Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in Western and Asian countries(Reference Loomba and Sanyal1). A meta-analysis reported that the prevalence of NAFLD in Iran was 33·95 % and that it was increasing at an alarming rate(Reference Moghaddasifar, Lankarani and Moosazadeh2). It has been estimated that NAFLD will be a major cause of liver-related morbidity and mortality by 2030(Reference Fleischman, Budoff and Ifran Zeb3). NAFLD describes a common clinic-pathological condition characterised by a large number of fatty deposits in hepatocytes, and it is detected by either histology or imaging(Reference Chalasani, Younossi and Lavine4). NAFLD has been linked to elevation of aminotransferases, dyslipidaemia, insulin resistance, hypertension and obesity(Reference Tsochatzis, Papatheodoridis and Manesis5,Reference Gao and Fan6) . Moreover, NAFLD in the hepatic steatosis stage can be reversed by lifestyle modification such as weight loss, regular physical activity and adherence to a healthy diet(Reference Rabl and Campos7).
Whole grains are highly recommended as the inseparable part of a healthy diet, and their significance in chronic disease management is interesting. According to the definition proposed by American Association of Cereal Chemists, whole grains are the intact, ground, cracked or flaked kernel of grains, whose principal anatomical components are the starchy endosperm, germ and bran(8). The 2012 Dietary Guidelines for Americans recommend consuming at least half of the daily grain servings as whole grains with gram recommendations ranging from 48 g/d to 75 g/8368 kJ to reduce the risk of chronic diseases(9).
Previous studies carried out on animal models suggested that dietary whole grains may have protective effects against chronic diseases(Reference Anderson and Hanna10). Furthermore, it has been claimed that consumption of whole-grain products may facilitate management of metabolic disorders(Reference Esmaillzadeh, Mirmiran and Azizi11). However, the results of a whole-grain-based case–control study found an inverse association between whole-grain consumption and NAFLD(Reference Georgoulis, Kontogianni and Tileli12). On the other hand, a large cohort study showed a negative significant correlation between whole-grain intake and hyperlipidaemia and insulin resistance(Reference McKeown, Meigs and Liu13). Similarly, some previous studies have suggested that whole-grain foods may improve glucose and lipid biomarkers in healthy obese or hyperglycaemic adults(Reference Angelino, Martina and Rosi14). Many randomised controlled trials have also evaluated the effects of whole-grain products on blood glucose concentrations and serum lipids, but they have found inconsistent results(Reference Tovar, Nilsson and Johansson15–Reference Sandberg, Björck and Nilsson18).
Despite acknowledgement of the importance of whole grains in the diet by researchers, there is little evidence regarding the association between consumption of whole grains and management of NAFLD-related diseases(Reference Malin, Kullman and Scelsi19). Thus, it is hypothesised in the present study that dietary whole grains might be effective in the management of NAFLD. To evaluate this hypothesis, this randomised controlled clinical trial was designed to assess whether consumption of whole grains is effective in the management of NAFLD in adults or not.
Materials and methods
Recruitment and eligibility screening
A total of 202 adult patients diagnosed with NAFLD, who had visited Ghods Clinic in Urmia, Iran, between March and May 2017, were enrolled in the study, consecutively. NAFLD diagnosis was based on the presence of ultrasonographic evidence of fatty liver and no other type of liver injury or steatosis. All patients with NAFLD were asked to participate and were recruited, provided they met the inclusion criteria and accepted to participate in the study. The reason behind this was to eliminate any bias in patient selection. Men and women at the age of fertility, that is, 18 years or older, with fatty liver grades 1, 2 or 3 and BMI ≤ 40 kg/m2 were included in the study. Excluding factors consisted of a history of alcohol abuse; evidence of any acute and chronic gastrointestinal diseases other than fatty liver; autoimmune disease; type 1 or 2 diabetes; uncontrolled thyroid conditions; psychiatric disorders; impaired renal function; use of drugs to treat NAFLD; use of drugs having the potential to affect glucose and lipid metabolism during the study and over the last 6 months; history of using medications that increase the risk of NAFLD, such as perhexiline, methotrexate, amiodarone, diltiazem, tamoxifen, nifedipine, oestrogens, chloroquine and other possible hepatotoxic agents and pregnancy and breast-feeding for women. Participants who took at least half of their daily grain servings as whole grains before the start of the study were also excluded from the study.
