Introduction
The main features of the targeted routine mosquito control with Bacillus thuringiensis var. israelensis (Bti) in the Upper Rhine Valley at the time of this investigation, were following: detailed mapping of mosquito breeding places, trained personnel, defined thresholds and purposeful dosage adaption of Bti, no large-scale application by plane, but targeted application by foot and by helicopter and an accompanying scientific research program. Recently, these methods have been further developed, including GPS-guided application, the exclusion of aquatic mass breeding sites of Chironomidae, and the use of sterile Bti formulations. Thus, only the endotoxins of Bti and no living spores or bacteria are applied (Becker, Reference Becker2002). Among other topics, the accompanying research program comprised studies investigating the role of mosquitoes in the diet of dragonflies (Pfitzner et al., Reference Pfitzner, Beck, Weitzel and Becker2015), amphibians (Blum et al., Reference Blum, Basedow and Becker1997), and bats (Arnold et al., Reference Arnold, Braun, Becker and Storch2001; Kretschmer, Reference Kretschmer1997). None of these studies revealed negative impacts of Bti treatment on predators. However, the possibility that Bti treatment may have negative effects on the food web cannot be ruled out. Bti is known to be toxic to Culicidae and Simuliidae. Other Nematocera affected are, for example, Dixidae, Psychodidae, Chironomidae, Sciaridae, and Tipulidae, particularly when Bti is applied in higher dosages or when the more sensitive first instars e.g. of Chironomidae occur (Becker et al., Reference Becker, Petrić, Zgomba, Boase, Madon, Dahl and Kaiser2010). This effect could influence the availability of insects for aerial feeding predators in Bti treated floodplains, either directly or indirectly via the food web. The investigation of the diet of Delichon urbicum (House Martin) nestlings in France by Poulin et al. (Reference Poulin, Lefebvre and Paz2010) showed a negative impact on the birds' reproductive performance, which was linked to the influence of Bti treatment on the availability of insects as potential prey. However, the availability of insects was not investigated in this study.
The diet of aerial feeding predators (e.g. birds, bats, and insects) depends on food availability and on choice of prey predators make from available food sources. Previous investigation of the diet of aerial feeding birds in other parts of the world has shown diverse dominant taxa. D. urbicum favoured Hymenoptera in Algeria (Merzouki et al., Reference Merzouki, Souttou, Sekour, Daoudi-Hacini and DoumandJi2014) and Diptera and Hymenoptera in France (Poulin et al., Reference Poulin, Lefebvre and Paz2010). It was shown in England that relevant taxa in the diet of D. urbicum changed with the season (Bryant, Reference Bryant1973). The authors related these changes to prey abundance in the respective areas, but even in the same area, bird colonies can show significant differences in prey composition, as observed by Beck et al. (Reference Beck, Hopkins and Jackson2013) for Tree Swallows (Tachycineta bicolour), especially regarding Chironomidae and Hemiptera.
The present study focuses on food availability by investigating the presence of potential prey in both areas treated with Bti and untreated areas in the Upper Rhine Valley. Additionally, food selection was examined using D. urbicum as an example. The investigation was part of the scientific research program accompanying routine mosquito control activities with Bti in the Upper Rhine Valley, in effect since 1980. The investigation was conducted over three consecutive years (1989–1991) and the results were available as an academic thesis (Boggasch, Reference Boggasch1990) and two internal reports. The combined data have now been statistically analyzed for the first time.
Materials and methods
Investigated areas
The four treated areas (Petersau, Riedwiesen, Ketscher Rheininsel, Rheinhausen, and Maudacher Bruch) and two untreated areas (Biedensand and Schusterwoerther Altrhein) were located in the Upper Rhine Valley (for more details see fig. 1 and Supplementary table 1).
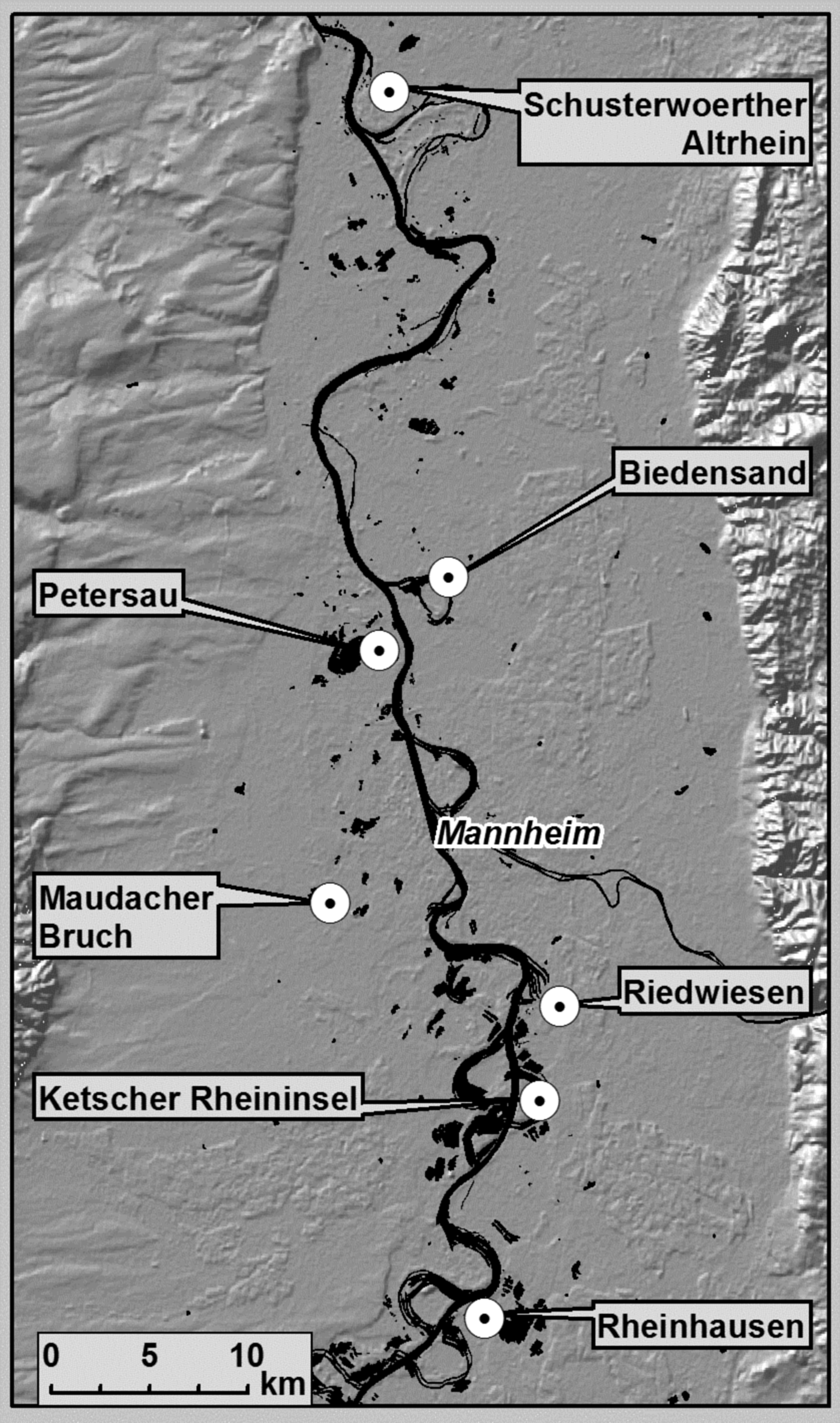
Fig. 1. Location of investigated areas in the Upper Rhine Valley (background: SRTM Shaded Relief ESRI® Data & Maps 2006)
Sampling methods
Car trap
The abundance of aerial insects was investigated using a car trap, an insect net fixed to the top of a car. This method has been successfully used in the past when investigating insect populations in agriculture (Stage et al., Reference Stage, Gjullin and Yates1952; Buttler, Reference Buttler1972; Almand et al., Reference Almand, Sterling and Green1974) and Culicidae populations (Summerman & Simmet, Reference Summerman and Simmet1965; Bidlingmayer, Reference Bidlingmayer1966, Reference Bidlingmayer1967a, Reference Bidlingmayerb, Reference Bidlingmayer1974; Knight & Henderson, Reference Knight and Henderson1967; Steelman et al., Reference Steelman, Richardson, Schaefer and Wilson1968). Service (Reference Service1977) includes an evaluation of vehicle-mounted traps for sampling populations of adult mosquitoes.
