Gehrels, Reference Gehrels1999).
Saltmarshes at near-field locations (climate not suitable for mangroves throughout the Holocene), those proximal to regions of maximum ice sheet extent at the last glacial maximum, exhibited a highly variable pattern of vertical growth and carbon accumulation dependent upon the influence of glacio-isostatic adjustment on relative sea-level rise (Khan et al., Reference Khan, Ashe, Shaw, Vacchi, Walker, Peltier, Kopp and Horton2015). Analyses of radiocarbon dated saltmarsh sequences in the UK differentiated both transgressive sequences (i.e. ‘catch-up’), representing increasing marine influence and regressive sequences (i.e. ‘progradation’ or ‘emergent’) indicating increasing terrestrialisation (Horton et al., Reference Horton, Shennan, Bradley, Cahill, Kirwan, Kopp and Shaw2018). The presence of these sequences aligned with Holocene sea-level history, with ‘catch-up’ transgressive sequences predominating in southern England where relative sea-level rise exhibited a pattern of deceleration, while regressive sequences were more prominent in Scotland, where relative sea level fell in both the early- and late-Holocene. Complex spatio-temporal patterns of postglacial relative sea-level change throughout the mid- to late-Holocene are also preserved in saltmarsh peats of near-field locations across the coastline of the North Atlantic (Vacchi et al., Reference Vacchi, Engelhart, Nikitina, Ashe, Peltier, Roy, Kopp and Horton2018; Cohen et al., Reference Cohen, Cartelle, Barnett, Busschers and Barlow2022; Creel et al., Reference Creel, Austermann, Khan, D’Andrea, Balascio, Dyer, Ashe and Menke2022).
Patterns of relative sea-level change over the Holocene and its influence on the distribution of BCEs have provided important lines of evidence about the future of BCEs. Global analyses of soil organic carbon stocks in saltmarshes indicate that regions where sea level has a longer history of rising over the mid- to late-Holocene, that is intermediate and some near-field locations, exhibit higher soil organic carbon stocks and deeper soil organic carbon pools than far-field locations (Rogers et al., Reference Rogers, Kelleway, Saintilan, Megonigal, Adams, Holmquist, Lu, Schile-Beers, Zawadzki, Mazumder and Woodroffe2019a). This has been linked to the influence of relative sea-level rise on accommodation for blue carbon within substrates, the largest carbon pool within BCEs. Where sea level has been rising at a moderate rate for a few millennia, vertical space is created for storage of blue carbon within below-ground biomass and soils (Figure 4C). In contrast, where sea level has been relatively stable over the mid- to late-Holocene, that is across much of the Southern Hemisphere, carbon pools may be depleted and shallower (although the depth is dependent upon tidal range), and this has been related to the limitations placed on accommodation as substrates become increasingly dominated by mineral sediments (Figure 4D). Where sea level has been falling, substrates become increasingly terrestrialised with brackish to fresh substrate salinities that are aerobic and favour decomposition and methanogenesis (Figure 4E). These differences in organic matter content between far-, intermediate- and near-field locations are also reflected in the character of contemporary sediment accumulating in wetlands. Intermediate- and near-field locations have higher organic carbon accumulation above artificial marker horizons (Saintilan et al., Reference Saintilan, Khan, Ashe, Kelleway, Rogers, Woodroffe and Horton2020), reflecting both the comparatively higher contemporary rates sea-level rise, and possibly the more organic sub-tidal reservoirs of sediment contributing to marsh accretion (Hopkinson et al., Reference Hopkinson, Morris, Fagherazzi, Wollheim and Raymond2018).
