Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Brookes, C. P.
and
Loxdale, H. D.
1985.
A device for simultaneously homogenizing numbers of individual small insects for electrophoresis.
Bulletin of Entomological Research,
Vol. 75,
Issue. 3,
p.
377.
Bowden, J.
Sherlock, P. L.
Digby, P. G. N.
Fox, J. S.
and
Rhodes, J. A.
1985.
Studies of elemental composition as a biological marker in insects. II. The elemental composition of apterae of Rhopalosiphum padi (L.) and Metopolophium dirhodum (Walker) (Hemiptera: Aphididae) from different soils and host-plants.
Bulletin of Entomological Research,
Vol. 75,
Issue. 1,
p.
107.
Carter, Nick
1985.
Simulation modelling of the population dynamics of cereal aphids.
Biosystems,
Vol. 18,
Issue. 1,
p.
111.
Loxdale, H. D.
Rhodes, Jackie A.
and
Fox, J. S.
1985.
Electrophoretic study of enzymes from cereal aphid populations.
Theoretical and Applied Genetics,
Vol. 70,
Issue. 4,
p.
407.
Wöhrmann, K.
Tomiuk, J.
and
Weber, G.
1986.
The search for hidden enzymatic variation in the aphid Macrosiphum rosae (L.).
Theoretical and Applied Genetics,
Vol. 73,
Issue. 1,
p.
77.
Brookes, C. P.
and
Loxdale, H. D.
1987.
Survey of enzyme variation in british populations of Myzus persicae (Sulzer) (Hemiptera: Aphididae) on crops and weed hosts.
Bulletin of Entomological Research,
Vol. 77,
Issue. 1,
p.
83.
ffrench-Constant, R. H.
and
Devonshire, A. L.
1987.
A multiple homogenizer for rapid sample preparation in immunoassays and electrophoresis.
Biochemical Genetics,
Vol. 25,
Issue. 7-8,
p.
493.
Loxdale, H. D.
and
Brookes, C. P
1988.
Electrophoretic study of enzymes from cereal aphid populations. V. Spatial and temporal genetic similarity of holocyclic populations of the bird-cherry oat aphid, Rhopalosiphum padi (L.) (Hemiptera: Aphididae), in Britain.
Bulletin of Entomological Research,
Vol. 78,
Issue. 2,
p.
241.
Hales, Dinah F.
and
Lardner, R. M.
1988.
GENETIC EVIDENCE FOR THE OCCURRENCE OF A NEW SPECIES OF NEOPHYLLAPHIS TAKAHASHI (HOMOPTERA: APHIDIDAE) IN AUSTRALIA.
Australian Journal of Entomology,
Vol. 27,
Issue. 2,
p.
81.
Newton, Chris
and
Dixon, A. F. G.
1988.
A preliminary study of variation and inheritance of life-history traits and the occurrence of hybrid vigour inSitobion avenae(F.) (Hemiptera: Aphididae).
Bulletin of Entomological Research,
Vol. 78,
Issue. 1,
p.
75.
LOXDALE, H. D.
and
BROOKES, C. P.
1989.
Separation of three Rubus‐feeding species of aphid — Sitobion fragariae (Wlk.), Macrosiphum funestum (Macch.) and Amphorophora rubi (Kalt.) (Hemiptera: Aphididae) — by electrophoresis.
Annals of Applied Biology,
Vol. 115,
Issue. 3,
p.
399.
HAND, S. C.
1989.
The overwintering of cereal aphids on Gramineae in southern England, 1977–1980.
Annals of Applied Biology,
Vol. 115,
Issue. 1,
p.
17.
Wöhrmann, K.
and
Hales, D.
1989.
Life cycle components and genetic variability in aphids.
Journal of Applied Entomology,
Vol. 107,
Issue. 1-5,
p.
71.
LUPOLI, R.
IRWIN, M. E.
and
VOSSBRINCK, C. R.
1990.
A ribosomal DNA probe to distinguish populations of Rhopalosiphum maidis (Homoptera: Aphididae).
Annals of Applied Biology,
Vol. 117,
Issue. 1,
p.
3.
Loxdale, Hugh D.
1990.
Estimating levels of gene flow between natural populations of cereal aphids (Homoptera: Aphididae).