The front opening of the car trap used in our investigation was measured 90 × 47 cm. The bottom edge of the net with a mesh size of 1 mm was 150 cm above the ground and 15 cm above the roof of the car. The rear of the car trap was measured 6.4 cm in diameter with a Velcro band to attach a small bag made from plankton net (fig. 2). A speed of 30 km h−1 was sufficient to trap flying insects into the attached plankton net bag. When the car stopped, the net of the car trap dropped over the roof rack, trapping the insects inside the plankton net bag. As the length of the car trap routes varied, the number of trapped insects was converted into number of insects per km. Car trap sampling was performed at different times of the day; samples were categorized according to sampling time in relation to the local time of sunset (RTS): RTS 0 = daytime (between noon and 1 h prior to sunset), RTS 1 = 30 min prior to sunset, RTS 2 = at sunset, RTS 3 = 30 min after sunset, RTS 4 = 1 h after sunset.
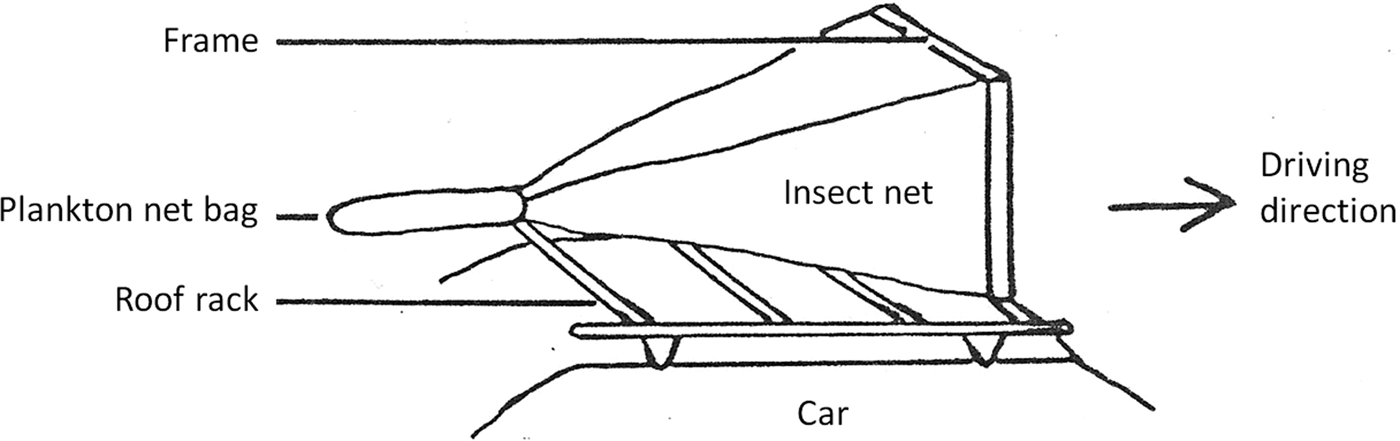
Fig. 2. Schematic diagram of the car trap
Air temperature was measured prior to each car trap sampling procedure. Samples were not performed in the morning, and were not taken on rainy days and days with strong wind.
Neck rings
The nestling diet of D. urbicum was examined with the help of neck rings during 1991. Neck rings made from insulated copper wire (0.15 mm) were fixed around the necks of 4–8 day old nestlings. The rings were fit in a way that allowed the birds to breathe easily, but they were unable to swallow the food bales. The neck rings remained on the nestlings for 30–45 min. This allowed ample time for the parents to feed their nestlings. Food bales were removed from the beaks of nestlings using a blade of grass and placed in 75% alcohol for storage. Afterwards, the nestlings were fed with dead Galleria melonella larvae. The spindle-shaped food bales were held together with saliva and measured 12.8 × 5.5 mm on average. They contained insects ranging from 1 mm (Hymenoptera) to 13.5 mm (Syrphidae) in length. The majority of insects measured between 2 and 5 mm (Timmermann & Becker, Reference Timmermann and Becker2003).
D. urbicum is a diurnal aerial hunter. The birds start flying at sunrise and stop at sunset. The exact activity time depends on season and individual preferences (Hoeser, Reference Hoeser1993; Saygili & Yigit, Reference Saygili and Yigit2007). Although the adult birds were active for longer periods, our observations revealed that they stopped feeding their nestlings 60–15 min before sunset. Based on this information, neck rings were placed on the nestlings approximately 2 h before sunset, when the adults visited their nesting box every 3–15 min.
An assessment of advantages and limitations of car trap and neck ring methods is reviewed in Timmermann & Becker (Reference Timmermann and Becker2003). To investigate food selection, the nestling diet samples were compared with the car trap samples conducted in parallel (at the same time of day) in the area surrounding the colony. Hunting birds were observed at a distance of approximately 2 km from the farm where the D. urbicum colony was located at heights ranging from a few centimetres above the ground up to heights that were not possible to estimate. The birds were also observed inside the farm and over the water.