The FUTURE: The present and past as a guide to blue carbon futures
The spectre of climate change has focussed attention on reducing atmospheric carbon concentrations and limiting warming well below 2 °C, a commitment established in the Paris Agreement at COP21 (Iyer et al., Reference Iyer, Edmonds, Fawcett, Hultman, Alsalam, Asrar, Calvin, Clarke, Creason and Jeong2015). Accordingly, the capacity of BCEs to draw carbon from the atmosphere is being leveraged as a climate mitigation strategy, and received considerable recognition in the latest IPCC report (Cooley et al., Reference Cooley, Schoeman, Bopp, Boyd, Donner, Ghebrehiwet, Ito, Kiessling, Martinetto, Ojea, Racault, Rost, Skern-Mauritzen, Pörtner, Roberts, Tignor, Poloczanska, Mintenbeck, Alegría, Craig, Langsdorf, Löschke, Möller, Okem and Rama2022). Conservation of BCEs aimed at minimising losses in extent through land use and land cover change (LULCC) and restoring condition through improved management will be important. The Reducing Emissions from Deforestation and Forest Degradation in Developing Countries (REDD+) program of the United Nations Framework Convention on Climate Change (UNFCCC) specifically targets the improved management of forests to minimise loss and release of greenhouse gases, and conservation efforts are increasing in developing countries (Ahmed and Glaser, Reference Ahmed and Glaser2016). Similar programs for non-forested ecosystems, such as saltmarshes, do not exist; however, there is a global peatland initiative that could be applied to saltmarshes. In many jurisdictions BCEs are already protected from loss because of the benefits they provide to society (Romañach et al., Reference Romañach, DeAngelis, Koh, Li, Teh, Barizan and Zhai2018). Despite these policies, loss of BCEs is ongoing (Gedan et al., Reference Gedan, Silliman and Bertness2009; Friess et al., Reference Friess, Rogers, Lovelock, Krauss, Hamilton, Lee, Lucas, Primavera, Rajkaran and Shi2019; Goldberg et al., Reference Goldberg, Lagomasino, Thomas and Fatoyinbo2020), and this increases the burden for carbon additionality by other mechanisms, such as restoration.
Restoring BCEs, achieved by planting vegetation, seeds or propagules, or managing barriers to tidal exchange that have been put in place to facilitate past LULCC, may enhance carbon sequestration as blue carbon vegetation re-establishes. Analyses suggest that restoration to recover BCE habitat that has been lost due to human activities in the coastal zone is potentially feasible for mangroves, less so for seagrasses and saltmarshes (Griscom et al., Reference Griscom, Adams, Ellis, Houghton, Lomax, Miteva, Schlesinger, Shoch, Siikamäki, Smith, Woodbury, Zganjar, Blackman, Campari, Conant, Delgado, Elias, Gopalakrishna, Hamsik, Herrero, Kiesecker, Landis, Laestadius, Leavitt, Minnemeyer, Polasky, Potapov, Putz, Sanderman, Silvius, Wollenberg and Fargione2017; Macreadie et al., Reference Macreadie, Costa, Atwood, Friess, Kelleway, Kennedy, Lovelock, Serrano and Duarte2021). However, the capacity for large-scale restoration is constrained by socio-economic factors, particularly where the coastal zone is critical for maintaining livelihoods and food security (Herr et al., Reference Herr, Blum, Himes-Cornell and Sutton-Grier2019). Efforts are in place globally to restore mangrove forests (Friess et al., Reference Friess, Rogers, Lovelock, Krauss, Hamilton, Lee, Lucas, Primavera, Rajkaran and Shi2019), but success is highly variable; often there is a lack of understanding of the geomorphological and hydrological controls on restoration success, and mangrove restoration efforts have been plagued with failure (Lee et al., Reference Lee, Hamilton, Barbier, Primavera and Lewis2019; Lovelock et al., Reference Lovelock, Barbier and Duarte2022c). Saltmarsh restoration is also occurring, but receives considerably less scientific attention beyond North America; this likely reflects an efficient policy environment and sufficient financial capacity for restoration (Billah et al., Reference Billah, Bhuiyan, Islam, Das and Hoque2022). Despite these challenges, restoration of BCEs is likely to accelerate, being buoyed by the United Nations declaration that 2021–2030 is the “United Nations Decade on Ecosystem Restoration” to help meet sustainable development goals (Billah et al., Reference Billah, Bhuiyan, Islam, Das and Hoque2022).
