Obesity develops when energy consumed in excess of daily metabolic requirements is stored as TAG in the adipose tissue. The prevalence of obesity and associated conditions has reached epidemic proportions worldwide(Reference Bruce and Byrne1, Reference Caterson, Hubbard and Bray2), and therefore there is a growing interest to identify nutritional factors that could influence energy balance and thus could reduce the susceptibility to develop obesity, such as that caused by a high-fat diet (HFD)(Reference Chalkley, Hettiarachchi and Chisholm3–Reference Lin, Thomas and Storlien5).
Whey protein isolate (WPI) is a mixture of milk proteins obtained after precipitation of casein during cheese production, and it contains β-lactoglobulin, α-lactalbumin, bovine serum albumin, glycomacropeptide, Ig, lactoferrin and other minor proteins. WPI has received a lot of attention recently because of its associated health benefits(Reference Krissansen6). Prolonged intake of whey proteins has been found to improve insulin sensitivity and glucose homeostasis(Reference Acheson, Blondel-Lubrano and Oguey-Araymon7–Reference Shertzer, Woods and Krishan10). Also, using healthy lean subjects or rodents, a number of studies have reported a short-term reduction in energy intake and an increase in thermogenesis following WPI intake in comparison with other dietary proteins including casein(Reference Acheson, Blondel-Lubrano and Oguey-Araymon7, Reference Hall, Millward and Long11–13). Although there are discrepancies(Reference Benton and Swan14–Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen17), it is largely recognised that WPI intake influences the balance between energy intake and energy expenditure in the lean state(Reference Luhovyy, Akhavan and Anderson18, Reference McAllan, Cotter and Roche19). In contrast, the effects of WPI on energy intake and energy expenditure in the HFD-induced state are less well known, although WPI has consistently been shown to reduce HFD-induced body-weight gain and adiposity(Reference Shertzer, Woods and Krishan10, Reference Pilvi, Harala and Korpela20–Reference Shi, Ahlroos-Lehmus and Pilvi23). However, three studies have reported no effects on energy intake in mice fed a HFD with WPI for 11(Reference Shertzer, Woods and Krishan10), 14(Reference Shi, Ahlroos-Lehmus and Pilvi23) or 21 weeks(Reference Pilvi, Korpela and Huttunen21) in comparison with the HFD-fed controls. In the study by Shertzer et al. (Reference Shertzer, Woods and Krishan10), WPI was found to increase metabolic activity, while Pilvi et al. (Reference Pilvi, Korpela and Huttunen21) found no changes in metabolic activity in mice fed a HFD with WPI compared with those fed a control diet. It is possible that these discrepancies arise partly due to the difference in the duration of HF feeding, with prolonged feeding-induced neuroendocrine changes having a greater impact on the ability of WPI to regulate energy balance. Thus, one could argue that a shorter duration of HF feeding (in comparison with the above-mentioned studies) may reveal how WPI affects physiological and cellular parameters important for energy balance regulation.
The present study sought to investigate the short-term (8 weeks) impact of WPI in conjunction with a HFD, specifically focusing on how the dietary protein influences HFD-induced (1) body-weight gain and body composition, (2) energy intake (total intake, meal number and meal size) and parameters related to energy expenditure (VO2, heat production and locomotor activity), (3) plasma levels of hormones and metabolites and (4) the hypothalamic and epididymal adipose tissue expression of genes involved in leptin and insulin signalling, inflammation and lipid metabolism.
Materials and methods
Animals, diets and reagents
Experiments involving mice were licensed under the Cruelty to Animals Act 1876 and received ethical approval from the University College Cork Animal Experimentation Ethics Committee (no. 2011/005). Male C57BL/6J mice (Harlan), 3–4 weeks old, were maintained at 20–22°C and 45–65 % humidity, with a 12 h light–12 h dark cycle (06.00–18.00 hours). The animals had ad libitum access to food and water throughout the trials and all diets were purchased from Research diets. The experimental diets were a low-fat diet (LFD; 10 % energy as fat and 20 % energy as casein; no. D12450), a HFD (45 % energy as fat and 20 % energy as casein; no. D12451) and a HFD with WPI (HF-WPI) (Alacen™ 895; NZMP) in place of casein (45 % energy as fat and 20 % energy as WPI). All reagents were purchased from Sigma unless stated otherwise.
Experimental protocol
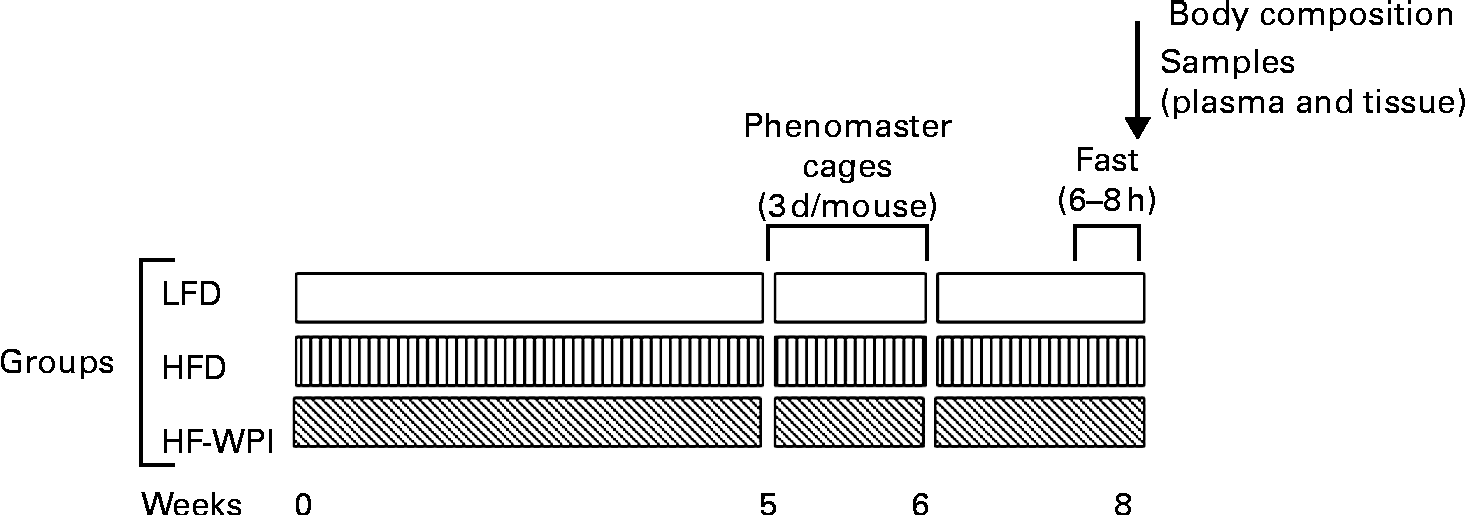
The dietary challenges were performed on group-housed mice (four per home cage) following a 4-week acclimatisation period, during which the animals were fed a LFD. As we intended to measure the effect of the above-mentioned diets on energy intake and metabolic parameters using TSE Phenomaster cages (TSE systems), by housing the mice individually in these specialised cages, it was important to first determine the length of time the animals may need to be acclimatised to this new cage environment. This was investigated in study 1. In study 2, we assessed the effect of the LFD, the HFD and the HF-WPI diet on energy intake and metabolic parameters using the above-mentioned cages, following the pre-established acclimatisation period.
Study 1
Mice were fed a LFD for 7 weeks (n 8). In weeks 5 and 6, the animals were individually housed in TSE Phenomaster cages (TSE systems) for 3 d and energy intake and metabolic parameters were measured on days 2 and 3. At the end of the third day, the animals were placed back in their appropriate home cages. Food intake in the Phenomaster cages was measured using high-precision weighing sensor-associated feeding stations. O2 consumption (ml/h per kg; VO2) and CO2 production (ml/h per kg, VCO2) were measured by indirect open-circuit calorimetry. The sensors measured the levels of food consumed and VO2 and VCO2 in each cage every 9 min. A meal was defined as an intake greater than 0·01 g. Energy intake was calculated from 16·10 kJ/g for the LFD. The RER was calculated from VCO2/VO2. Locomotor activity was measured using a multi-dimensional infrared beam system, and it is defined as the total number of infrared beam breaks in the X-axis and Y-axis.