Taxonomic classification
The taxonomic classification of all insects was determined to a minimum of the order level. If possible (depending on condition), Diptera, Coleoptera, and Planipennia were determined to the family level, and Culicidae to the species level by using a dissecting microscope and several identification keys and references, in particular Lindner (Reference Lindner1925), Enderlein (Reference Enderlein, Brohmer, Ehrmann and Ulmer1936), Mohrig (Reference Mohrig1969), Oldroyd (Reference Oldroyd1970), Unwin (Reference Unwin1981), Bährmann (Reference Bährmann1982), Brohmer (Reference Brohmer1982), Stresemann (Reference Stresemann1986), Cranston et al. (Reference Cranston, Ramsdale, Snow and White1987), and Harris & White (Reference Harris and White1987).
Statistics
For statistical analysis, unpublished and published data from 1989 to 1991 were combined.
Non-parametric multi-dimensional scaling ordination
Non-parametric multi-dimensional scaling ordination (NMDS) was applied to determine the similarity between taxa present at different sites and at different times of day. Similarity was calculated with a Bray–Curtis similarity measure on square-root transformed data (Clarke & Warwick, Reference Clarke and Warwick2001). The resemblance analysis illustrates the similarity of individual samples to each other. Each dot represents a sample and the distance between the dots represents the similarity regarding the composition of taxa: the closer the dots, the more similar the samples. A clear separation of a group of dots (cluster) from the other dots in the diagram would mean that the samples of the cluster are more similar to each other than all other samples. By labelling the dots according to a specific parameter, the similarity of samples regarding this parameter can be superimposed. ‘Stress’ has an inverse measure of fit to the data (measure of goodness-of-fit). An NMDS ordination with a stress value >0.2 is deemed suspect. Stress values equal to or <0.1 are considered fair. Stress values between 0.1 and 0.2 are useable.
The significance of differences between sites was tested with the ANOSIM (analysis of similarity) routine. The ANOSIM statistic compares the mean of ranked dissimilarities between groups to the mean of ranked dissimilarities within groups. A global R value close to 1 suggests dissimilarity between groups, while an R value close to 0 suggests an even distribution of high and low ranks within and between groups. R values <0 suggest that dissimilarities are greater within groups than between groups.
For resemblance analyses of the aerial insect abundance in different areas, only samples comparable between the sites regarding sampling time and month were used: samples collected from May to September in all years, taken at RTS 1, RTS 2, RTS 3, and RTS 4 (n = 161) were included. Samples taken at other times of day and in the areas Rheinhausen and Maudacher Bruch (numbers of suitable samples too low) were excluded. Differences between Bti treated and untreated areas were expected to be strongest around sunset, the main activity period of Aedes vexans (MEIGEN, 1830), the most abundant mosquito species in the Upper Rhine Valley and the primary target of mosquito control. In order to show differences as clearly as possible, daytime samples were excluded from the comparison of the areas.
The influence of sampling time in relation to the local sunset time was also investigated by resemblance analysis. All areas were included and samples taken in each area were combined, only distinguished by sampling time (n = 24). Samples taken between the 30 min intervals and those taken 1 h after sunset were excluded because the numbers of suitable samples were too low.
Diversity analyses
The same samples used for comparison of investigated areas by NMDS were also used for diversity analyses. Univariate diversity indices were calculated with the DIVERSE routine: taxa richness (S), Shannon's index (H′ loge), and Evenness (J′). Differences in mean values of diversity indices between sites were tested by ANOVA on log-transformed data. In the instance of significant results, a Tukey–Kramer HSD post hoc test was used to identify differing means (overall significance level, P < 0.05).
Generalized linear model
No distinction can be made between the availability (i.e. present as adult insects in the respective area) and activity (i.e. flying at the respective time of the day) of taxa; therefore, both aspects were analyzed in one model. Influences of temperature, season, RTS, and interaction of RTS and temperature on mean abundance of taxa were tested by generalized linear model (GLM) on log-transformed data. Samples from all months and years, taken at RTS 0, RTS 1, RTS 2, RTS 3, and RTS 4 (n = 237) were included. Samples taken between the 30 min intervals were excluded because the number of suitable samples was too low.
Savage's index
For the identification of preferred and avoided prey of D. urbicum, Savage's index was used as described in Merzouki et al. (Reference Merzouki, Souttou, Sekour, Daoudi-Hacini and DoumandJi2014): W i = A i/D i, where A i = relative abundance in the nestling diet (i.e. in the food bales), D i = relative availability in the environment (i.e. in the car trap samples), W i values <1 show avoidance and W i values >1 show preference. Statistical significance of W i mean values was tested using χ2 analysis after applying Bonferroni adjustment.
Software
NMDS and ANOSIM were carried out using the PRIMER software package V6.1.11 (PRIMER-E, Plymouth, UK, 2009). All other procedures were computed with JMP V10.0.1 (SAS Institute, Cary, NC, USA, 2012).
Results
A total of 145,570 insects were caught in 255 car trap samples. 9524 insects were found in 71 D. urbicum nestling diet samples. Eleven mosquito species, 14 Nematocera families, 36 Brachycera families, 22 Coleoptera families, 4 Planipennia families, Hemiptera (4 taxa), Hymenoptera (2 taxa), Ephemeroptera, Lepidoptera, Odonata, Psocoptera, Thysanoptera, and Trichoptera were identified (see Supplementary table 2).
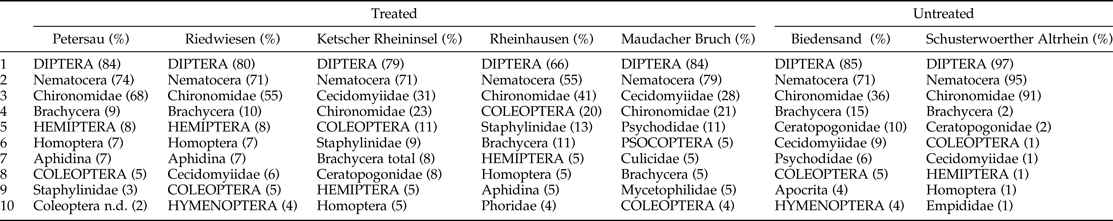
The ten most abundant taxa found in each area and their percentages of the total sample are shown in table 1. Diptera was the most abundant insect order in all investigated areas and Nematocera was the most abundant sub-order. In five of the seven areas, Chironomidae was the predominant Nematocera family and the most frequent aquatic Nematocera at all sites. In the wooded areas, Ketscher Rheininsel and Maudacher Bruch, Cecidomyiidae were more common among Nematocera than Chironomidae. In four areas, Brachycera were the next most common. Brachycera and Coleoptera were always among the most frequently available insects. Staphylinidae represented the most common Coleoptera family at all investigated sites, although they were not always among the ten most frequent taxa. The role of Aphidina was diverse. Whereas they made up 5–7% of the car trap samples in Petersau, Riedwiesen, Ketscher Rheininsel, and Rheinhausen (Hemiptera and Homoptera consisting mostly of Aphidina), they were not among the ten most abundant taxa in the other three areas.