Study design
This parallel, open-label randomised controlled clinical trial was conducted from June to August 2017. Written informed consents were obtained from all participants after they became informed about the aims and procedures of the study. The study protocol was approved by the Ethics Committee at Urmia University of Medical Sciences and was registered at the Iranian Registry of Clinical Trials website (IRCT20170206032417N3). Randomisation lists were computer-generated by a statistician, and the laboratory staff, radiologist and statistician were blinded to the intervention assignment until the end of the trial.
Intervention
At baseline, patients were randomly allocated to two groups to receive dietary advice either to obtain at least half of their cereal servings each day from whole-grain foods (intervention group (n 56)) or from usual cereals (control group (n 56)) for 12 weeks. This time period was designated based on those previous studies showing beneficial effects of whole grains on metabolic markers in 6 and 8 weeks(Reference Cooper, Kable and Marco16,Reference Kirwan, Malin and Scelsi17) . A dietitian met with participants at baseline to explain the 2012 Dietary Guidelines for Americans(9) used in the study and also to provide educational information to facilitate their understanding and adherence. Participants in the intervention group were given a list and description of whole-grain foods and were advised to consume at least half of the daily grain servings as whole grains based on recommendations in the 2012 Dietary Guidelines for Americans(9). In addition, participants in both groups were asked to eat, two to three servings of low-fat dairy products, five servings of fruits and vegetables and two servings of lean meat, poultry or fish on a daily basis, as recommended in the 2012 Dietary Guidelines for Americans(9). At baseline, patients were requested to maintain their levels of physical activity throughout the trial period. Compliance with the guidelines was determined every week by a telephone call and dietary assessment on four occasions (at weeks 0, 4, 8 and 12) during the study.
Liver ultrasonography
All participants underwent a liver ultrasonographic examination performed by one operator and one expert radiologist (intra-inter-observer variation approximately 20 and 25 %, respectively) at baseline and at the end of the study. NAFLD was defined as the presence of an ultrasonographic pattern according to the following criteria: liver-kidney echo discrepancy, attenuated echo penetration, visibility of diaphragm and narrowing of the lumen of the hepatic veins on ultrasonography (Philips Affiniti 50 Ultrasound). Fatty liver was graded as normal, grades 1, 2 and 3, according to the modified criteria of Kurtz et al.(Reference Kurtz, Dubbins and Rubin20).
Biochemical measurements
Biochemical testing was performed at weeks 0 and 12 to determine serum alanine aminotransferase, aspartate aminotransferase, γ-glutamyltransferase, total cholesterol, TAG, HDL-cholesterol, LDL-cholesterol, fasting blood sugar and insulin concentrations using routine automated assay methods at the Urmia University of Medical Sciences reference laboratory after an overnight fast. Serum concentrations of alanine aminotransferase, aspartate aminotransferase, ALP, total cholesterol, TAG, HDL-cholesterol, LDL-cholesterol and fasting blood sugar were measured using routine enzymatic assays with commercial kits (Pars Azmoon) and an automatic biochemical analyser (BT 4500, Biotechnica). Furthermore, serum concentrations of insulin were measured by the electrochemiluminescence method on the Cobas E411 analyser (Roche Diagnostics GmbH). Homeostatic model assessment of insulin resistance (HOMA-IR) and quantitative insulin sensitivity check index were also calculated based on the suggested formulas(Reference Sharma and Fleming21).
Blood pressure and anthropometric measurements
At weeks 0 and 12, systolic blood pressure and diastolic blood pressure were taken by the same person, during the same period of the day, and after 5 min of rest in the sitting position employing a validated mercury sphygmomanometer device (MicrolifeBP AG1-10). Furthermore, anthropometric measures were taken in the standing position, with participants wearing light clothing and no shoes, at baseline and week 12. BMI was calculated as weight in kilograms divided by height in metres squared (kg/m2); height and weight were measured using a digital scale and stadiometer (BSM370; Inbody Co.). Moreover, waist and hip circumferences were measured following recommendations by the WHO(22).The waist to hip circumference ratios (WHR) were calculated as well.