Study 2
The experimental protocol is outlined in Fig. 1. For 8 weeks, three weight-matched groups of mice were fed the LFD, the HFD or the HF-WPI diet (n 8). Body weights were measured weekly. In weeks 5 and 6, the energy intake and metabolic activity were measured in the individual mice using the TSE Phenomaster cages (TSE systems; Fig. 1). As mentioned earlier, the animals were housed in the Phenomaster cages for 3 d. Following a 2 d acclimatisation period (see the Results section for study 1), data were collected on the third day, after which the animals were placed back in their home cages. The parameters mentioned earlier were measured. Energy intake was calculated as described earlier for the LFD and using 19·80 kJ/g for the HFD and the HF-WPI diet. Heat production (kcal/h per kg) was calculated using the Weir equation(Reference Weir24) (3·941 × VO2+1·106 × VCO2) and converted to kJ/h per kg (1 kcal = 4·184 kJ). The food quotient (FQ), defined as the ideal diet-specific VCO2:VO2 ratio, was calculated for each diet as described previously(Reference Longo, Charoenthongtrakul and Giuliana25). In week 8, after a 6–8 h fast, body composition was determined by NMR using the Bruker minispec LF50H (Bruker Optics). Also, at that time point, plasma was collected from fasted anaesthetised mice to measure the glucagon-like peptide 1, insulin, glucose, leptin and TAG levels. The animals were then immediately culled by cervical dislocation, and tissues of interest were dissected and snap-frozen in liquid N2 (liver and adipose tissue) or on dry ice (brain).

Fig. 1 Timeline of the dietary treatments and experimental measurements. C57BL/6J mice were fed for 8 weeks with diets containing 10 % energy as fat (low-fat diet, LFD) or 45 % energy as fat (high-fat diet, HFD) with either 20 % energy as casein (LFD and HFD) or whey protein isolate (high fat (HF)-WPI). To measure metabolic parameters at weeks 5 and 6, mice were individually housed in TSE Phenomaster cages (TSE systems) for 3 d with data being collected in the final 24 h of the housing period. In week 8, body composition was measured following a 6–8 h fast, and then plasma and tissue samples were isolated.
Plasma hormones, glucose and TAG
Blood collected into BD Vacutainer EDTA tubes from fasted anaesthetised (65 mg/kg ketamine and 13 mg/kg xylazine) mice was treated with 70 mg/l aprotinin and 0·1 mm-diprotin A (Sigma) to prevent the degradation of plasma peptides by dipeptidyl aminopeptidase IV, trypsin and other related proteolytic enzymes. Blood was centrifuged at 2000 rpm and 4°C for 15 min, and plasma was isolated and stored at − 80°C. ELISA were used to measure plasma glucagon-like peptide-1 (Linco Research), leptin and insulin (Crystal Chem) levels. Plasma glucose and TAG levels were measured using colorimetric assay kits (glucose: Calibochem; TAG: Wako Chemicals). Homeostatic model assessment of insulin resistance values were determined using the following formula(Reference Matthews, Hosker and Rudenski26):
Fasting plasma insulin (μU/ml) × fasting plasma glucose (mmol/l)/22·5.
Liver TAG
Lipids from frozen liver samples (50 mg) were extracted according to the Folch method(Reference Folch, Lees and Sloane Stanley27). Briefly, lipids in each sample were extracted using a 2:1 (v/v) solution of chloroform–methanol and aliquots of the organic phase were collected, dried and resuspended in duplicate in the infinity TAG lipid-stable reagent (Thermo Scientific) or the LabAssay TAG kit reagent (Wako Chemicals). TAG levels in the samples were determined according to the manufacturer's instructions, and for samples resuspended in the infinity TAG lipid-stable reagent, lipid quantification was performed using a TAG chemistry calibrator (Pointe Scientific).
RNA extraction and complementary DNA synthesis
Total RNA was isolated from the hypothalamic and epididymal adipose tissues with diethylpyrocarbonate-treated distilled water using the RNeasy lipid tissue mini kit (Qiagen) according to the manufacturer's instructions. To remove any potential genomic DNA contamination, on-column DNase treatment (Qiagen) was performed during RNA isolation. Complementary DNA was synthesised from 1 μg of total RNA using 2·5 ng/μl of random hexamer primers (Bioline), 0·5 mm-deoxyribonucleotide triphosphate (Promega), 32 nKat/μl of RNase inhibitor (Promega) and the First Strand Synthesis System containing the SuperScript II RT (Invitrogen), according to the manufacturer's instructions. A parallel reaction without the inclusion of the RT enzyme was also performed as a negative control.
Real-time PCR
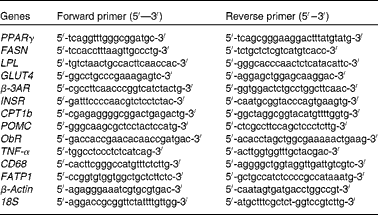
The amplification of complementary DNA was performed in the Lightcycler 480 system (Roche) using 0·25 μm primers (Eurofins MWG Operon), 1 μl complementary DNA and the Lightcycler 480 SYBR Green I Master kit (Roche), according to the manufacturer's instructions. Real-time PCR conditions were as follows: 95°C for 10 min followed by fifty cycles at 95°C for 10 s; 60 or 55°C for 5 s; 72°C for 15 s. The primer sequences used are given in Table 1. Melting curve analysis allowed the validation of the authenticity of the real-time PCR products. Automated sequencing was performed to verify the sequences of these PCR products. Data obtained as C p values were normalised to the expression of 18S and β-actin according to ΔΔC p= ΔC p target gene − ΔC p housekeeping gene. For both the adipose tissue(Reference Guo, Xia and Zou28–Reference Yasmeen, Reichert and Deiuliis31) and the hypothalamus(Reference Giraudo, Della-Fera and Proctor32–Reference Sellayah, Sek and Anthony34), 18S and β-actin have been shown to be the appropriate housekeeping genes. The relative gene expression was calculated using ![]() $$2^{ - \Delta \Delta C _{p}} $$, and it is shown in comparison with that of the LFD group.
$$2^{ - \Delta \Delta C _{p}} $$, and it is shown in comparison with that of the LFD group.
Table 1 Sequences of primers used for real-time PCR

PPARγ, peroxisome proliferator activated receptor; FASN, fatty acid synthase; LPL, lipoprotein lipase; GLUT4, Glucose transporter 4; β-3AR, β-3 adrenergic receptor; INSR, insulin receptor; CPT1b, carnitine palmitoyltransferase 1b; POMC, pro-opiomelanocortin; ObR, leptin receptor; TNF-α, Tumour necrosis factor alpha; CD68, cluster of differentiation 68; FATP1, fatty acid transporter 1.
Immunoblot analysis
Adipose tissue samples (approximately 1 ml of lysis buffer per 200 mg, n 3) were homogenised in a lysis buffer (50 mm-HEPES, 150 mm-NaCl, 1 mm-EDTA, 1 mm-ethylene glycol tetraacetic acid, 1 % Nonident P40, 0·5 mm-dithiothreitol and 0·1 mm-Na3VO4), containing protease inhibitors (0·1 mm-phenylmethylsulphonyl fluoride, 2 μg aprotinin/ml, 2 μg leupeptin/ml, 0·02 mm-NaF and 0·025 mm-NaPPi), and centrifuged at 12 000 g for 10 min to remove insoluble debris. Protein concentrations were analysed using the bicinchoninic acid reagent (Pierce Biotechnology). Protein lysate (20 μg) was mixed with a 4 × sample buffer (333 mm-Tris–HCl, 3 % SDS, 26·7 % glycerol, 130 mm-dithiothreitol and 0·2 % Bromophenol Blue) and heated for 10 min at 95°C before loading onto a NuPAGE® 4–12 % Bis–Tris gel (Invitrogen). Protein bands were separated using SDS-PAGE in the 3-(N-morpholino) propanesulfonic acid (MOPS) running buffer and transferred onto polyvinylidene difluoride membranes (BioRad). A Tris-buffered saline solution containing 0·1 % Tween-20 was used as the wash buffer, which was supplemented with 2 % bovine serum albumin (Sigma) and 3 % non-fat dry milk (Marvel; Premier Foods Limited) for the blocking solution and with 1 % bovine serum albumin and 1 % non-fat dry milk for the antibody diluent. The blocked membranes were exposed to a 1:500 dilution of the primary antibody against the insulin receptor (INSR) β (sc-711; Santa Cruz Biotechnology). Horseradish peroxidase-conjugated secondary antibody (1:8000 dilution; Jackson Immuoresearch) was used to visualise the bound primary antibody. The membranes were stained with a 1:15 000 dilution of a β-actin–horseradish peroxidase antibody (A3854; Sigma) to correct for sample loading. Visualisation was performed using the enhanced chemiluminescence Western blotting Substrate Kit (Pierce Biotechnology).
Statistical analysis
All data are presented as means with their standard errors. Differences between the experimental groups were analysed by a one-way or two-way ANOVA with Tukey's post hoc pairwise comparisons. Body weight differences over 8 weeks were analysed by a two-way repeated-measures ANOVA with post hoc pairwise comparisons. Statistical analysis of immunoblot analysis data was performed using a multiple-comparisons Kruskal–Wallis test followed by Mann–Whitney U tests for individual comparisons. Significance was accepted at P< 0·05, and the statistical analysis was performed using the GraphPad Prism version 3.03 (GraphPad Software, Inc.), Minitab version 15 (Minitab, Inc.) and Sigma Stat version 3.1 (SyStat Software, Inc.).