Table 1. Relative abundance of the ten most common taxa. This table includes all samples taken using the car trap during the three years of this investigation. It displays all levels of identification (apart from species level), and thus one individual may be included in up to three categories (e.g. family, sub-order, and order).
Comparison of investigated areas
Biodiversity
The results of the biodiversity analysis are shown in table 2. Details of ANOVA results for taxa richness and diversity can be found in Supplementary figs 1–3. There were no significant differences between taxa richness in the investigated areas, except for the treated areas Petersau and Riedwiesen (ANOVA, Tukey–Kramer post hoc test; F = 3.91, P < 0.0039). The number of individuals per km was negatively related to diversity (Shannon's index and Evenness). The untreated area, Biedensand had the highest Shannon's index and Evenness, but the lowest number of individuals per km. The lowest diversity was calculated for the other untreated area, Schusterwoerther Altrhein, which had the highest number of individuals per km. The low Evenness reflects the dominance of Chironomidae in this area (>90%). With regard to diversity and individuals per km, the treated areas lay between those extremes (see table 2). Evenness values of the two untreated areas Biedensand and Schusterwoerther Altrhein were significantly different from each other and from all other sites (ANOVA, Tukey–Kramer post hoc test; F = 18.67, P < 0.0001). Petersau, Riedwiesen, and Ketscher Rheininsel showed no significant differences (ANOVA, Tukey–Kramer post hoc test; P > 0.5). Significant differences for Shannon's Index were very similar to those for Evenness. However, the difference between Riedwiesen and Biedensand was significant (ANOVA, Tukey–Kramer post hoc test; F = 14.17, P < 0.0001); all other differences P > 0.5).
Table 2. Individual numbers, taxa numbers and biodiversity (mean values of samples).

Similarity
Essentially, resemblance analysis showed no clear grouping of samples. The similarity of all investigated areas was confirmed by ANOSIM analysis (global R = 0.336, P = 0.001). Superimposing the data according to the area investigated (fig. 3) showed a vague stratification analogous to the differences in diversity and individual numbers per km described earlier. The highest significant difference was found between the two untreated areas Schusterwoerther Altrhein and Biedensand (R = 0.683, P = 0.002), the lowest difference was between Riedwiesen and Petersau (R = 0.126, P = 0.024). Resemblance analysis showed no significant differences between Bti-treated and untreated sites (global R = 0.056, P = 0.032).
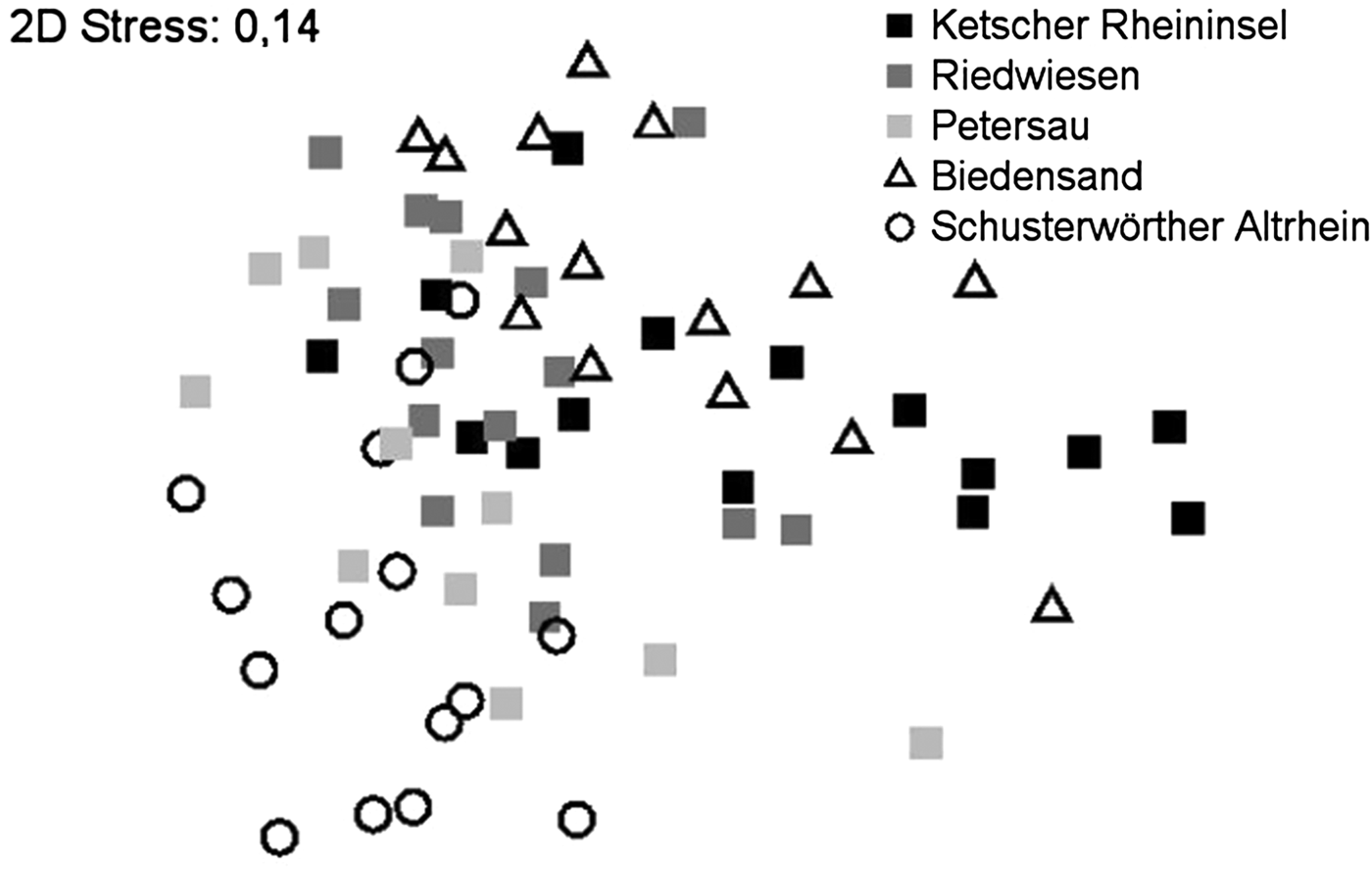
Fig. 3. Resemblance analysis (NMDS) superimposed according to investigated areas. No clear grouping of samples but stratification is visible. The untreated areas Biedensand and Schusterwoerther Altrhein are at both ends of the scatter-plot.