Market mechanisms have been developed to incentivise BCE restoration, and currently there are two primary markets; the compliance and voluntary markets (Sapkota and White, Reference Sapkota and White2020). Compliance markets are underpinned by regulations to offset greenhouse gas emissions and typically require offsets to be accounted for under existing standards and using approved methodologies, such as the Verified Carbon Standard (VCS) methodology for tidal wetlands (VM0033) (Emmer et al., Reference Emmer, Needelman, Emmett-Mattox, Crooks, Megonigal, Myers, Oreska, McGlathery, Shoch and Washington2015a; Emmer et al., Reference Emmer, von Unger, Needelman, Crooks and Emmett-Mattox2015b). In some jurisdictions, the voluntary market is also highly regulated; for example, the Australian Government administers a voluntary market, the Emissions Reduction Fund, which provides tradeable credits (Australian Carbon Credit Units) for tidal restoration activities that increase blue carbon storage (Lovelock et al., Reference Lovelock, Adame, Bradley, Dittmann, Hagger, Hickey, Hutley, Jones, Kelleway and Lavery2022a; Lovelock et al., Reference Lovelock, Adame, Butler, Kelleway, Dittmann, Fest, King, Macreadie, Mitchell and Newnham2022b). The payment period for these programs is well-defined as the carbon benefits are likely to diminish in the above-ground carbon pool once vegetation has reached maturity, and in the below-ground pool when substrates are saturated with mineral and organic material (i.e. when accommodation is limited). The 25-year permanence time frame aligns with the period for which woody vegetation is anticipated to reach maturity and exhibit high rates of carbon addition to substrates (Osland et al., Reference Osland, Feher, Spivak, Nestlerode, Almario, Cormier, From, Krauss, Russell and Alvarez2020), while the 100-year timeframe aligns with what is regarded to be permanently sequestered soil organic carbon (i.e. permanence) (Dynarski et al., Reference Dynarski, Bossio and Scow2020). Payments are dependent upon forward projections and ongoing verification.
Use of BCEs as a mechanism for carbon removal has received some criticism (Williamson and Gattuso, Reference Williamson and Gattuso2022) due to the high variability and errors in carbon burial rates, lateral carbon transport (Maher et al., Reference Maher, Call, Santos and Sanders2018), methane and nitrous oxide fluxes (Rosentreter et al., Reference Rosentreter, Al‐Haj, Fulweiler and Williamson2021a; Malerba et al., Reference Malerba, Friess, Peacock, Grinham, Taillardat, Rosentreter, Webb, Iram, Al-Haj and Macreadie2022), carbonate formation and dissolution (Saderne et al., Reference Saderne, Geraldi, Macreadie, Maher, Middelburg, Serrano, Almahasheer, Arias-Ortiz, Cusack and BDJNc2019; Van Dam et al., Reference Van Dam, Zeller, Lopes, Smyth, Böttcher, Osburn, Zimmerman, Pröfrock, Fourqurean and Thomas2021), vulnerability to future climate change and non-climatic factors, and cost-effectiveness and scaleability (Macreadie et al., Reference Macreadie, Costa, Atwood, Friess, Kelleway, Kennedy, Lovelock, Serrano and Duarte2021). Confidence in forward projections is likely to be improved as data collection continues and knowledge gaps are addressed (Macreadie et al., Reference Macreadie, Anton, Raven, Beaumont, Connolly, Friess, Kelleway, Kennedy, Kuwae, Lavery, Lovelock, Smale, Apostolaki, Atwood, Baldock, Bianchi, Chmura, Eyre, Fourqurean, Hall-Spencer, Huxham, Hendriks, Krause-Jensen, Laffoley, Luisetti, Marbà, Masque, KJ, Megonigal, Murdiyarso, Russell, Santos, Serrano, Silliman, Watanabe and Duarte2019).
Permanence (i.e. beyond the 25-year and 100-year time frames) of blue carbon is fundamental to the success of BCEs as a natural climate solution. Critically, conservation and restoration activities will not occur in the absence of sea-level rise, warming and elevated atmospheric carbon dioxide concentrations, placing considerable uncertainty regarding permanence. The fate of sequestered carbon from BCEs once they succumb to sea-level rise is difficult to project, but likely to be highly variable. Palaeo-records provide the opportunity to validate projections of the response of BCEs to sea-level rise; however, do not fully indicate blue carbon futures as sea-level rise is now occurring on coastal landscapes that developed throughout the Holocene, have been highly modified and do not have a historic analogue (Woodroffe and Murray-Wallace, Reference Woodroffe and Murray-Wallace2012). Projecting blue carbon futures therefore requires integration of information from the past and present behaviour.