Results
Body weight and composition
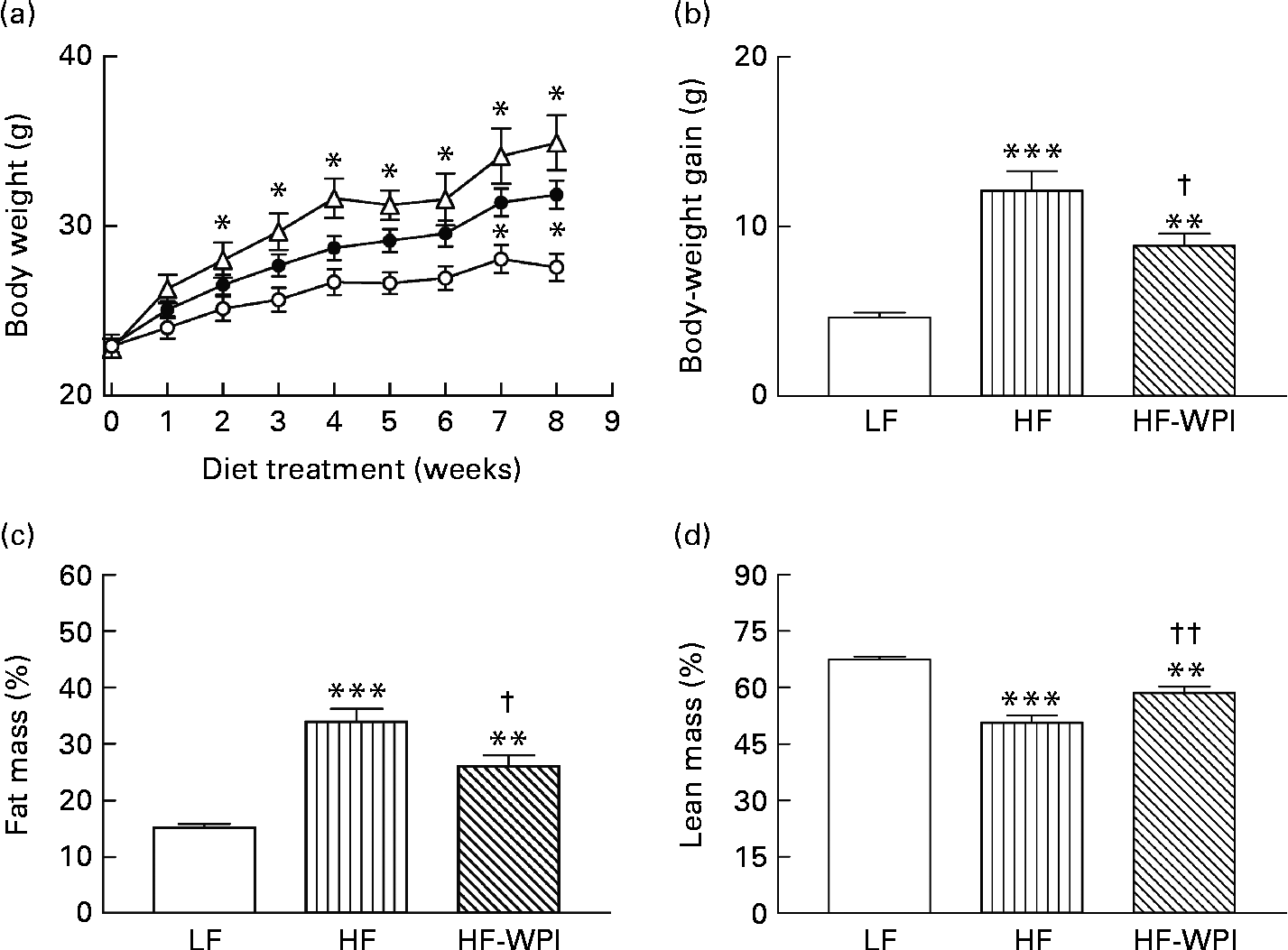
Body weight trajectories over the 8-week treatment period showed that the mice on the HFD and the HF-WPI diet were significantly heavier than the LFD group from the second and seventh weeks, respectively (Fig. 2(a)). Importantly, the HF-WPI group gained significantly less body weight than the HFD group (P< 0·05; Fig. 2(b)). In week 8, the total body fat (%) in the HF-WPI diet-fed mice was significantly lower than that in the HFD-fed mice (P< 0·05; Fig. 2(c)), but the total body fat (%) was still greater than that in the LFD-fed mice (P< 0·001). Furthermore, the total lean mass (%) was higher in the HF-WPI group than in the HFD-fed group (P< 0·05; Fig. 2(d)), and as anticipated it was lower in the HFD- and HF-WPI diet-fed mice than in the LFD group (P< 0·001).

Fig. 2 Effect of feeding a low-fat (LF, ![]() ), a high-fat (HF,
), a high-fat (HF, ![]() ) or a HF with whey protein isolate (HF-WPI,
) or a HF with whey protein isolate (HF-WPI, ![]() ) diet for 8 weeks upon (a) body weight, (b) body-weight gain, (c) total fat mass and (d) total lean mass in C57BL/6J mice. Values are means (n 8 per group), with standard errors represented by vertical bars. Mean value was significantly different from that of the group fed the LF diet: * P< 0·05, ** P< 0·01, *** P< 0·001. Mean value was significantly different from that of the group fed the HF diet: † P< 0·05, †† P< 0·01.
) diet for 8 weeks upon (a) body weight, (b) body-weight gain, (c) total fat mass and (d) total lean mass in C57BL/6J mice. Values are means (n 8 per group), with standard errors represented by vertical bars. Mean value was significantly different from that of the group fed the LF diet: * P< 0·05, ** P< 0·01, *** P< 0·001. Mean value was significantly different from that of the group fed the HF diet: † P< 0·05, †† P< 0·01.
Plasma hormone, glucose and TAG, and hepatic TAG levels
In week 8, plasma leptin concentrations were higher in the HFD and HF-WPI groups than in the LFD group (P< 0·05), while plasma TAG levels were greater in the HF-WPI group than in both the LFD and HFD groups (P< 0·05; Table 2). There was no significant difference in the plasma glucose, insulin, homeostatic model assessment of insulin resistance and glucagon-like peptide-1 levels among the three diet groups (Table 2). Hepatic TAG levels (mg/g liver) were determined using the infinity TAG lipid-stable reagent or the LabAssay TAG kit (Wako Chemicals) following the extraction of the lipids. The data generated using the former revealed that the HFD-fed mice had a higher hepatic TAG level than the LFD group (P< 0·05; Table 2). While hepatic TAG levels in the HF-WPI diet-fed mice were lower than those in the HFD group, the values did not differ significantly from those of either the LFD or HFD group. In contrast, the data generated using the LabAssay TAG kit (Wako Chemicals) showed that the hepatic TAG values (mg/g liver) for the HF-WPI diet (58·68 (sem 3·92))-fed mice were significantly lower than those for the HFD group (90·9 (sem 8·65)) (P< 0·05), while the values for the HF-WPI group were not significantly different from those for the LFD group (46·65 (sem 3·09)).
Table 2 Plasma levels of hormones and TAG and hepatic TAG accumulation (Mean values with their standard errors, n 7–8)

WPI, whey protein isolate; GLP-1, glucagon-like peptide 1; HOMA-IR, homeostatic model assessment of insulin resistance.
a,bMean values with unlike superscript letters were significantly different (P< 0·05).
Energy intake, energy expenditure and RER
Study 1
The LFD-fed mice were individually housed in the Phenomaster cages during weeks 5 and 6 of the 7-week dietary challenge. In the 3 d housing period in the Phenomaster cages, the energy intake, VO2, RER and locomotor activity in the animals were similar on days 2 and 3 (Fig. S1, available online). We interpreted these data to suggest that by day 2 the mice had been acclimatised to the new Phenomaster cage environment and that data gathered on day 3 represent accurate measures of their energy intake and metabolic activity. Previous studies have also used a 48 h period for acclimatisation, before measuring the metabolic and feeding behaviours in mice using similar specialised cages(Reference Czyzyk, Nogueiras and Lockwood35, Reference Dubois, Kishimoto and Lillis36).