Factors influencing taxa presence
As no significant differences regarding the availability of flying insects between treated and untreated areas could be detected, the factors influencing taxa presence have been more closely investigated. The effect of season, air temperature, RTS, and the interaction between temperature and RTS on the presence of the most important taxa are summarized in table 3 (including statistical P-values); additional details of GLM results can be found in Supplementary figs 4–10. The analysis revealed that different taxa are differently influenced by the investigated factors, and RTS had an effect on all analyzed taxa.
Table 3. GLM results.
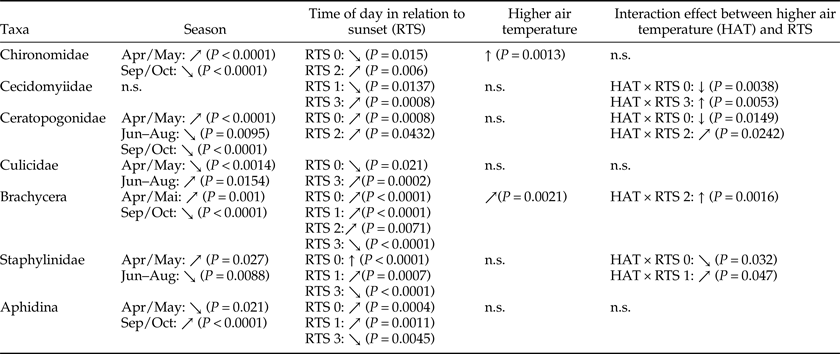
Influence of season, RTS, and air temperature on most abundant taxa (only significant values). ↑ = strong positive effect, ↗ = moderate positive effect, ↓ = strong negative effect, ↘ = moderate negative effect, n.s. = not significant. RTS 0 = daytime (between noon and 1 h prior to sunset), RTS 1 = 30 min prior to sunset, RTS 2 = at sunset, RTS 3 = 30 min after sunset.
Higher air temperature had the strongest positive effect on the presence of Chironomidae; early season had a moderate positive effect and late season had a moderate negative effect. The presence of Cecidomyiidae was most positively influenced by higher air temperature after sunset and most negatively by higher air temperature during daytime. The presence of Ceratopogonidae was particularly negatively influenced by higher air temperature during daytime. For Culicidae, the time after sunset had a positive effect and daytime had a negative effect. A strong positive effect of higher air temperature at sunset was observed for Brachycera. Daytime had a strong positive effect on the presence of Staphylinidae. For Aphidina, positive effects were observed for daytime and 30 min prior to sunset, whereas the time after sunset had a negative effect.
Similarity of taxa composition with regard to RTS
In a further step, the similarity of taxa composition with regard to RTS was evaluated by NMDS. Superimposing the data according to RTS (fig. 4) indeed displayed stratification. The insect composition at RTS 0 and RTS 3 were at both ends of the scatter-plot and showed no overlap. ANOSIM analysis revealed a high level of significance for this result (R = 0.936, P = 0.002). Samples taken at RTS 1 were also significantly different from those taken at RTS 3 (R = 0.661, P = 0.002). Samples taken at RTS 1 lay between RTS 0 and RTS 2 samples and overlapped with both. Samples taken at RTS 2 overlapped with both RTS 1 and RTS 3. Differences between these samples were not significant. In general, samples were relatively similar with regard to sampling time in relation to sunset (global R = 0.333, P = 0.001).
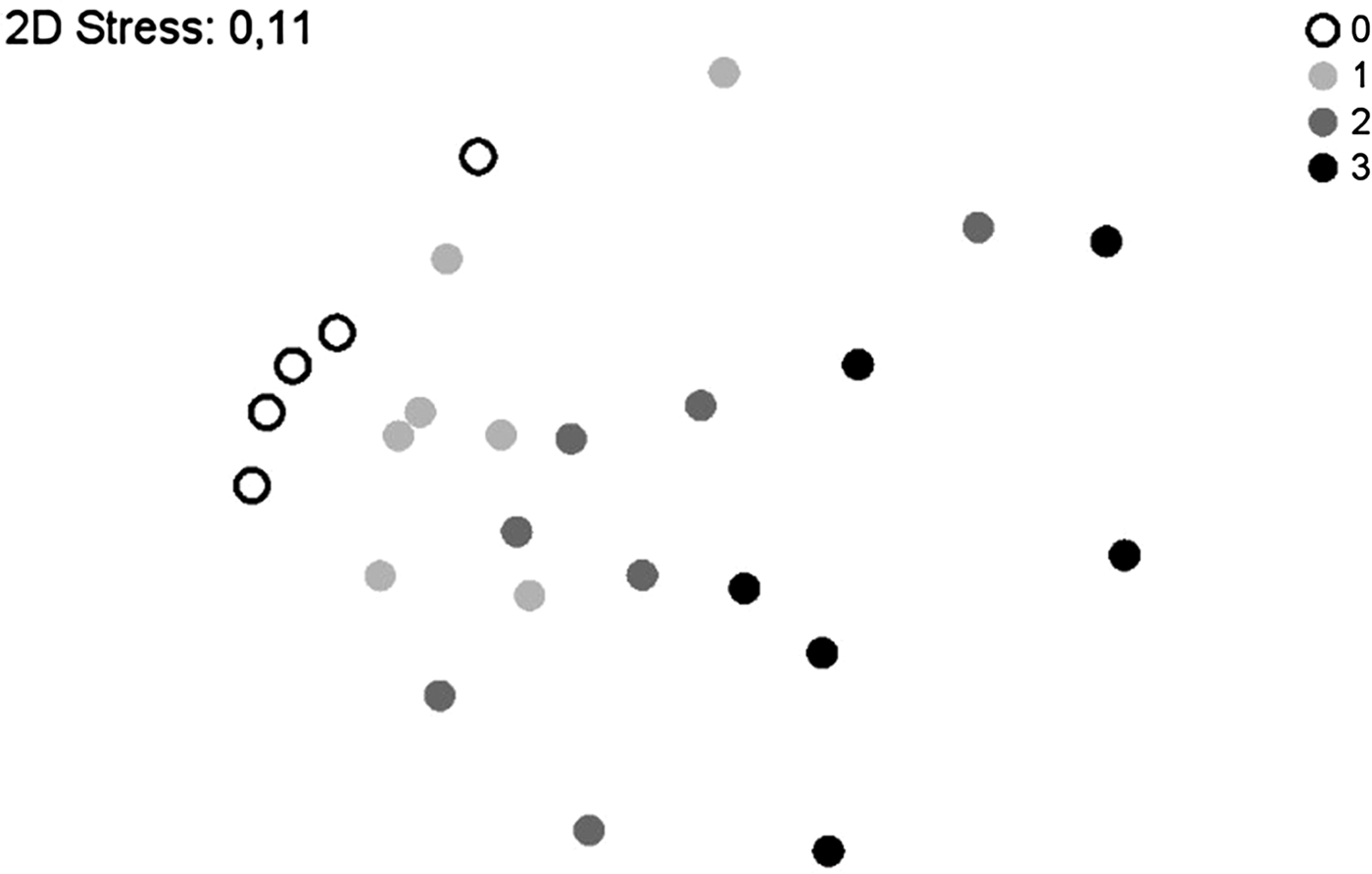
Fig. 4. Resemblance analysis (NMDS) superimposed according to sampling time in relation to local time of sunset: 0 = daytime, 1 = 30 min prior to sunset, 2 = at sunset, 3 = 30 min after sunset. The insect composition during daytime and 30 min prior to sunset is significantly different from the insect composition 30 min after sunset.