Recent analyses have indicated that rising seas associated with ice melt following the last glacial maximum exceeded the capacity of tropical mangroves (Saintilan et al., Reference Saintilan, Khan, Ashe, Kelleway, Rogers, Woodroffe and Horton2020) and saltmarshes (Horton et al., Reference Horton, Shennan, Bradley, Cahill, Kirwan, Kopp and Shaw2018; Törnqvist et al., Reference Törnqvist, Cahoon, Morris and Day2021) to remain in situ (i.e. ‘keep-up’) when sea level increased at rates exceeding ~5–7 mm yr.−1. However, mangrove and saltmarsh sediments have been preserved since the Holocene (Hanebuth et al., Reference Hanebuth, Stattegger and Grootes2000; McKee et al., Reference McKee, Cahoon and Feller2007; Wang et al., Reference Wang, Sun, Wang and Stattegger2009) following sea-level rise at rates higher than currently encountered (Redfield, Reference Redfield1972; McKee et al., Reference McKee, Cahoon and Feller2007; Saintilan et al., Reference Saintilan, Khan, Ashe, Kelleway, Rogers, Woodroffe and Horton2020). Long-periods of sea-level stability across much of the Southern Hemisphere have contributed to the development of broad, mature coastal floodplains (i.e. considerable elevation capital) when conditions are conducive. These floodplains are not typically saturated with carbon (Rogers et al., Reference Rogers, Kelleway, Saintilan, Megonigal, Adams, Holmquist, Lu, Schile-Beers, Zawadzki, Mazumder and Woodroffe2019a), may be hot spots for potential and realised acid sulphate soils (Michael, Reference Michael2013), and could become extensive BCEs, much like those of the mid-Holocene (Woodroffe et al., Reference Woodroffe, Thom and Chappell1985). Many BCEs in the northern hemisphere have been adapting to sea-level rise for millennia and are likely to be lower-lying (i.e. less elevation capital) and have limited capacity to adapt to anticipated sea-level rise as the coastal zone is highly contested and coastal squeeze is likely.
The permanence of organic material exposed to drowning is an important consideration. Since it takes some time for mature trees and tidal marshes, that are high in the tidal frame to drown with incremental increases in sea level, it is feasible that submergence and death may occur beyond the minimum 25-year permanency time frame applied in managed blue carbon markets. Loss of standing biomass that is currently lower in the tidal frame or in the interior of saltmarshes may be high (Kearney et al., Reference Kearney, Stevenson and Ward1994), and depending on exposure to erosion, soil carbon may be variably preserved (Krauss et al., Reference Krauss, Demopoulos, Cormier, From, McClain-Counts and Lewis2018; Rogers et al., Reference Rogers, Kelleway, Saintilan, Megonigal, Adams, Holmquist, Lu, Schile-Beers, Zawadzki, Mazumder and Woodroffe2019a) or reworked and transported elsewhere (DeLaune and White, Reference DeLaune and White2012; Haywood et al., Reference Haywood, Hayes, White and Cook2020). Increasing atmospheric carbon dioxide concentrations may enhance productivity of BCE vegetation, with enhanced root allocation contributing to sea-level rise adaptation and carbon sequestration via addition of carbon volume to substrates (Ball et al., Reference Ball, Cochrane and Rawson1997; Langley et al., Reference Langley, McKee, Cahoon, Cherry and Megonigal2009; McKee et al., Reference McKee, Rogers, Saintilan and Middleton2012; Reef et al., Reference Reef, Spencer, Mӧller, Lovelock, Christie, McIvor, Evans and Tempest2017). Widespread mangrove dieback has been associated with short-term methane flux to the atmosphere (Jeffrey et al., Reference Jeffrey, Reithmaier, Sippo, Johnston, Tait, Harada and Maher2019); this has potential implications on atmospheric carbon budgets in the event of broadscale mangrove mortality under high rates of sea-level rise, or short-term sea-level fluctuations. Figure 5 conceptualises the hypothesised carbon storage and greenhouse gas flux outcomes under scenarios of atmospheric carbon dioxide concentrations, warming and relative sea-level rise.