Study 2
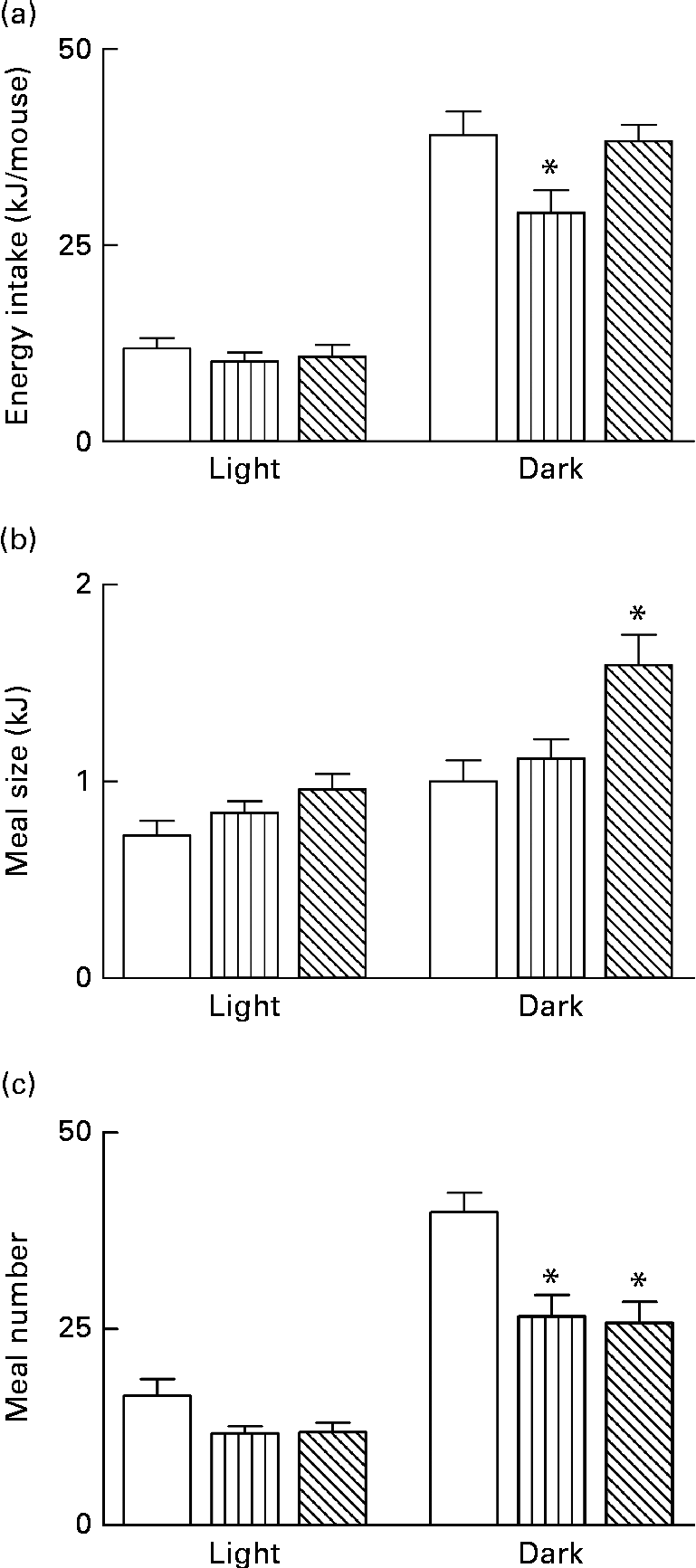
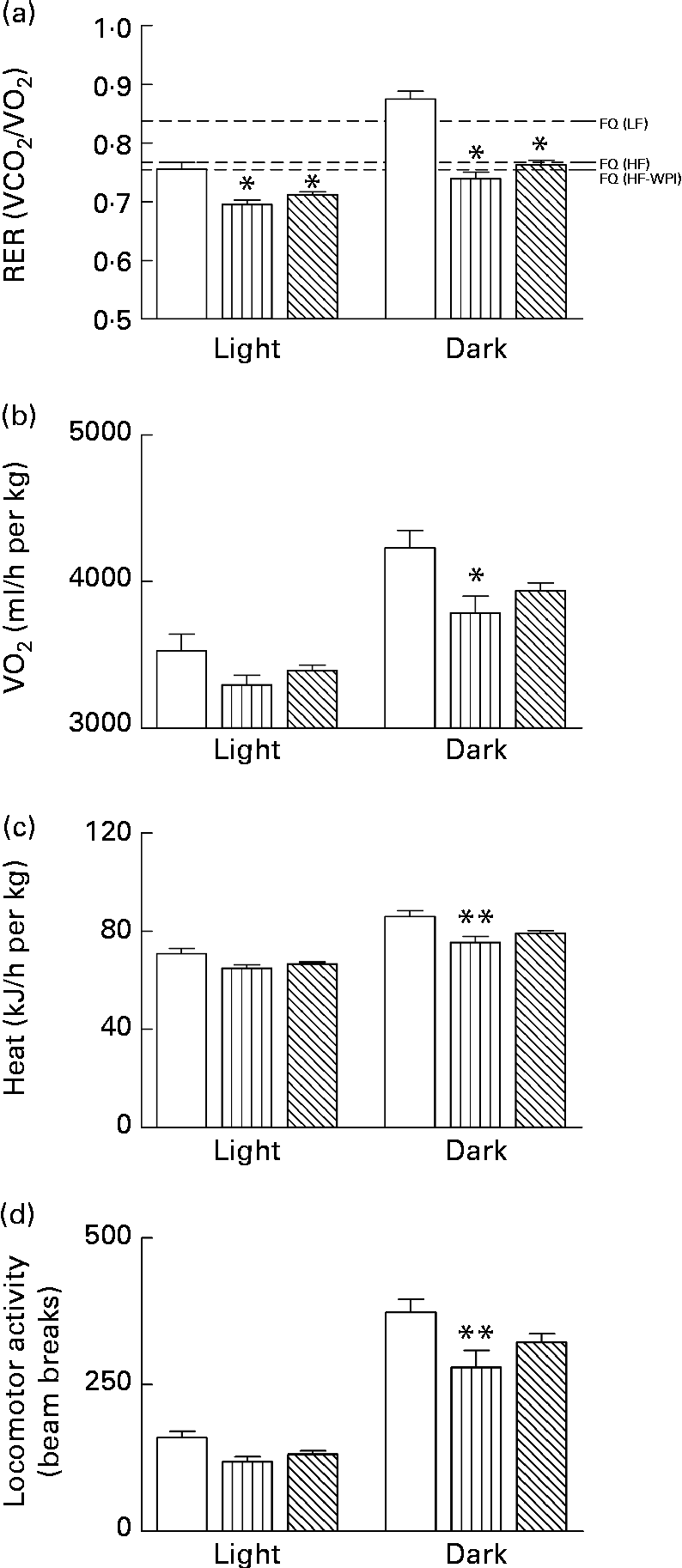
Energy intake and metabolic activity were measured as described earlier in weeks 5 and 6 of the 8-week dietary challenge. Energy intake was lower during the dark phase for the HFD group compared with both the HF-WPI diet- and LFD-fed mice (P< 0·05; Fig. 3(a)). The average meal size over the same period was not significantly different between the LFD- and HFD-fed mice, while the HF-WPI diet-fed mice had a greater average meal size than both the LFD and HFD groups (P< 0·05; Fig. 3(b)). The meal number during the dark phase was reduced for both the HFD and HF-WPI groups compared with the LFD-fed mice (P< 0·001; Fig. 3(c)). No difference in energy intake-related parameters was found between all groups during the light phase (Fig. 3(a)–(c)). The FQ calculated for the LFD, HFD and HF-WPI diet are shown in Fig. 4(a). The RER of both the HFD- and HF-WPI diet-fed groups were lower than that of the LFD group in both the light and dark phases (P< 0·05; Fig. 4(a)). The VO2, heat production and locomotory activity during the dark phase of the HFD-fed mice were lower than those of the LFD group (P< 0·05; Fig. 4(b)–(d)). In the HF-WPI diet-fed mice, these parameters did not differ significantly from those of either the LFD or HFD group during the dark phase. No differences in the above-mentioned parameters were observed between all groups during the light phase (Fig. 4(b)–(d)).

Fig. 3 Effect of feeding a low-fat (LF, □), a high-fat (HF, ![]() ) or a HF with whey protein isolate (HF-WPI,
) or a HF with whey protein isolate (HF-WPI, ![]() ) diet for 5 to 6 weeks upon (a) energy intake (b) meal size and (c) meal number in C57BL/6J mice. Experimental data, collected from individual mice at 9 min intervals over a 24 h period using TSE Phenomaster cages (TSE systems), are shown for the light and dark phases. Values are means (n 8 per group), with their standard errors represented by vertical bars. (a) * Mean value was significantly different from those of the LF and HF-WPI groups (P< 0·05). (b) * Mean value was significantly different from those of the LF and HF groups (P< 0·05). (c) * Mean value was significantly different from that of the LF group (P< 0·05).
) diet for 5 to 6 weeks upon (a) energy intake (b) meal size and (c) meal number in C57BL/6J mice. Experimental data, collected from individual mice at 9 min intervals over a 24 h period using TSE Phenomaster cages (TSE systems), are shown for the light and dark phases. Values are means (n 8 per group), with their standard errors represented by vertical bars. (a) * Mean value was significantly different from those of the LF and HF-WPI groups (P< 0·05). (b) * Mean value was significantly different from those of the LF and HF groups (P< 0·05). (c) * Mean value was significantly different from that of the LF group (P< 0·05).