Taxa presence with regard to RTS
For the evaluation of taxa presence with regard to RTS, samples taken at RTS 0, RTS 1, RTS 2 and RTS 3 were summarized over all areas (N = 205), and the percentage of taxa from total was calculated. The comparison of active insects at each point in time showed clear differences. The taxa responsible for these differences are displayed in fig. 5. The percentage of Diptera changed from 57% during daytime to 97% 30 min after sunset. Primarily responsible were Nematocera (37–95%), but Nematocera families showed diverse tendencies. The percentage of Chironomidae, the most abundant family, increased until sunset (21–62%) and decreased afterwards (53.5%). The percentage of Cecidomyiidae increased until 30 min after sunset (4–28%). Culicidae represented only a small proportion of the total sample. They showed a rise in percentage in the evening (0.1–3.4%). Mosquitoes were caught at a rate of 90% at sunset or later. The activity pattern was reversed for Brachycera (19–1.5%), Staphylinidae (13–0.1%) and Aphidina (12–0.1%).
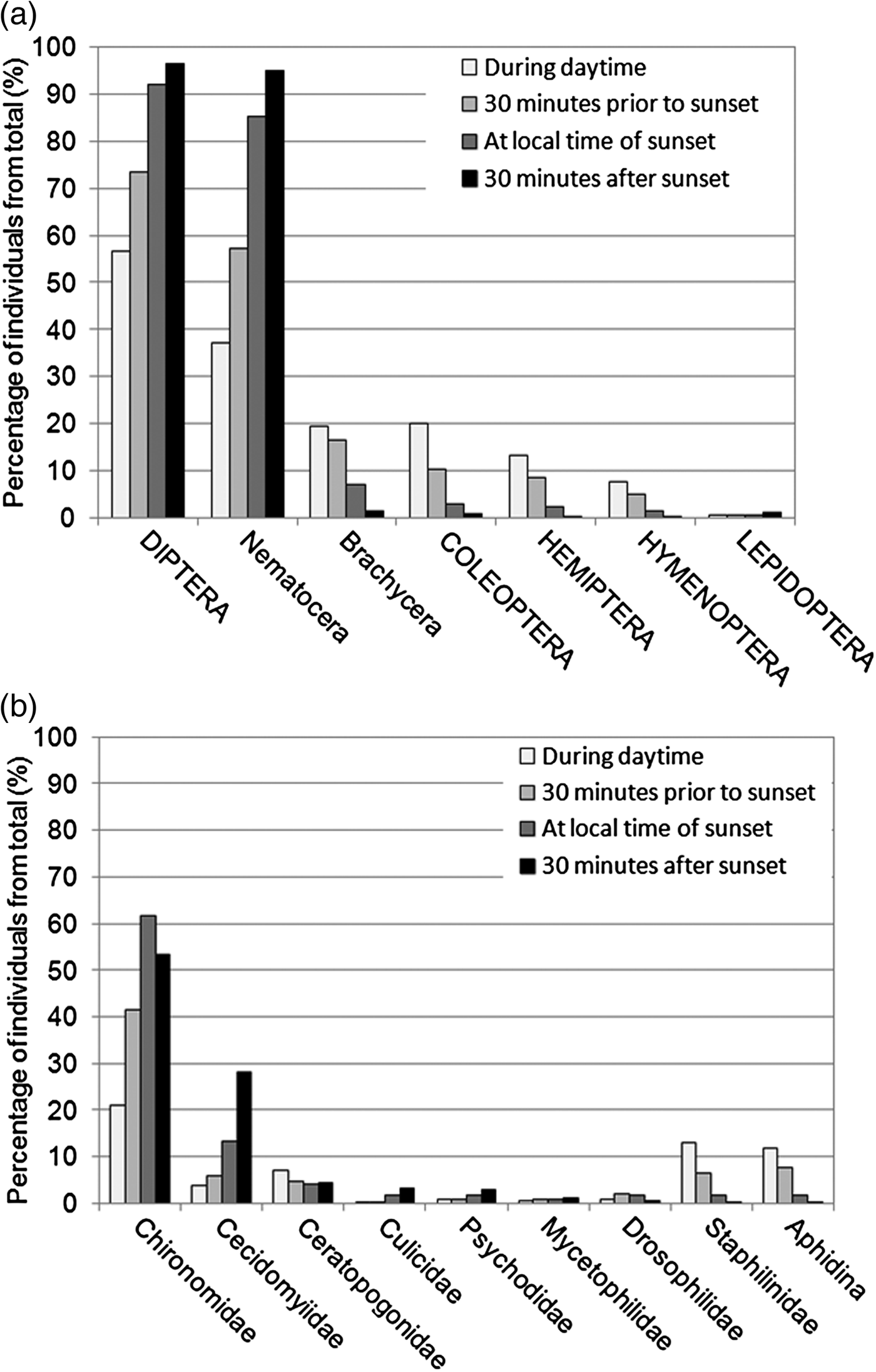
Fig. 5. Percentages of the most abundant insect orders plus sub-orders for Diptera (a) and of most abundant Diptera and Coleoptera families plus Aphidina (b) from total sample according to relation to sunset. All car trap samples from all years and areas taken during daytime (n = 37), 30 min prior to sunset (n = 59), at sunset (n = 60), and 30 min after sunset (n = 49) are included, only distinguished by sampling time.