Dietary intake and physical activity assessment
At baseline and every 4 weeks, 3-d (one weekend day and two nonconsecutive week days) 24-h diet recall interview was performed by a trained dietitian. The dietary recalls were analysed using the Nutritionist 4 (N4) software (First Databank, Inc.; Hearst Corporation) modified for Iranian foods. The obtained data were then used to estimate the mean of consumed dairy products, vegetables, fruits, cereals, whole grains, meat and fat group servings. If a whole-grain food did not exist in the N4 software, it would be replaced with a proper whole grain with similar micro- and macronutrient and dietary fibre contents. According to the 2012 dietary guidelines for Americans, grain servings were defined as 1/2 cup (120 ml) of cooked rice, pasta or cereal; one slice of bread (30 g) and one ounce (28 g) of ready-to-eat cereals such as puffed products, biscuits, shred products and other conventional cereal piece shapes. Physical activities were investigated using the metabolic equivalent of task (MET) questionnaire(Reference Ainsworth, Haskell and Whitt23) at weeks 0, 6 and 12.
Primary and secondary outcomes
The primary outcome measures were changes in liver steatosis grades and serum concentrations of liver enzymes. Secondary outcome measures were changes in serum concentrations of lipid profiles, glycaemic variables, blood pressure and anthropometric variables.
Statistical analysis
The obtained data were analysed using SPSS software version 21 (SPSS, Inc.). For all analyses, a P value <0·05 was considered to be significant. Continuous and categorical data were presented as mean values and standard deviation and frequency, respectively. To calculate the sample size, the standard formula suggested for parallel clinical trials was applied, considering type 1 error (α) of 0·05 and type 2 error (β) of 0·10 (power = 90 %) (based on a study conducted by Katcher et al.(Reference Katcher, Legro and Kunselman24) and a mean change in the total cholesterol). Based on this, the sample size was estimated to be forty-seven per study group. Assuming 19 % dropouts from each group, the final sample size was estimated to be fifty-six per group. Differences in general characteristics between groups at baseline were compared by applying independent-samples Student’s t tests (for continuous variables) and χ 2 (for categorical variables). Marginal model and generalised estimating equations with a cumulative logit link function were used to compare changes in the fatty liver grade among groups during the intervention. To assess confounders, the changes in fatty liver grade for baseline value of the outcome, mean change in food groups, MET value, energy intake, weight, BMI and WHR were adjusted by using the generalised estimating equations models. To identify within-group differences, paired-samples t tests were used. Repeated-measures ANOVA, using the general linear model with group (intervention and control) as the between-subjects factor, was conducted to identify changes in dietary intakes and serum concentrations of liver enzymes, lipid profile, glycaemic variables, blood pressure over time and also differences between the groups. Moreover, to assess confounders, all analyses for baseline value of the outcome, mean change in food groups, MET value, energy intake, weight, BMI and WHR were adjusted by applying the ANCOVA test.
Results
A total of 112 participants were randomly assigned to two groups. After randomisation, eighteen participants were excluded, due to failure to follow-up. In total, ninety-four participants completed the trial: the intervention group (n 47) and control group (n 47) (Fig. 1). No significant differences were found between the two groups regarding baseline demographic characteristics (Table 1). On the basis of 3-d 24-h dietary recalls obtained at study baseline and throughout the intervention, significant changes between the groups, in terms of energy intake and consumed food groups, with the exception of dairy and fruit intakes were observed (Table 2).