Fig. 4 Effect of feeding a low-fat (LF, □), a high-fat (HF, ![]() ) or a HF with whey protein isolate (HF-WPI,
) or a HF with whey protein isolate (HF-WPI, ![]() ) diet for 5 to 6 weeks upon (a) RER, (b) oxygen consumption (VO2), (c) heat production and (d) locomotor activity in C57BL/6J mice. Experimental data, collected from individual mice at 9 min intervals over a 24 h period using TSE Phenomaster cages (TSE Systems), are shown for the light and dark phases. The food quotient (FQ;
) diet for 5 to 6 weeks upon (a) RER, (b) oxygen consumption (VO2), (c) heat production and (d) locomotor activity in C57BL/6J mice. Experimental data, collected from individual mice at 9 min intervals over a 24 h period using TSE Phenomaster cages (TSE Systems), are shown for the light and dark phases. The food quotient (FQ; ![]() ) used in (a) is defined as the ideal diet-specific VCO2:VO2 ratio, and it was calculated for each diet as described previously(Reference Longo, Charoenthongtrakul and Giuliana25). Values are means (n 8 per group), with standard errors represented by vertical bars. Mean value was significantly different from that of the group fed the LF diet: * P< 0·05, ** P< 0·01.
) used in (a) is defined as the ideal diet-specific VCO2:VO2 ratio, and it was calculated for each diet as described previously(Reference Longo, Charoenthongtrakul and Giuliana25). Values are means (n 8 per group), with standard errors represented by vertical bars. Mean value was significantly different from that of the group fed the LF diet: * P< 0·05, ** P< 0·01.
Adipose tissue gene and protein expression
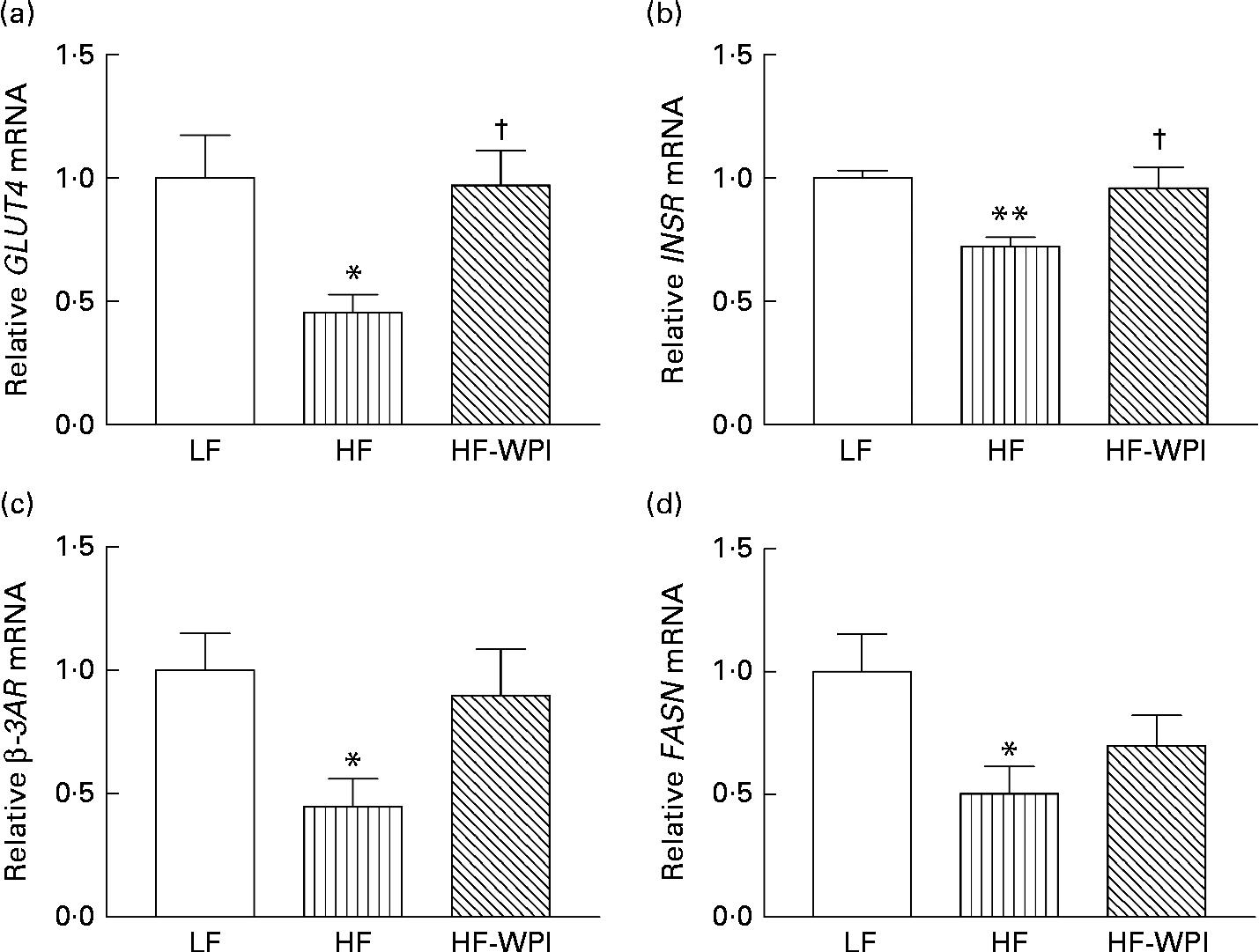
The mRNA expression of GLUT4 (Fig. 5(a)), INSR (Fig. 5(b)), β-3 adrenergic receptor (β-3AR; Fig. 5(c)) and fatty acid synthase (FASN; Fig. 5(d)) was significantly lower in the HFD group than in the LFD-fed mice (P< 0·05). The mRNA expression of INSR and GLUT4 was increased in the HF-WPI diet-fed mice compared with the HFD-fed mice (P< 0·05), such that the expression was not significantly different from that of the LFD group (Fig. 5(a) and (b)). Similarly, the mRNA expression of β-3AR in the HF-WPI group was increased compared with the HFD group, but did not reach statistical significance. The mRNA expression of FASN was not affected by the HF-WPI diet (Fig. 5d). No significant difference in the expression of the following genes was found between all the three diet groups: PPARγ (1·00 (sem 0·17) in the LFD group v. 0·93 (sem 0·24) in the HFD group v. 1·09 (sem 0·18) in the HF-WPI group); lipoprotein lipase (LPL: 1·00 (sem 0·16) in the LFD group v. 1·37 (sem 0·21) in the HFD group v. 1·54 (sem 0·20) in the HF-WPI group); carnitine palmitoyltransferase 1b (CPT1b: 1·00 (sem 0·15) in the LFD group v. 1·24 (sem 0·22) in the HFD group v. 1·24 (sem 0·14) in the HF-WPI group); fatty acid transporter protein 1 (FATP1: 1·00 (sem 0·13) in the LFD group v. 0·83 (sem 0·13) in the HFD group v. 0·93 (sem 0·10) in the HF-WPI group); TNF-α (1·00 (sem 0·14) in the LFD group v. 2·35 (sem 0·82) in the HFD group v. 1·43 (sem 0·31) in the HF-WPI group); cluster of differentiation 68 (CD68: 1·00 (sem 0·17) in the LFD group v. 2·95 (sem 1·05) in the HFD group v. 1·37 (sem 0·28) in the HF-WPI group).

Fig. 5 Effect of feeding a low-fat (LF), a high-fat (HF) or a high-fat with whey protein isolate (HF-WPI) diet for 8 weeks upon the epididymal adipose tissue mRNA expression of (a) GLUT4, (b) insulin receptor (INSR) (c) β-3 adrenergic receptor (β-3AR) and (d) fatty acid synthase (FASN) in C57BL/6J mice. The mRNA expressions were normalised using 18S and β-actin according to ΔΔC p= ΔC p target gene − ΔC p housekeeping gene. The gene expressions were calculated using ![]() $$2^{ - \Delta \Delta C _{p}} $$, and they are shown in comparison with that of the LF group. Values are means (n 7–8 per group), with their standard errors represented by vertical bars. Mean value was significantly different compared with the LF group: * P< 0·05, ** P< 0·01. † Mean value was significantly different compared with the HF group (P< 0·05).
$$2^{ - \Delta \Delta C _{p}} $$, and they are shown in comparison with that of the LF group. Values are means (n 7–8 per group), with their standard errors represented by vertical bars. Mean value was significantly different compared with the LF group: * P< 0·05, ** P< 0·01. † Mean value was significantly different compared with the HF group (P< 0·05).
