No CrossRef data available.
Published online by Cambridge University Press: 02 December 2024
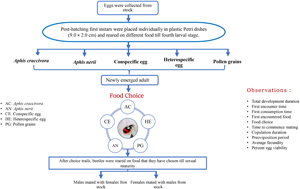
Understanding why animals choose one food over another is one of the key questions underlying the fields of behaviour ecology. This study aims to test if ladybird beetle, Propylea dissecta Mulsant (Coleoptera: Coccinellidae) can forage selectively for nutrients in order to redress specific nutritional imbalances to maximise their fitness. We hypothesised that the presence of more food choices leads to bad decisions in terms of their food selection which ultimately negatively affects the mating and reproductive parameters of P. dissecta. To test this, we first manipulated the predator's nutritional status by rearing them in five separate dietary groups from first instar larvae to newly emerged adult stage. Thereafter, we tested their food choice between five different foods, i.e. Aphis craccivora Koch, Aphis nerii Boyer de Fonsclombe, conspecific eggs, heterospecific eggs and mixed pollen grains, equidistantly placed in a Petri dish. Based on the food choice of the newly emerged adults, they were reared on the chosen diet for 10 days. Thereafter, adults were paired with their opposite sex (collected from stock culture reared on A. craccivora) and mating and reproductive parameters were recorded. Our results suggested that the variety of food did not affect the food choice of ladybird beetle, P. dissecta. They tend to choose their natural diet, i.e. aphid in each dietary regime. We found that previous dietary regime, i.e. larval dietary regime, significantly influences the mating and reproductive parameters of both the male and female except for the time to commence mating by the male. Food choices of adult beetles were found to significantly influence the time to commence mating, average fecundity and per cent egg viability in males and only mating duration in females. Our findings suggest that P. dissecta consistently made optimal decisions when facing various food choices. They consistently preferred their natural and preferred food choice over others, indicating a strong food selection behaviour.