Bulletin of Entomological Research,
Vol. 80,
Issue. 3,
p.
331.
Loxdale, H. D.
and
Brookes, C. P.
1990.
Prevalence of Sitobion fragariae (Walker) over S. avenae (Fabricius) (Hemiptera: Aphididae) on wild cocksfoot grass (Dactylis glomerata) in south-east England.
Bulletin of Entomological Research,
Vol. 80,
Issue. 1,
p.
27.
WALTON, M. P.
LOXDALE, H. D.
and
WILLIAMS, L. ALLEN
1990.
Electrophoretic ‘keys’ for the identification of parasitoids (Hymenoptera: Braconidae: Aphelinidae) attacking Sitobion avenae (F.) (Hemiptera: Aphididae).
Biological Journal of the Linnean Society,
Vol. 40,
Issue. 4,
p.
333.
Hales, D. F.
Chapman, R. L.
Lardner, R. M.
Cowen, R.
and
Turak, E.
1990.
APHIDS OF THE GENUS SITOBION OCCURRING ON GRASSES IN SOUTHERN AUSTRALIA.
Australian Journal of Entomology,
Vol. 29,
Issue. 1,
p.
19.
Lázzari, Sonia M. N.
1992.
Characterization and discrimination of three Rhopalosiphum species (Homoptera: Aphididae) based on isozymes.
Revista Brasileira de Zoologia,
Vol. 9,
Issue. 1-2,
p.
139.
Black, William C.
DuTeau, Nancy M.
Puterka, Gary J.
Nechols, James R.
and
Pettorini, Jennifer M.
1992.
Use of the random amplified polymorphic DNA polymerase chain reaction (RAPD-PCR) to detect DNA polymorphisms in aphids (Homoptera: Aphididae).
Bulletin of Entomological Research,
Vol. 82,
Issue. 2,
p.
151.
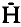 ) was low (ca. 2%) in both years and confined mainly to one locus, EST-1. The prevalence of homozygous allozyme variation supports ecological findings suggesting S. avenae to be largely anholocyclic in Britain. In 1981 and 1982, large populations, sampled from 11 sites in Britain and Spain, were examined at 13 loci.
) was low (ca. 2%) in both years and confined mainly to one locus, EST-1. The prevalence of homozygous allozyme variation supports ecological findings suggesting S. avenae to be largely anholocyclic in Britain. In 1981 and 1982, large populations, sampled from 11 sites in Britain and Spain, were examined at 13 loci. 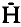 ranged from 2 to 7·6%, with heterozygosity restricted again mainly to EST-1. Some alleles were unique to certain geographical regions (including Britain or Spain); others showed significant spatial, and in two British populations examined in successive years, temporal frequency differences. Calculating Nei’s genetic identity (I) and distance (D) coefficients for each population pair mostly gave I>0·8, D<0·2 with overall means (± s.e.m.) of 0·896 ± 0·006 and 0·111 ± 0·006, respectively, which are comparable with geographical population values for other insects. D was poorly correlated with geographical distance, although values were slightly greater (ca. 0·025) for international population comparisons and did not overlap on a principal coordinates plot. The overall population similarity suggests substantial inter-population gene flow; geographical barriers may have restricted movements but seemingly have not led to allopatric race formation. P and
ranged from 2 to 7·6%, with heterozygosity restricted again mainly to EST-1. Some alleles were unique to certain geographical regions (including Britain or Spain); others showed significant spatial, and in two British populations examined in successive years, temporal frequency differences. Calculating Nei’s genetic identity (I) and distance (D) coefficients for each population pair mostly gave I>0·8, D<0·2 with overall means (± s.e.m.) of 0·896 ± 0·006 and 0·111 ± 0·006, respectively, which are comparable with geographical population values for other insects. D was poorly correlated with geographical distance, although values were slightly greater (ca. 0·025) for international population comparisons and did not overlap on a principal coordinates plot. The overall population similarity suggests substantial inter-population gene flow; geographical barriers may have restricted movements but seemingly have not led to allopatric race formation. P and 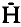 values resemble those for many other aphids, but H values are lower than typically found in other insects. Possible causes of this heterozygote deficiency are discussed.
values resemble those for many other aphids, but H values are lower than typically found in other insects. Possible causes of this heterozygote deficiency are discussed.