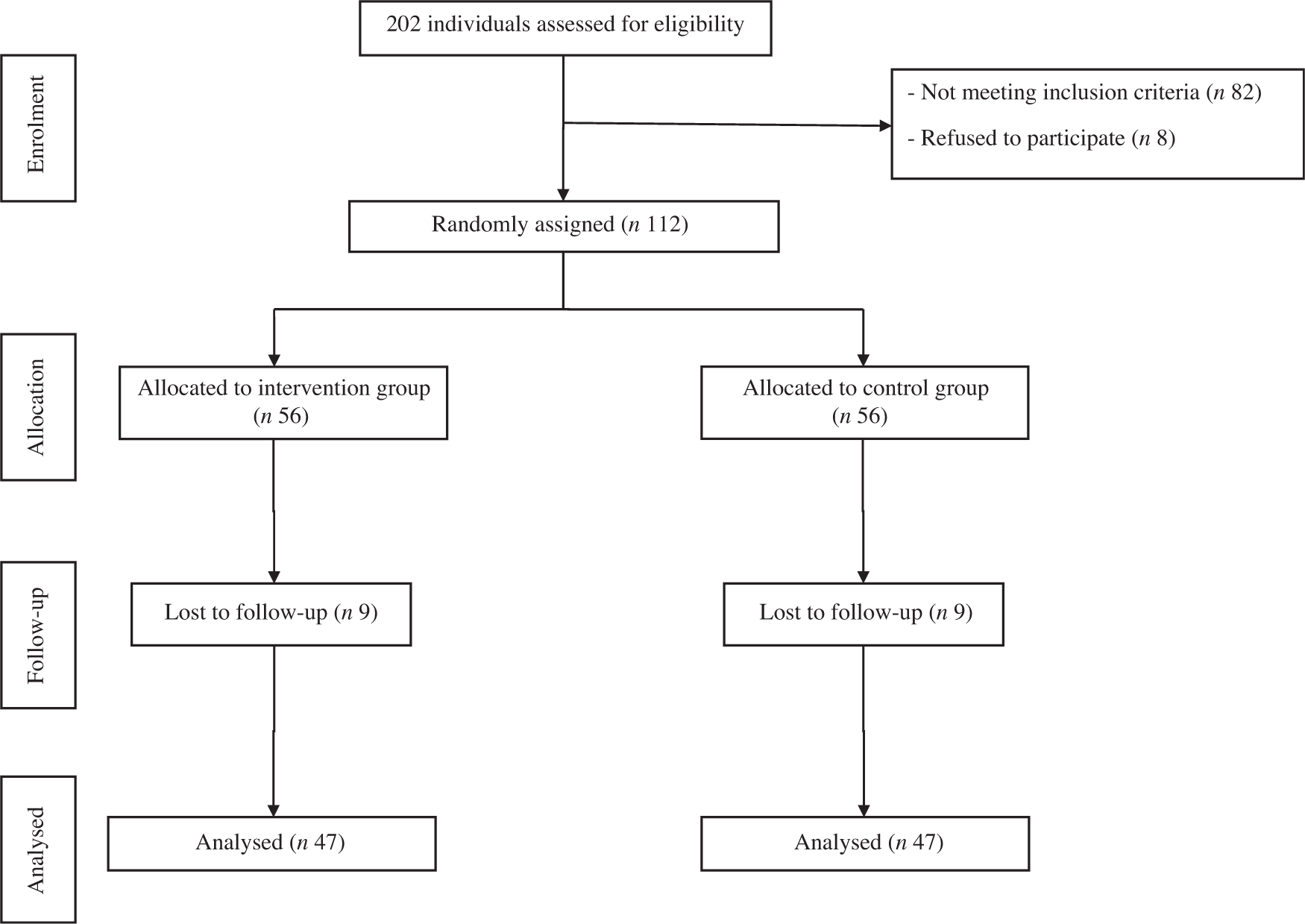
Fig. 1. Summary of patient flow diagram.
Table 1. Demographic characteristic of patients with non-alcoholic fatty liver disease (n 47)
(Mean values and standard deviations)
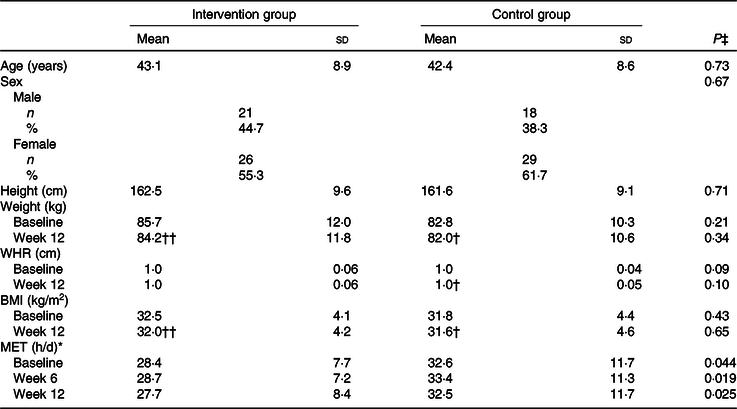
WHR, waist to hip circumference ratio; MET, metabolic equivalent of task.