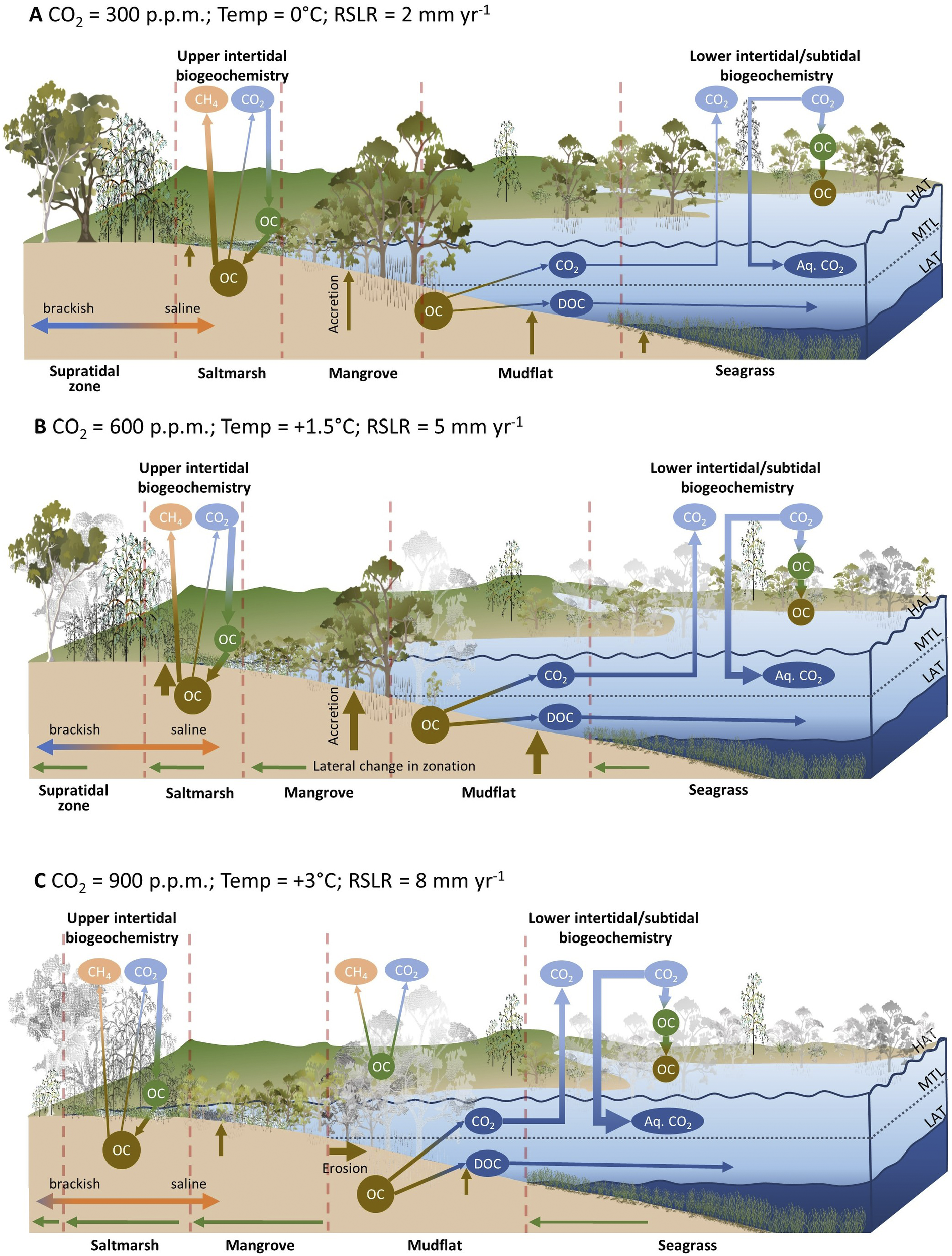
Figure 5. Conceptualisation of the interacting effects of atmospheric carbon dioxide, warming and relative sea-level rise on BCE projected to occur under a range of emissions scenarios. Under the baseline scenario (A) carbon is fixed by in situ vegetation and contributes to soil carbon accumulation and substrate volume via accretion. The landward margin under brackish conditions is a source of methane. Under the mid-range emissions scenario (B) the feedbacks between elevated atmospheric CO2 and organic carbon sequestration and between relative sea-level rise (RSLR) and organic carbon production, preservation, and vertical accretion are strengthened. Saline intrusion reduces methane emissions in the landward fringe, although this may be counterbalanced by increased emissions resulting from increases in NPP induced by CO2 and warming. Under the high-range emission scenario (C) a tipping point is reached where RSLR exceeds vertical accretion leading to mortality and shoreline retreat. Mortality of terrestrial vegetation contributes to elevated methane production in the short term. Note that the relative strength of interactions with greenhouse gases is indicated by the thickness of lines, and greyed-out vegetation is indicative of dieback or loss associated with the effects of rising sea levels.