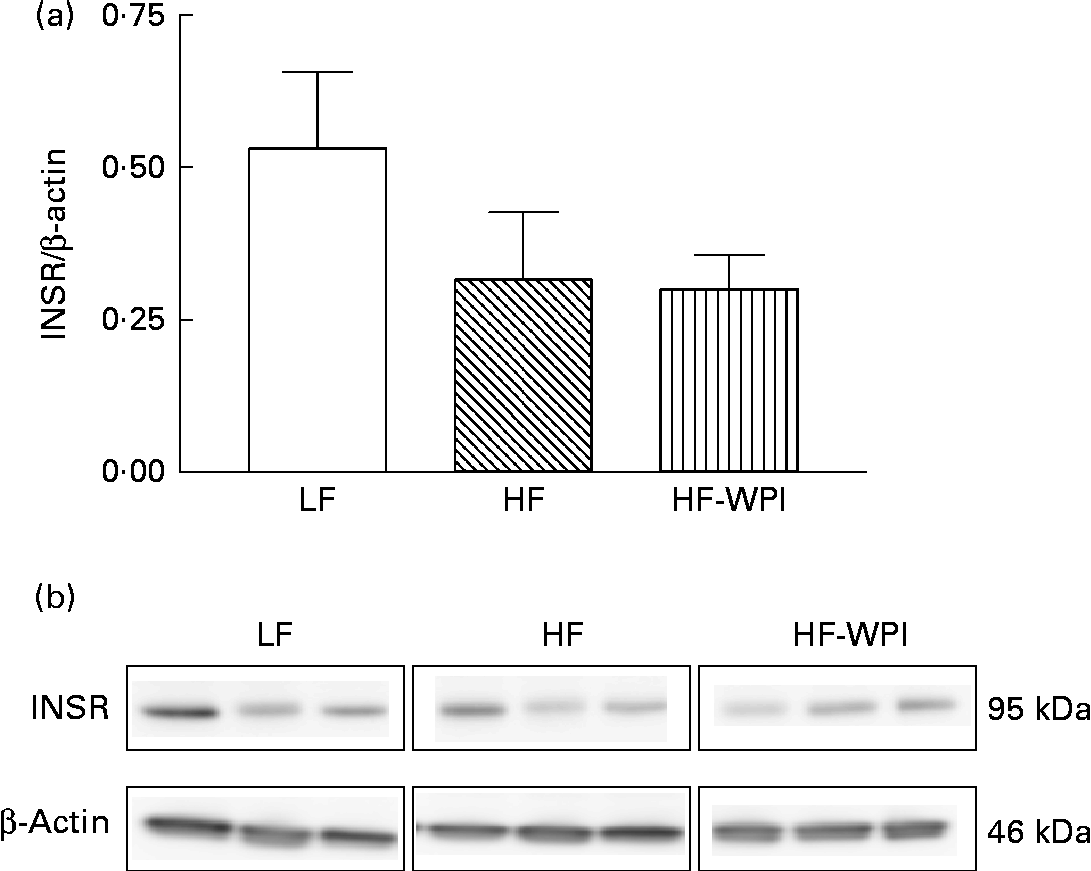
The association between the diet and the expression of INSR was further explored by investigating the protein expression of INSR-β in the epididymal adipose tissue (Fig. 6). The intake of the HFD decreased the protein expression of INSR-β compared with the intake of the LFD, similar to mRNA expression, although the decreased protein expression did not reach statistical significance (Fig. 6). The protein expression of INSR-β did not differ between the HFD and HF-WPI groups (Fig. 6).

Fig. 6 Effect of feeding a low-fat (LF), a high-fat (HF) or a high-fat with whey protein isolate (HF-WPI) diet for 8 weeks upon the epididymal adipose tissue expression of insulin receptor-β (INSR-β), as measured by the immunoblot analysis. The protein expression of INSR-β relative to the expression of β-actin is shown in (a) and the images of the corresponding immunoblot are shown in (b). Values are means (n 3 per group), with their standard errors represented by vertical bars.
Hypothalamic tissue gene expression
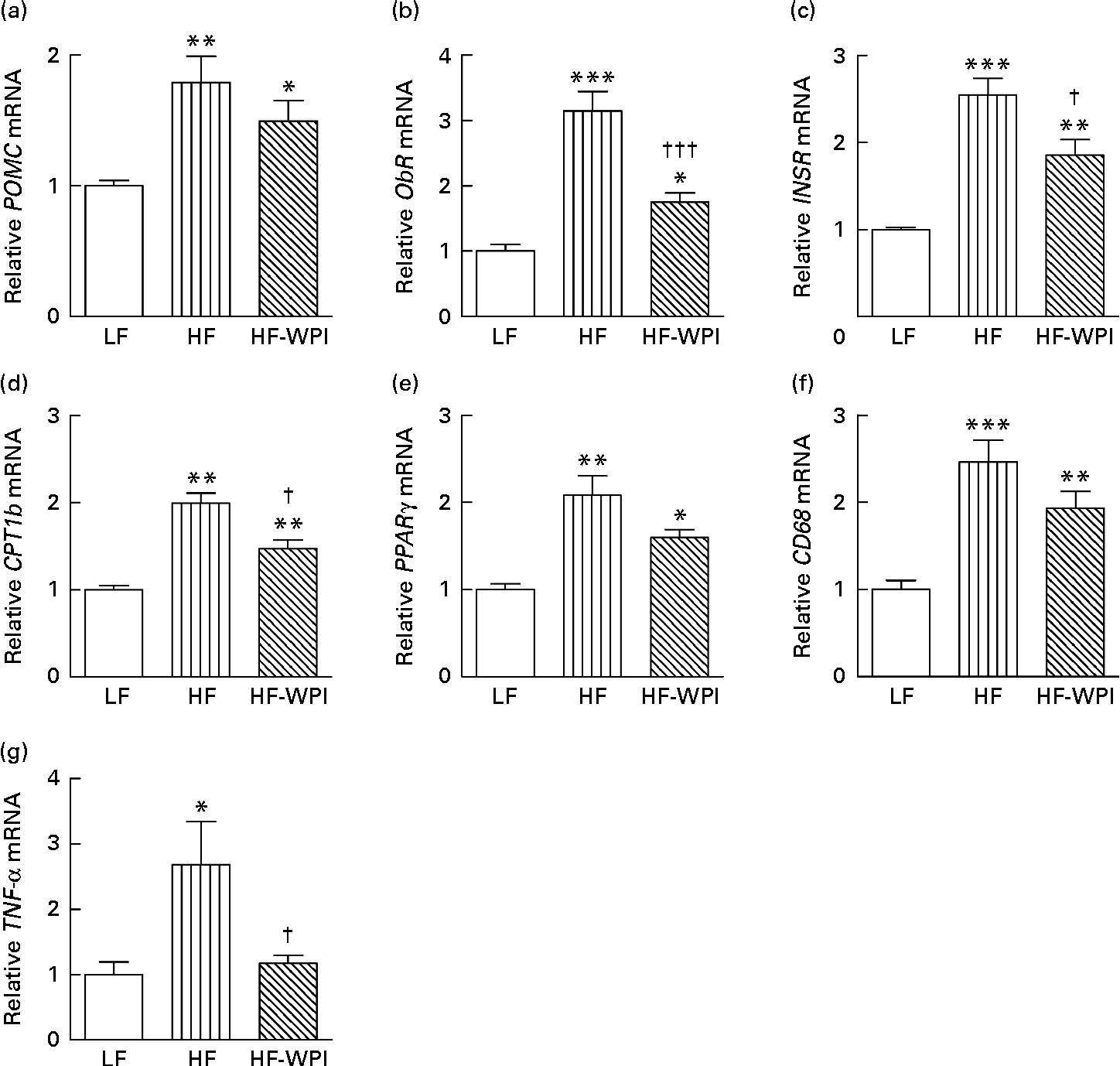
Given the key central effects on feeding and adipocyte metabolism(Reference Nogueiras, Lopez and Dieguez37, Reference Nogueiras, Wiedmer and Perez-Tilve38), we next focused our attention on the hypothalamus and investigated whether the diets affected the expression of energy balance-related genes in this region. The data showed that the HFD group had increased mRNA expression of pro-opiomelanocortin (POMC), leptin receptor (ObR), INSR, CPT1b, PPARγ and CD68 compared with the LFD-fed mice (P< 0·001; Fig. 7(a)–(f), respectively). Similarly, an increase in the mRNA expression of TNF-α was observed with HFD feeding (P< 0·05; Fig. 7(g)). The mRNA expression of ObR, INSR, CPT1b and TNF-α was significantly lower in the HF-WPI-fed mice than in the HFD group (P< 0·05; Fig. 7(b)–(d) and (g)), with no significant difference in expression being observed for POMC and CD68 between the two groups. The mRNA expression of PPARγ in the HF-WPI diet-fed mice also showed a trend towards a decrease compared with the HFD values (P= 0·06). The observed mRNA expression of ObR, INSR, CPT1b, PPARγ and CD68 was still elevated in the HF-WPI group compared with the LFD group (P< 0·05).