* P values were computed by analysis of general linear model ANOVA for repeated measurements: P Time = 0·10, P Group = 0·024, P Time × Group = 0·21.
P values were computed by paired-samples t test: † P < 0·05, †† P < 0·001.
‡ P values were computed by the independent-samples t test for continuous variables and by χ 2 for categorical variables.
Table 2. Dietary intakes of study participants (servings) at weeks 0, 4, 8 and 12 of the study (n 47)
(Mean values and standard deviations)
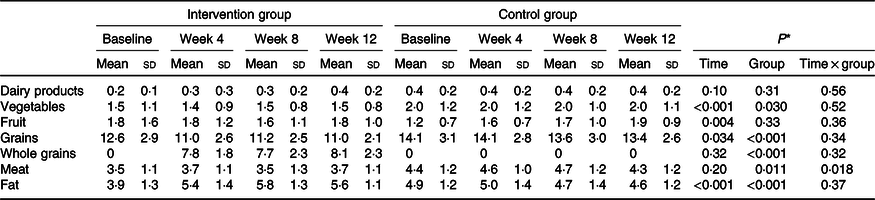
* P values represent the effect of time, group, and time × group interaction (computed by analysis of general linear model ANOVA for repeated measurements).
Primary outcomes
Ultrasound grades decreased significantly in the intervention group compared with the control group (P < 0·001). In the intervention group, 66 % of patients showed a reduction in grade of fatty liver (Table 3).
Table 3. Changes in fatty liver grade during the 12-week diet period in patients with non-alcoholic fatty liver disease in the intervention and control groups*
(Numbers and percentages)
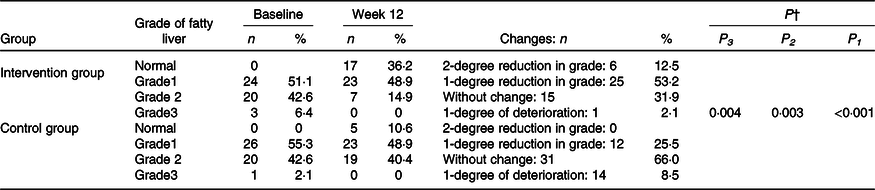
MET, metabolic equivalent of task; WHR, waist to hip circumference ratio.
* Intervention group: baseline, n 56 and week 12, n 47. Control group: baseline, n 56 and week 12, n 47.
† Based on marginal model and generalised estimating equations with a cumulative logit link function, there were significant differences between groups regarding grades of liver steatosis (P < 0·001). On the basis of generalised estimating equations, there were significant differences between groups in grades of liver steatosis after adjusting for baseline value of the outcome, mean change in food groups and MET value (P = 0·003) and after more adjusting for mean change in energy intake, weight, BMI and WHR (P = 0·004).
Changes in serum concentrations of alanine aminotransferase (P < 0·001), aspartate aminotransferase (P < 0·001) and γ-glutamyltransferase (P = 0·009) were significantly different between the two groups. The intervention group showed a greater reduction in serum concentrations of these enzymes than did the control group (Table 4). These significant differences between the groups were observed even after adjustments were made to the baseline value of the outcome, mean changes in food groups and MET value, and after more adjustments were made to the mean changes in energy intake, weight, BMI and WHR (Table 5).