The degree to which climate change modifies BCEs is difficult to project as shoreline erosion is poorly preserved in the stratigraphy of depositional environments, limiting the capacity to parameterise models. In addition, multiple coastal processes contribute to shoreline change and observations indicate considerable local and regional variability in the operation of processes. Studies that project BCE dynamics with sea-level rise and infer carbon implications are therefore typically undertaken at the local scale, and at data-rich sites, and the outcomes can rarely be extrapolated to other locations. Otherwise, projections are dependent upon simplification of processes and apply reductive estimates of carbon concentrations (Lovelock and Reef, Reference Lovelock and Reef2020; Wang et al., Reference Wang, Sanders, Santos, Tang, Schuerch, Kirwan, Kopp, Zhu, Li and Yuan2021), or explore future scenarios using simplified idealised models (Kirwan and Mudd, Reference Kirwan and Mudd2012). Spatial models typically require decisions regarding whether landward retreat of BCEs is parameterised, and, invariably, the projected outcomes are highly dependent upon model parameterisation. For example, recent projections estimated net gains in blue carbon in the order of 1.5 Pg to 2100 when coastal squeeze impacts are minimised and climate change impacts are high (i.e. RCP8.5 scenario), while net blue carbon gains are in the order of 0.8 Pg to the year 2100 when under moderate climate change scenario (i.e. RCP4.5 scenario) (Lovelock and Reef, Reference Lovelock and Reef2020).
Models have consistently indicated the importance of minimising coastal squeeze to enhance climate adaptation and mitigation benefits from BCEs (Schuerch et al., Reference Schuerch, Spencer, Temmerman, Kirwan, Wolff, Lincke, McOwen, Pickering, Reef, Vafeidis, Hinkel, Nicholls and Brown2018; Lovelock and Reef, Reference Lovelock and Reef2020; Wang et al., Reference Wang, Sanders, Santos, Tang, Schuerch, Kirwan, Kopp, Zhu, Li and Yuan2021). Managing structures that modify tidal exchange and sediment supply will be crucial for maximising blue carbon benefits. Indeed, storm surge barriers are already managed to reduce coastal flooding impacts (Haigh et al., Reference Haigh, Dornbusch, Brown, Lyddon, Nicholls, Penning-Roswell and Sayers2022) and engineering structures could be modified to meet design requirements anticipated with sea-level rise and to manage for blue carbon services (Sadat-Noori et al., Reference Sadat-Noori, Rankin, Rayner, Heimhuber, Gaston, Drummond, Chalmers, Khojasteh and Glamore2021; Haigh et al., Reference Haigh, Dornbusch, Brown, Lyddon, Nicholls, Penning-Roswell and Sayers2022). In many cases ‘holding back the tide’ will become challenging and costly, and it is likely that difficult land use decisions will be made to increase BCE extent and the provision of co-benefits (Rogers et al., Reference Rogers, Lal, Asbridge and Dwyer2022). Sea-level rise will reduce the viability of large tracts of low-lying coastal land for agriculture and grazing purposes, and the efficacy of existing structures to hold back tides, many of which were designed when rates of sea-level rise were negligible, will be tested. In these circumstances, the benefits of land cover conversion for BCEs should therefore be weighed against the costs associated with upgrading existing structures to meet future design requirements that account for the effects of sea-level rise and storm surges on tidal regimes. Momentum towards recognising BCE co-benefits for biodiversity, coastal fisheries and water quality is increasing (Rahman et al., Reference Rahman, Zimmer, Ahmed, Donato, Kanzaki and Xu2021; Hagger et al., Reference Hagger, Waltham and Lovelock2022), and efforts are underway to develop a ‘blue chip’ carbon markets that provide payments for blue carbon additionality and co-benefits (Macreadie et al., Reference Macreadie, Robertson, Spinks, Adams, Atchison, Bell-James, Bryan, Chu, Filbee-Dexter and Drake2022). These blue-chip markets may sufficiently incentivise land managers to reconsider upgrades of tidal barriers and instead receive a blue carbon income as the land is restored to a BCE (Rogers et al., Reference Rogers, Lal, Asbridge and Dwyer2022).