Fig. 7 Effect of feeding a low-fat (LF), a high-fat (HF) or a high-fat with whey protein isolate (HF-WPI) diet for 8 weeks upon the hypothalamic tissue mRNA expression of (a) pro-opiomelanocortin (POMC), (b) leptin receptor (ObR), (c) insulin receptor (INSR), (d) carnitine palmitoyltransferase 1b (CPT1b), (e) PPARγ, (f) cluster of differentiation 68 (CD68) and (g) TNF-α in C57BL/6J mice. The mRNA expressions were normalised using 18S and β-actin according to ΔΔC p= ΔC p target gene − ΔC p housekeeping gene. The relative gene expressions were calculated using ![]() $$2^{ - \Delta \Delta C _{p}} $$, and they are shown in comparison with that of the LF group. Values are means (n 6–8 per group), with their standard errors represented by vertical bars. Mean value was significantly different compared with the LF group: * P< 0·05, ** P< 0·01, *** P< 0·001. Mean value was significantly different compared with the HF group: † P< 0·05, ††† P< 0·001.
$$2^{ - \Delta \Delta C _{p}} $$, and they are shown in comparison with that of the LF group. Values are means (n 6–8 per group), with their standard errors represented by vertical bars. Mean value was significantly different compared with the LF group: * P< 0·05, ** P< 0·01, *** P< 0·001. Mean value was significantly different compared with the HF group: † P< 0·05, ††† P< 0·001.
Discussion
Mammals are able to defend perturbations in their body weight and adiposity by a complex interaction between central and peripheral tissues. The intake of a HFD is known to shift the defended body weight to a higher set point through changes in the mechanisms regulating energy balance(Reference Ryan, Woods and Seeley39). To better understand how WPI may influence this shift in energy balance during an 8-week HFD feeding trial, we compared energy balance-related parameters in LFD-, HFD- and HFD with WPI (HF-WPI)-fed mice at weeks 5 and 6 and at week 8. In agreement with recent findings(Reference Shertzer, Woods and Krishan10, Reference Pilvi, Harala and Korpela20–Reference Shi, Tauriainen and Martonen22), WPI reduced HFD-induced weight gain and adiposity. Interestingly, the HF-WPI diet-fed mice had increased energy intake but unaltered energy expenditure-related parameters compared with the HFD-fed mice. WPI also influenced hypothalamic and adipose tissue gene expression in the latter mice. To our knowledge, the present study is the first to show that WPI has an impact on energy intake by affecting the meal size and also causes changes to hypothalamic cellular activity in HFD-fed mice.
High-fat feeding in mice has been shown to have a differential impact upon energy intake in a temporal fashion, with no difference being observed in weeks 1–4, a significant reduction in energy intake during weeks 5–12 and a progressive increase in energy intake in week 13 onwards compared with the LFD-fed controls(Reference Lin, Thomas and Storlien5). Consistent with these findings, during weeks 5 and 6, we found a reduced energy intake in the HFD-fed mice compared with the mice fed the LFD. We further observed that this reduction is due to the dark-phase consummatory behaviour, possibly resulting from the reduction in meal number. The data suggest that mice fed the HFD are attempting to regulate their energy intake at weeks 5 and 6 during the diet-induced shift in body weight (set point). The intake of WPI, in place of casein, increased HFD-induced dark-phase energy intake to a level comparable with that observed in the LFD group, probably by increasing the meal size. The differential effects of WPI and casein on HFD-induced energy intake may arise due to the differential kinetics of digestion and/or metabolism of the two proteins and the associated neuroendocrine changes, given that whey proteins are known to be digested fast, thus releasing the associated bioactive components into the circulatory system much quicker than that observed with casein digestion(Reference Hall, Millward and Long11, Reference Luhovyy, Akhavan and Anderson18). Previous studies have shown that WPI reduces energy intake compared with other dietary proteins in the lean state(Reference Zhou, Keenan and Losso12, 13), with one study suggesting that whey proteins increase intermeal interval compared with soya proteins(13). In the obese state, the effects of WPI on energy intake appear to be lost, yet dietary proteins still attenuate HFD-induced obesity(Reference Shertzer, Woods and Krishan10, Reference Pilvi, Harala and Korpela20–Reference Shi, Ahlroos-Lehmus and Pilvi23). The discrepancies between these data and those of the present study, in particular, with regard to energy intake, may be related to the differences in dietary macronutrient and micronutrient composition (45 % energy as fat v. 60 % energy as fat, casein v. soya as the control and altered Ca content in the diet) and/or the study duration (8 weeks v. up to 20 weeks) that were used to assess the energy balance impact of whey proteins(Reference McAllan, Cotter and Roche19). It is also possible that some of these discrepancies may have arisen due to the fact that we subjected the group-housed mice to a single housing environment of the TSE Phenomaster cages (TSE systems) to measure their energy intake. Indeed, individual housing can induce stress in C57BL/6J mice, resulting in a decreased food intake by 3 h following exposure to the new environment(Reference Saegusa, Takeda and Muto40). However, the effect of the novelty stress is lost after 6 h in the new environment as the food intake in these mice is similar to that of the controls. To minimise any such effect of single housing on physiological parameters in the present study, we allowed the mice a 2 d acclimatisation period in the new TSE Phenomaster cages (TSE systems) before measuring their energy balance-related parameters. Indeed, our preliminary investigation (study 1) revealed that on days 2 and 3 of the housing period, the mice consumed a similar energy content and exhibited a similar metabolic activity (Fig. S1, available online).
We, as others(Reference Longo, Charoenthongtrakul and Giuliana25, 41), have found an association between FQ, RER and energy balance. In a state of ‘perfect’ energy and nutrient balance (energy homeostasis), the FQ equals the RER. In the present study, the RER was much lower than the FQ for all dietary groups during the light phase. This is expected given that during this phase the mice consumed less energy and had reduced metabolic (VO2 and heat) and locomotor activities compared with those observed during the dark phase. The potential mismatch between energy intake and energy expenditure that causes fat oxidation, as indicated by the RER being lower than the FQ, is likely to represent a mechanism to obtain the required energy to sustain metabolic activity in the mice during the light phase(41). During the dark phase, the RER of the HFD group was close to the FQ, but the RER of the LFD group was much higher than the FQ. This indicates that for the HFD group, energy intake is closer to energy utilisation, while for the LFD group, a much greater energy intake is required to meet the energy demands. This suggestion is supported by the data showing that both energy intake and overall metabolic (VO2 and heat) and locomotor activities in the mice on the HFD were much lower than those observed in the LFD-fed mice. The data support the suggestion that mice fed the HFD for 5 to 6 weeks are attempting to regulate their energy supply with their energy demands to maintain their body weight, in a way similar to what the LFD-fed mice do, but at a set point much higher than that of the latter group(Reference Ryan, Woods and Seeley39). Interestingly, during the dark phase, the mice on WPI had a RER almost equal to their theoretical FQ, yet the expected association between energy intake and parameters linked to energy expenditure did not exist, as these mice had a much higher energy intake than the HFD-fed mice, but had metabolic and locomotor activities similar to those of the HFD-fed mice. The present data suggest that WPI does not have a significant impact upon the metabolic activity in the HFD-fed mice. This is not in agreement with the data from another study(Reference Shertzer, Woods and Krishan10), in which it has been shown that HFD-fed mice drinking WPI-supplemented water had increased VO2 levels and mitochondrial respiration rates compared with the HFD controls. However, unlike in the present study, the two dietary groups in the Shertzer et al. (Reference Shertzer, Woods and Krishan10) study were consuming disproportionate amounts of protein due to WPI being present in the drinking water in addition to the protein received in the diet. Therefore, the increased metabolic activity observed in the WPI group in the above-mentioned study may simply have resulted from the increased protein intake-induced metabolism rather than due to an effect of the protein source. In the absence of an elevated metabolic activity, energy consumed by the HF-WPI-fed mice in the present study could be utilised for another biological activity, such as muscle metabolism, resulting in an increased total lean mass (%) in the HF-WPI group compared with the HFD group by week 8.