Table 4. Changes in liver enzymes, lipid profile, glycaemic variables and blood pressure during the 12-week diet period in patients with non-alcoholic fatty liver disease in the intervention and control groups (n 47)
(Mean values and standard deviations)
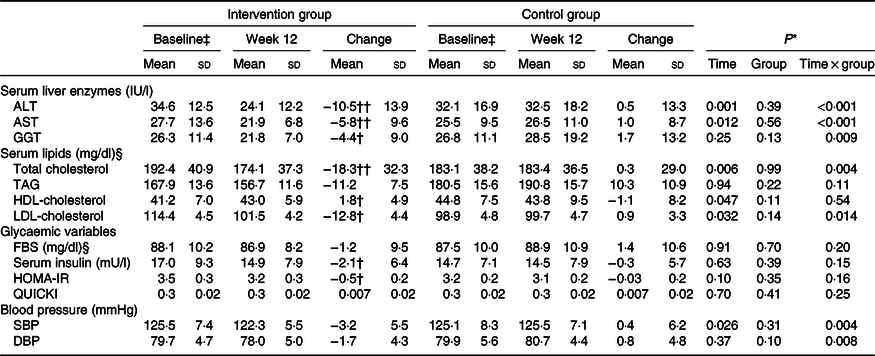
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase; FBS, fasting blood sugar; HOMA-IR, homoeostasis model of assessment-estimated insulin resistance; QUICKI, quantitative insulin sensitivity check index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
* P values represent the effect of time, group and time × group interaction (computed by analysis of general linear model ANOVA for repeated measurements).
P values were computed by paired-samples t test: † P < 0·05, †† P < 0·001.
‡ On the basis of independent-samples t test, there were no significant differences between groups with regard to baseline values (P > 0·05).
§ To convert cholesterol in mg/dl to mmol/l, multiply by 0·0259. To convert TAG in mg/dl to mmol/l, multiply by 0·0113. To convert FBS in mg/dl to mmol/l, multiply by 0·0555.
Table 5. Adjusted changes in liver enzymes, lipid profile, glycaemic variables and blood pressure during the 12-week diet period in patients with non-alcoholic fatty liver disease in the intervention and control groups (n 47)
(Mean values and standard deviations)
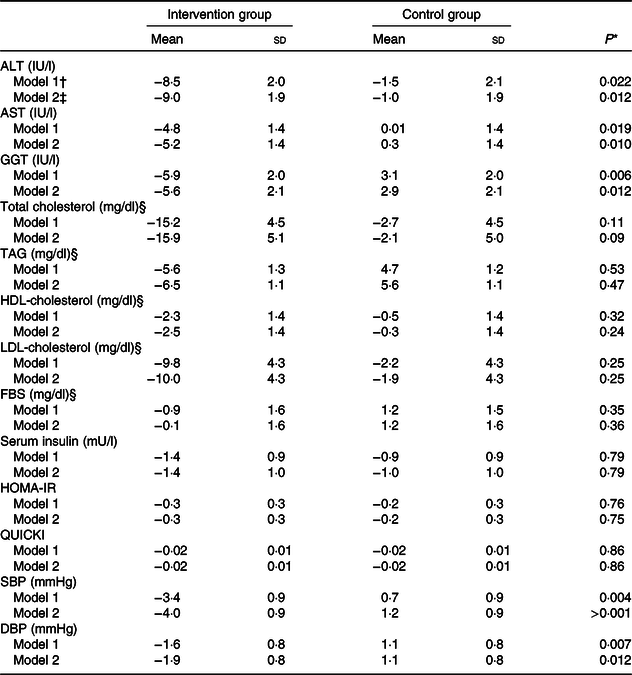
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase; FBS, fasting blood sugar; HOMA-IR, homoeostasis model of assessment-estimated insulin resistance; QUICKI, quantitative insulin sensitivity check index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MET, metabolic equivalent of task; WHR, waist to hip circumference ratio.
* P values were computed by univariate ANCOVA.
† Adjusted for baseline value of the outcome, mean change in food groups and MET value.
‡ Adjusted for baseline value of the outcome, mean change in food groups, energy intake, weight, BMI, WHR and MET value.
§ To convert cholesterol in mg/dl to mmol/l, multiply by 0·0259. To convert TAG in mg/dl to mmol/l, multiply by 0·0113. To convert FBS in mg/dl to mmol/l, multiply by 0·0555.
Secondary outcomes
Changes in serum concentrations of total cholesterol (P = 0·004), LDL-cholesterol (P = 0·014), systolic blood pressure (P = 0·004) and diastolic blood pressure (P = 0·008) were significantly different between the two groups. The intervention group showed a greater reduction in these parameters than did the control group. In addition, no significant differences were found between the two groups in terms of serum concentrations of TAG, HDL-cholesterol, insulin and fasting blood sugar and homeostatic model assessment of insulin resistance, weight, BMI or WHR.