Food selection by D. urbicum
Results of the Savage index calculation (including P-values) are shown in table 4. Aphidina (Hemiptera, Homoptera) were the most abundant prey in June and were significantly preferred by the birds for both broods. For all other taxa found in the diet of the first brood, the variation was too high to allow informed statements. The composition of taxa in the diet of the second brood in August was different from the first brood, and preference was not as clearly shown as for the first brood. The number of available Aphidina was considerably lower than in June. Brachycera (Diptera) and Staphylinidae (Coleoptera) were preferred for the second brood. Chironomidae were the most commonly available food source among Nematocera (and also within Diptera). They comprised >40% of the potential prey for the second brood, but were significantly avoided.
Table 4. Savage's index and relative abundance of insect taxa in nestling diet and environment.
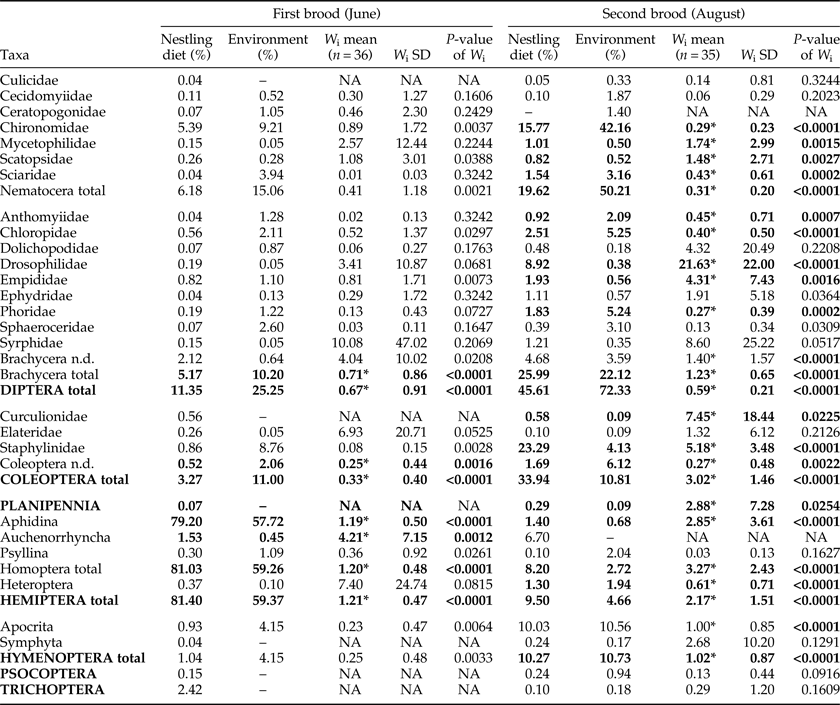
Taxa with W i mean values <1 were avoided, taxa with W i mean values >1 were preferred; asterisks (*) indicate significant P-values according to χ2 analysis after applying Bonferroni adjustment.
Some taxa found in the nestling diet were not available in the car trap samples and vice versa. However, the proportion of the total sample represented by such taxa was always <1%, apart from Anthicidae (2.5%) and Chrysomelidae (2.9%) (both Coleoptera) found in the nestling diet of the second brood.
Discussion
Although mosquito control with Bti was conducted during the three years of this investigation, the similarity between the investigated areas suggests that routine Bti treatment had no indirect effect on non-target insects. Not even the direct effect – the reduction of mosquitoes – made a difference to insect abundance, due to the low overall percentage of mosquitoes from the total number of insects. Theoretically, this could be different in years with extreme floods and mass emergence of floodwater mosquitoes. However, as such years are rare, it would be expected to have only temporary effects, if any. Apart from Chironomidae, Nematocera families known to be sensitive to Bti, did not belong to the most abundant taxa – neither in treated, nor in untreated areas. During the last two decades, the insect population of the Upper Rhine Valley has been monitored almost every year by monthly light trap catches, as part of the research program accompanying routine mosquito control. The results suggest that our conclusions regarding the effect of Bti on non-target insects are still valid (Steuerwald, 2016, internal report).
According to Borrvall et al. (Reference Borrvall, Ebenman and Jonsson2000) high biodiversity may lessen the risk of radical ecosystem changes and the risk of additional species extinction, following the loss of one species, decreases with the number of species per functional group. Diversity analysis of the investigated areas did not reveal lower biodiversity of treated sites in comparison with untreated sites. Our results suggest that treated areas are not more at risk than untreated sites with regard to species extinction. High or low biodiversity showed no correlation to Bti treatment, but depended on environmental factors not explored in this study, e.g. structure of habitats or land use. This result is in line with conclusions of other researchers who found the effects of regular Bti treatment to be minute in comparison with other environmental factors that structure the insect fauna of temporary wetlands in Sweden (Persson Vinnersten et al., Reference Persson Vinnersten, Lundström, Schäfer, Petersson and Landin2010) and affect the evolution of invertebrate communities in Bti-treated brackish water coastal wetlands in France (Lagadic et al., Reference Lagadic, Roucaute and Caquet2014). In both studies, the authors reasoned that Bti treatment did not negatively affect food resources for birds.
This study shows that food availability to aerial feeding predators changes according to season, time of day, and temperature. This likely also applies to other factors that have not been analyzed, such as frequency and extent of flooding, water quality or air humidity. Differing effects of the investigated factors on various taxa were observed, which could influence their availability as a food resource. There is a high probability that other such influences would be revealed when insects were identified to the species level. The aerial insect composition after sunset was significantly different from that observed during daytime. After sunset, Nematocera were by far the most dominant insects in all areas. Insects with a mainly crepuscular activity phase but low emergence during daytime, such as Culicidae, Cecidomyiidae and Psychodidae, cannot be primarily relevant as food sources for diurnal hunters. Samples taken in different areas during the daytime were similar to each other in composition. The proportion of Brachycera, Coleoptera and Hemiptera was considerably higher than in evening samples, although Nematocera were still the most abundant group. When evaluating these observations, it must always be considered that insects were not identified to the species level. It is possible that the above mentioned taxa could show different activity patterns in areas with different species.