Feeding the HFD for 8 weeks increased the overall fat mass compared with LFD feeding, which concurs with previous studies(Reference Chalkley, Hettiarachchi and Chisholm3, Reference Lin, Thomas and Storlien5). Feeding WPI to the HFD-fed mice decreased the overall fat content in the body, with decreased TAG accumulation in the liver, although the statistical significance of this decrease varied depending upon the type of assay kit used to measure the lipid content in the liver or upon the efficiency of the Folch lipid extraction method(Reference Hanson and Lester42). The results are nonetheless consistent with previous findings reported in the literature(Reference Shertzer, Woods and Krishan10, Reference Shi, Tauriainen and Martonen22) that WPI reduces liver TAG levels. The effects of the dietary challenges on tissue lipid metabolism were also reflected at the level of gene expression. We showed that HFD feeding decreased the expression of INSR, GLUT4 and FASN in the epididymal adipose tissue compared with LFD feeding. Given the involvement of these genes in adipocyte lipogenesis(Reference Herman, Peroni and Villoria43, Reference Kersten44), the data suggest that a HFD decreases endogenous TAG production in the epididymal adipose tissue. Interestingly, we, as others(Reference Collins, Daniel and Petro45–Reference Sawa and Harada47), observed a reduction in the expression of β-3AR in the epididymal adipose tissue with HFD feeding. As β-3AR plays an important role in energy homeostasis due to its impact on lipolysis and thermogenesis(Reference Collins, Daniel and Petro45–Reference Sawa and Harada47), it is likely that this reduction in gene expression coupled with the reduced activity of the protein product that is known to occur in HFD-fed mice(Reference Collins, Daniel and Petro45–Reference Sawa and Harada47) contributes to the increased TAG accumulation and hence adiposity in these mice. Similarly, the HFD-induced increase in the expression of lipid-responsive PPARγ (Reference Ryan, Li and Grayson48, Reference Diano, Liu and Jeong49) and CPT1b (Reference Bonnefont, Djouadi and Prip-Buus50, Reference Gao, Chen and Kong51) in the hypothalamus is consistent with the recent findings that the HFD increases fatty acid accumulation in the brain(Reference Borg, Omran and Weir52, Reference Posey, Clegg and Printz53). Thus, the present data suggest that HFD-fed mice have greater lipid storage in multiple tissues compared with the LFD-fed mice, potentially leading to the shift in their defended body weight to a higher level. The reduction in the overall body and hepatic fat content described here with the HF-WPI diet feeding has been reported by other studies, which have shown the effects of WPI on adipocyte cross-sectional area and liver TAG levels in HFD-fed mice(Reference Shi, Tauriainen and Martonen22). In fact, compared with a diet with casein as the protein source, a whey protein diet fed to rats reduced the expression and activity of key lipogenic enzymes in the liver(Reference Morifuji, Sakai and Sanbongi54). We further suggest that lipid accumulated in the hypothalamus may also be decreased with WPI intake because of the significantly lower expression of CPT1b in the hypothalamus coupled with a trend towards a decreased expression of PPARγ (P= 0·06). These cellular changes occurred in a background of elevated plasma TAG levels, suggesting reduced plasma lipid uptake and/or storage in the tissues in the HF-WPI-fed mice compared with the HFD controls. The present investigation revealed that WPI does not affect the expression of LPL or FATP1 that are involved in fatty acid extraction(Reference Fielding and Frayn55) and transport(Reference Gimeno56) to the epididymal adipose tissue, respectively, although we cannot rule out the possibility that WPI may have affected the protein expression or activity of the above-mentioned genes, causing the HF-WPI-fed mice to have elevated plasma TAG levels but reduced adiposity compared with the HFD-fed mice. To explore the possibility that WPI may have influenced lipid storage, we investigated genes involved in lipid metabolism within the adipose tissue. The lack of a WPI effect on the adipose tissue expression of FASN and CPT1b may suggest that adipose tissue-specific lipogenesis and β-oxidation pathways(Reference Bonnefont, Djouadi and Prip-Buus50) are unaffected by WPI intake. Interestingly, we observed a trend towards an increase in the expression of β-3AR in the epididymal adipose tissue of the HF-WPI-fed mice. A longer duration of WPI intake (21 weeks) was found to significantly increase the adipose tissue expression of β-3AR compared with that of the control HFD group(Reference Pilvi, Storvik and Louhelainen57). These data suggest the possibility that WPI increases lipolysis in adipocytes via the activation of β-3AR, possibly arising as a consequence of the effects of dietary protein on the central nervous system, given that central nervous system outflow to the adipose tissue activates β-3AR(Reference Nogueiras, Lopez and Dieguez37, Reference Nogueiras, Wiedmer and Perez-Tilve38, Reference McAllan, Cotter and Roche58, Reference Nogueiras, Perez-Tilve and Veyrat-Durebex59).
HFD-induced development of obesity is associated with increased inflammation(Reference Gustafson, Gogg and Hedjazifar60–Reference Harford, Reynolds and McGillicuddy62) and is accompanied by functional peripheral and central resistance to both leptin(Reference Lin, Thomas and Storlien5, Reference Lin, Storlien and Huang63–Reference Levin and Dunn-Meynell66) and insulin(Reference Chalkley, Hettiarachchi and Chisholm3, Reference Posey, Clegg and Printz53, Reference Felig67–Reference Pedersen, Kahn and Flier69). Evidence from the present study also suggests that the components of the leptin and insulin signalling pathways acting in the hypothalamus and the adipose tissue may be affected by the HFD. Feeding of the HFD for eight weeks increased the expression of CD68 and TNF-α in the hypothalamus. In parallel, we observed an increased expression of INSR in the hypothalamus and a reduced expression of INSR and GLUT4 in the adipose tissue with HFD feeding. These findings are consistent with the suggestion that insulin resistance is partly instigated by an inflammatory response to a HFD(Reference Pedersen, Kahn and Flier69–Reference McGillicuddy, Harford and Reynolds71). The increased expression of the leptin receptor and its downstream target POMC in the hypothalamus of the HFD-fed mice compared with the LFD-fed controls corroborates with previous data(Reference Lin, Storlien and Huang63, Reference Townsend, Lorenzi and Widmaier72, Reference Nilaweera, Ozanne and Wilson73). The changes in the expression of the leptin receptor may be a homeostatic mechanism attempting to defend against HFD-induced body-weight gain, in a background of elevated plasma leptin, hypothalamic inflammation and increased PPARγ expression, which contributes to central leptin resistance(Reference Ryan, Li and Grayson48, Reference Lu, Sarruf and Talukdar74). The increased hypothalamic tissue expression of genes linked to the leptin and insulin signalling pathways in response to a HFD may thus represent an elevated threshold needed to initiate physiological responses of these hormones(Reference Ryan, Woods and Seeley39, Reference Posey, Clegg and Printz53, Reference Van Heek, Compton and France65). In the epididymal adipose tissue and in the hypothalamus, WPI reversed the HFD-associated effects on the mRNA expression of INSR (both tissues), GLUT4 (adipose) and leptin receptor (hypothalamus), although the expression of the INSR-β isoform in the adipose tissue did not differ from that of the HFD group. Differences in INSR mRNA and INSR-β protein expression could have arisen due to the effects of the diet on the transcription, translation and/or processing of the mature precursor protein or may have also arisen due to the differences in the sensitivity of the techniques (real-time PCR v immunoblotting) used to measure gene expression. Despite these differences, WPI-induced changes in the expression of genes linked to the leptin and insulin signalling pathways were observed to occur in concert with reduced tissue lipid accumulation and potentially lower inflammation, specifically in the hypothalamus, since the expression of TNF-α in the hypothalamus was significantly lower in the HF-WPI diet-fed mice than in the HFD-fed mice. The gene expression profile in the WPI-fed mice may thus represent a lower threshold (or higher sensitivity) to leptin and insulin action, which may allow the central nervous system outflow to the adipose tissue to activate and normalise the expression of β-3AR as that observed in week 8 of the HF-WPI dietary challenge. The investigation of gene expression in specific nuclei within the hypothalamus may allow future investigators to further define the role of this region in mediating the effects of WPI on the adipose tissue.
In summary, we showed that WPI causes an overall reduction in fat mass in HFD-fed mice, normalises their energy intake, possibly by affecting the meal size, and influences their hypothalamic and adipose tissue expression of genes linked to leptin and insulin signalling pathways and lipid metabolism. Further work is needed to determine how WPI appears to prevent plasma TAG storage in the tissues and whether this contributes to potentially increased leptin and insulin sensitivity in the hypothalamus and the adipose tissue. Overall, the present data collected at two different time points (at weeks 5/6 and week 8) are consistent with the suggestion that WPI partially or completely reverses the HFD-induced physiological and cellular changes, which may therefore represent a state of reduced susceptibility to weight gain on a HFD.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114513001396
Acknowledgements
K. N. N. was supported by the Teagasc Vision Programme on Obesity, which also funded the work detailed in this manuscript. L. M. was supported by a Teagasc PhD Walsh Fellowship. H. M. R. was supported by SFI PI (11/PI/1119). The authors' contributions are as follows: L. M., K. N. N., J. F. C., H. M. R. and R. K. designed the research; L. M. performed the research; D. K. performed the plasma analysis; H. S. performed the immunoblot analysis; L. M. wrote the paper; K. N. N., J. F. C., H. M. R., R. K. and H. S. corrected the manuscript. The authors declare that there are no conflicts of interest.