After adjusting for baseline value of the outcome, mean changes in food groups and MET value and after adjusting more for mean changes in energy intake, weight, BMI and WHR, the differences in changes of serum, total cholesterol and LDL-cholesterol concentrations between the two groups became non-significant. Moreover, other findings were not significantly changed after the adjustments were made (Table 4).
Discussion
NAFLD poses substantial challenges to public health across the globe. Therefore, to improve NAFLD risk factors, researchers have become interested in the effectiveness of different dietary patterns. Consequently, in the present study, it is hypothesised that patients with NAFLD who included whole-grain foods in their diet would show a more considerable improvement in laboratory liver tests.
In the present study, we showed that the consumption of whole grains for 12 weeks led to statistically and clinically significant improvements in grade of steatosis and liver enzymes. Significant differences in hepatic steatosis and liver enzymes were observed between the groups, even after adjustment with covariates. This indicates that whole grain by itself and independent of weight loss improved the above outcomes. Data on the effects of whole grains on hepatic steatosis and liver enzymes among NAFLD patients, however, are scarce. Results from a case–control study with thirty-four non-alcoholic steatohepatitis patients suggested that whole grains were potentially useful for improving hepatic steatosis and liver enzymes(Reference Georgoulis, Kontogianni and Tileli12). Also, in a study by Abdelmalek et al.(Reference Abdelmalek, Sanderson and Angulo25), it was found that betaine, as a beneficial factor in whole grain, improved hepatic steatosis. Tovar et al.(Reference Tovar, Nilsson and Johansson15) observed a significant decrease in γ-glutamyltransferase concentrations after consumption of whole-grain barley product supplementation, compared with a control diet, in overweight women. The results of study conducted by Angelino et al.(Reference Angelino, Martina and Rosi14), however, showed that whole-grain consumption for 12 weeks had no effect on liver enzymes. The discrepancy was probably due to an approximate doubling in total daily fibre intake in the study by Tovar et al.(Reference Tovar, Nilsson and Johansson15). This efficacy can at least be partially attributed to the effects of the whole grains on modulation of the microbiota composition in humans, with corresponding changes to gut microflora metabolites including SCFA(Reference Martínez, Lattimer and Hubach26).
At an anatomical location, the liver is directly supplied with blood flow from the intestine. As a result, changes in intestinal permeability caused by dysbiosis gut microbiota allow the translocation of bacterial products into the portal vein and development of obesity-related metabolic diseases such as NAFLD. The portion of gut microbiota in the progression of NAFLD is mainly based on increased hepatic reactive oxygen species due to the increased pro-inflammatory markers in the intestinal lumen. Some studies have shown associations between whole-grain consumption and inflammatory markers(Reference Hajihashemi, Azadbakht and Hashemipor27–Reference Qi, Van Dam and Liu30). It is assumed in our study that the reduction in oxidative stress and inflammatory markers due to the consumption of whole grains has been responsible for the decrease in liver enzymes and steatosis; nonetheless, further investigations are needed. On the other hand, whole-grain foods are a good source of micronutrients such as Cu, which is identified as a key player in various metabolic disorders(Reference Tarantino, Porcu and Arciello31). Reduced serum and hepatic Cu concentrations characterise NAFLD patients and seem to have an important role in the progression of metabolic diseases.
Moreover, findings from the present study showed that the consumption of whole grains did not affect serum fasting blood sugar and insulin concentrations. In a report from the Framingham Offspring Cohort Study, the number of daily servings of whole-grain foods was inversely related to insulin resistance and fasting insulin concentrations(Reference McKeown, Meigs and Liu13). In the Insulin Resistance Atherosclerosis Study, a greater intake of whole grains was associated with higher insulin sensitivity(Reference Liese, Roach and Sparks32). Similar results were observed in a cross-sectional study of 285 adolescents(Reference Steffen, Jacobs and Murtaugh33). This discrepancy might be due to the number of daily servings of whole-grain foods, nature of the disease and duration of intervention.