Even though Chironomidae showed a peak in activity at sunset, they were available during daytime in considerable numbers and could represent a worthwhile food source for diurnal predators. This role has been discussed by several authors in the past, especially as some Chironomidae species are affected by Bti when applied at higher dosages. Yiallouros et al. (Reference Yiallouros, Storch and Becker1999) showed that Chironomus thummi thummi Kieffer (subfamily Chironominae) and Psectrocladius psilopterus Kieffer (subfamily Orthocladiinae) are 40- to 60-fold less sensitive to Bti than Aedes aegypti. Ali (Reference Ali1981) showed that species of the subfamily Chironominae are susceptible to Bti at about ten times the mosquito larvicidal rates. Although the safety of Chironomidae is largely guaranteed due to their lower sensitivity to the bacterial toxins, aquatic mass breeding sites of Chironomidae are not treated during routine mosquito control in the Upper Rhine Valley. This study shows that the number of Chironomidae was not decreased in treated areas, which was also reasoned by Lundström et al. (Reference Lundström, Schäfer, Petersson, Persson Vinnersten, Landin and Brodin2010a), who additionally observed that the number of Chironomidae species was higher in Bti-treated areas of Sweden (Lundström et al., Reference Lundström, Brodin, Schäfer, Persson Vinnersten and Östman2010b). Due to the above mentioned reasons, Chironomidae numbers are not expected to decline considerably as a result of the targeted mosquito control in the Upper Rhine Valley.
Since the last two decades, not only the accuracy of the application of Bti-formulations could be increased by means of GPS-based applications, but also the dosage of Bti used per hectare could be reduced by improvement of the Bti-formulation. Presently, most of the areas are treated with Bti-ice-granules, which can be precisely applied by helicopters equipped with an isolated seeder. The amount of Bti used per hectare could be reduced by half (Becker, Reference Becker2003). In the 1990s the breeding sites were treated with Bti preparations (Bactimos primary powder, activity: 9000 ITU mg−1) at rates of 500 g ha−1 corresponding to 4.5 × 109 ITU ha−1. Presently, 800 g ha−1 of Vectobac WG (activity: 2.700 ITU mg−1 after sterilization) are applied corresponding to 2.16 × 109 ITU ha−1. Thus, the risk of overdosing of Bti has been reduced significantly since the time of this investigation. The Bti formulations are sterilized, and no living spores are applied. It could be shown that no accumulation appears, but the proteins are decomposed within days (Becker, Reference Becker2002).
We found no other study investigating the nestling diet of aerial feeding birds in which the methods allowed such a direct comparison with aerial insect abundance as in the present study. Usually, either the nestling diet was investigated indirectly using faecal samples (Bryant, Reference Bryant1973; Merzouki et al., Reference Merzouki, Souttou, Sekour, Daoudi-Hacini and DoumandJi2014), which combine prey fed during a period of time, or aerial insect abundance was studied with suction traps (Bryant & Turner, Reference Bryant and Turner1982), which combine insects available over a period of time. Using these methods, it is not possible to conduct parallel samples at a particular time of the day. In some studies, insects were captured in sweep nets (Poulin, Reference Poulin2012; Merzouki et al., Reference Merzouki, Souttou, Sekour, Daoudi-Hacini and DoumandJi2014), a method that makes the aerial insect abundance difficult to quantify, includes insects roused or attracted by the collector, and covers only small areas. Emergence traps are inappropriate to investigate food availability for aerial feeding predators, as they only capture insects hatching in their respective habitat. Insects emerging in other habitats or migrating between areas are not captured. By the combination of methods used in the present study, activity patterns could be taken into account and the condition of most insects was good enough for reliable identification. With the car trap it was possible to explore a large area within a short time. It also allowed the exact spatiotemporal assignment of insects, suitable for answering both questions of insect availability in a certain area, and on the timing of availability. Insects are not attracted by the car trap, and the movement that leads to capture originates from the car rather than from the insects. Therefore, the car trap is not only unselective, but also independent of the influences of a single, small-scale habitat (Bidlingmayer, Reference Bidlingmayer1967a, Reference Bidlingmayerb).
The comparison of simultaneously conducted car trap and diet samples revealed that the taxa were corresponding to a high degree. The insects primarily consumed by the birds were different for the first (Aphidina) and second brood (Brachycera and Staphylinidae), but all were principally active during daytime and, with the exception of some Brachycera, they had terrestrial larvae. This means that they could not be directly affected by Bti treatment of mosquito breeding sites. Chironomidae were consumed, but not preferred. The high consumption rates of Chironomidae observed in some studies could have different explanations. For example, Chironomidae species may have been different from those in our investigation, there may have been a lack of more attractive prey in the respective area, or there may have been specialization of birds or competition between birds (Waugh, Reference Waugh1978). Comparisons of the diets of aerial feeding birds should be made with great caution, not only when comparing different areas, but also when drawing comparisons within the same area. Food selection by the observed D. urbicum colony in the present study is a valuable piece of information, but should not simply be transferred to other colonies. Investigating the availability of food resources independent of a specific predator can be useful, as food selection can differ between individuals and it cannot be generally assumed that prey composition always reflects the available food resource.
Our results are not in line with those of Poulin et al. (Reference Poulin, Lefebvre and Paz2010), who reported that direct and indirect impacts of Bti treatment on the abundance of Nematocera were responsible for lower breeding performance of D. urbicum in France. However, prey availability was not investigated by Poulin et al. (Reference Poulin, Lefebvre and Paz2010). Rather, the authors assumed that the number of Nematocera and their arthropod predators was lower in treated areas because their number was lower in the birds' diet. According to our observation, these conclusions seem questionable; especially as Lagadic et al. (Reference Lagadic, Roucaute and Caquet2014) pointed out that there were considerable environmental discrepancies between treated and untreated areas in Poulin et al. (Reference Poulin, Lefebvre and Paz2010).
Essentially, two conclusions can be derived from the results of this study. Firstly, the car trap method can be highly recommended for studies investigating aerial insect abundance. It allows the identification of flying insects at defined points in time and enables direct comparisons with food caught by any aerial feeding predator at the same time. Secondly, the manner in which mosquito control was conducted in the Upper Rhine Valley showed no direct or indirect effects on aerial insect abundance, which would indicate a negative impact on food resources for aerial feeding predators.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485317000141.
Acknowledgements
The authors are very grateful to their colleagues who compensated for the limitations in time and knowledge: Jan Timmermann digitized the ‘old’ data, André Witthoeft-Muehlmann performed the statistical analyses, and Minoo B. Madon did the proof reading. Many thanks! Additionally, the authors would like to thank the Gesellschaft zur Foerderung der Stechmueckenbekaempfung (GFS) and the Institute for Dipterology (IfD) for their constant support.