Conclusion
In the short- to medium-term, climate change may increase the capacity of BCEs to capture and store atmospheric CO2, largely due to processes that respond to elevated CO2 and temperature, and influence carbon capture and storage. These processes include in situ responses such as CO2 fixation, biomass storage, biogeochemical enhancement of burial efficiency, as well as the expansion of BCEs at local and global scales. The magnitude and duration of the negative feedback on climate may vary between hemispheres. Climate change is expected to squeeze mangrove and saltmarsh areas in the Northern Hemisphere between accelerating relative sea-level rise and hard barriers. In the Southern Hemisphere, opportunities for landward expansion of mangrove and saltmarsh may be available where late-Holocene sea-level history coupled with ongoing sediment supply and lower contemporary rates of relative sea-level rise has facilitated the development of broad coastal floodplains and where coastal squeeze effects are minimised. The long-term future for these negative feedbacks on radiative forcing is dependent upon decisions made in the coming decades. At the global scale, the rate of sea-level rise projected under high emissions scenarios will lead to degradation and loss of existing coastal wetlands. Sea-level rise of ~5–7 mm yr.−1 is likely to be a critical tipping point at which the predominantly negative climate feedbacks driven by blue carbon sequestration become positive feedbacks driven by plant decomposition and remineralisation. This tipping point will be surpassed under high emissions scenarios within the next century.
The threat to in situ coastal wetlands makes local land-use and coastal protection the key determinant of long-term survival, driven by retreat of BCEs to higher elevations. Investment in coastal wetland conservation and restoration provides benefits not only for the preservation of ecosystem services such as coastal fisheries, but also a promising opportunity for nature-based mitigation. National governments are developing a broad spectrum of climate adaptation and mitigation responses with innovative approaches to financing these activities, including some focused specifically on blue carbon. Carbon markets are rapidly expanding as a tool for governments, private corporations, and individuals to reduce greenhouse gas emissions. While currently only a few blue carbon projects have reached the point of generating finance through carbon markets, projects are in development in Mexico, Kenya, Colombia, Madagascar and other locations, and blue carbon credits are in high demand due to the multitude of co-benefits provided. This suggests that carbon markets are promising to finance coastal restoration, climate adaptation, and livelihoods for coastal communities.
Critical knowledge gaps need to be overcome before the full benefit of blue carbon can be realised. Priorities include: establishing the full global extent of BCEs and developing ongoing monitoring at management-relevant resolutions; addressing the permanence and temporal continuity of blue carbon storage and sequestration subject to SLR, changing climatic conditions and their impact on the distribution of mangroves, tidal wetlands, seagrass and macroalgae at high latitudes; establishing the factors that determine the carbon storage and sequestration capacity at the site scale and how these might be managed to increase mitigation benefit; and establishing the carbon mitigation potential and pathways for other coastal and marine ecosystems such as macroalgae and tidal forests. Given the recent momentum in blue carbon research, scientists and policy makers are well placed to address these gaps, providing research is sufficiently supported. Crucial to the effectiveness of blue carbon research for policy and management application is actively focussing on the highly under-studied regions, particularly in the global south, where the distribution of mangrove forests is greatest.
Open peer review
To view the open peer review materials for this article, please visit http://doi.org/10.1017/cft.2023.17.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/cft.2023.17.
Data availability statement
Data were not generated to prepare this literature review.
Acknowledgements
The authors wish to acknowledge the traditional custodians of blue carbon estate globally and recognise their contribution to management and stewardship for millennia. We advocate for their leadership as blue carbon managers. Many collaborators have contributed to the research undertaken by the authors, and we appreciate their knowledge sharing.
Author contribution
Conception, writing and preparation of figures were principally undertaken by Rogers with substantial input for Kelleway and Saintilan. Figure 1 was prepared by Kelleway and Figures 4 and 5 by Rogers.
Financial support
We appreciate the financial support provided by respective institutions (University of Wollongong and Macquarie University), the Australian Research Council, and federal, state, and local governments who have supported the work of the authors.









Comments
No accompanying comment.