Whole-grain foods are thought to have a beneficial effect on glycaemic variables due to their greater fibre content, which leads to a slower or reduced digestion of macronutrients, reduced blood glucose burden and lower insulin concentrations when supplemented in clinical trials(Reference Jensen, Koh-Banerjee and Franz34). Also, bioactive compounds in whole-grain foods, such as phenolic acids and alkylresorcinols, seem to contribute to the antioxidant and anti-inflammatory effects(Reference Lutsey, Jacobs and Kori29).
The lipid profile results of the present analyses are in line with the Framingham Offspring Cohort Study that showed whole-grain foods were related to lower LDL-cholesterol and total cholesterol concentrations and not to HDL-cholesterol or TAG concentrations(Reference Angelino, Martina and Rosi14). The Tehran, Lipid and Glucose Study with 827 men and women revealed that intake of traditional Iranian whole-grain foods was associated with lower TAG concentrations but not with any other lipid variables(Reference Esmaillzadeh, Mirmiran and Azizi11). Similarly, an improvement in the lipid profiles has also been found in overweight or obese subjects, after consumption of oat-based products for 12 weeks(Reference Davy, Davy and Ho35) and in subjects with intake of β-glucan-rich products for 8 weeks(Reference Tovar, Nilsson and Johansson15). The beneficial effects of whole-grain foods have been, at least partly, ascribed to their lipid-lowering properties, particularly for whole grains containing relatively high amounts of viscous soluble fibre(Reference Ross, Godin and Minehira36).
In the present study, we found a 3 mmHg reduction in systolic blood pressure and a 1 mmHg reduction in diastolic blood pressure. These results are also consistent with results of previous studies that reported the beneficial effects of whole grains on blood pressure(Reference Tighe, Duthie and Vaughan37,Reference Streppel, Arends and van’t Veer38) . Contrary to our study, Angelino et al.(Reference Angelino, Martina and Rosi14) found no significant variations in systolic and diastolic blood pressure, probably because of low consumption of daily whole grains. The beneficial effects of whole grains on blood pressure could be explained by potential bioactivity of micronutrients such as Ca, Mg, Zn, K and vitamin D(Reference Tarantino, Porcu and Arciello31).
Based on an assessment of dietary intake at baseline and every 4 weeks, participants in the intervention group had a greater reduction in energy intake than did participants in the control group. This might be explained by the fact that whole grains could reduce appetite; however, further investigations are required. Participants in the intervention group significantly improved their diet quality by increasing their intake of dietary fibres to almost 14 g/4184 kJ, which is the amount recommended in the 2012 dietary guidelines for Americans(9). Participants in the whole-grain group also increased their intake of beneficial micronutrients which potentially contribute to the management of NAFLD(Reference Tarantino, Porcu and Arciello31). Thus, the consumption of whole-grain foods would be expected to benefit metabolic diseases, such as obesity, type 2 diabetes and NAFLD.
The strength of the present study rests on its high completion and compliance rate. Furthermore, consumption of whole-grain food is a low-cost management strategy for NAFLD patients.
A limitation of the present study is its short duration of interventions.
In conclusion, this open-label randomised controlled clinical trial provided some evidence that consumption of whole-grain foods improved hepatic features in patients with NAFLD. Nevertheless, future clinical studies examining larger sample sizes for longer periods are required to determine the long-term health benefits of whole grains.
Acknowledgements
The authors would like to express their gratitude towards the Urmia University of Medical Sciences, for the facilities and financial support. The authors would also like to thank the patients who participated in the present study. This article is based on data obtained from a master’s thesis in Nutrition.
The present study was supported by Urmia University of Medical Sciences. This trial was registered at the Iranian Registry of Clinical Trials website as IRCT20170206032417N3.
The authors’ responsibilities were as follows: M. A. and M. D. conceived and designed the study and analysed the data; J. A. provided material and technical support; F. B. wrote the manuscript; M. A. critically revised the manuscript for important intellectual content and M. A. had primary responsibility. All authors read and approved the final manuscript.
There are no conflicts of interest.
The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported. The reporting of this work is compliant with CONSORT guidelines. The lead author affirms that no important aspects of the study have been omitted and that any discrepancies from the study as planned have been explained.









