Whey protein concentrate (WPC) is a protein-enriched powder made from whey during the process of cheese making. It is commonly used in the manufacturing of foods for infants and young children. Emerging evidence has demonstrated that WPC is useful for the treatment of a wide variety of gastrointestinal disorders such as inflammatory bowel disease and necrotising enterocolitis( Reference Sprong, Schonewille and van der Meer 1 , Reference Playford, Macdonald and Johnson 2 ). It has been found that the beneficial role of WPC in the intestine is closely related to its numerous bioactive compounds including functional amino acids, lactoferrin (LF) and growth factors, which is largely attributed to the stimulation of mucin synthesis and modification of immune response( Reference Playford, Macdonald and Johnson 2 , Reference Marshall 3 ). Recently, it has also been reported that WPC improves intestinal epithelial barrier function in vitro ( Reference Hering, Andres and Fromm 4 ). However, the molecular mechanisms underlying the protective effects are poorly understood.
WPC contains abundant bioactive compounds that are vital for immune and gut development early in life( Reference Penttila 5 , Reference Cross and Gill 6 ). Among the most relevant substances in WPC are Ig, LF and growth factors (e.g. transforming growth factor β (TGF-β) and epidermal growth factor (EGF)). Nevertheless, investigations directly examining the role of WPC in affecting the barrier integrity in vivo have not been reported. It is also of great interest to investigate whether bioactive compounds in WPC can be partly involved in WPC-induced prevention of intestinal epithelial barrier disruption. Until now, there are little data about the role of WPC in restitution of intestinal epithelium after injury( Reference Hering, Andres and Fromm 4 ).
Mammalian milk and WPC are rich in TGF-β including TGF-β1( Reference Hering, Andres and Fromm 4 , Reference Penttila, van Sprie and Zhang 7 ). TGF-β1 is also the most abundant isoform in the mucosa of the gut( Reference Barnard, Warick and Gold 8 ) and may play an important role in postnatal adaptation of the gastrointestinal tract in suckling animals( Reference Mei and Xu 9 ). TGF-β1 is of particular interest as it has known effects in remarkable number of biological processes, including epithelial cell growth and differentiation( Reference Godlewski, Hallay and Bierła 10 , Reference van’t Land, Meijer and Frerichs 11 ), restitution of intestinal epithelium after injury( Reference Mei and Xu 9 , Reference Xiao, Song and Jiao 12 ) and immune regulation( Reference Penttila 5 ). Thus far, different signalling pathways have been reported to be involved in TGF-β action, including Smad-dependent and Smad-independent pathways( Reference Weiss and Attisano 13 ). The canonical TGF-β signalling pathway is mediated by Smad family proteins( Reference Weiss and Attisano 13 ). Besides the canonical Smad pathways, there have been a number of non-Smad signalling pathways described, including mitogen-activated protein kinase (MAPK pathways) (extracellular signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK) and p38 MAPK pathways) in TGF-β1 actions( Reference Derynck and Zhang 14 ). We hypothesised that TGF-β1 in WPC might be involved in those barrier-protection processes and would lead to changes of these TGF-β signalling pathways. In the present study, we used a piglet model challenged with lipopolysaccharide (LPS) to investigate the beneficial effects of WPC on intestinal epithelial barrier function. Moreover, intracellular signals through which WPC and its active components might exert beneficial barrier effects were studied.
Methods
Animal care and experimental design
This experiment was approved by the Animal Care and Use Committee, Zhejiang University. A total of eighteen 35-d-old weaned barrows (Duroc×Landrace×Yorkshire, weaned at 21 d of age), with an average weight of 9·5 kg, were allocated to three groups, each consisting of six animals. One group served as the control group, whereas the other two groups were subjected to intestinal injury by injecting LPS. Animals were fed diets according to their groups: (1) control group (piglets fed the control diet); (2) LPS group (piglets fed the control diet and LPS); (3) LPS+WPC group (piglets fed the diet inclusion of 5 %WPC; WPC was provided by Open Country Dairy Ltd). Pigs were individually housed in pens with dimensions of 1·8×1·1 m2 in an environmentally controlled nursery barn. The room temperature was maintained at 25–27°C. Each pen contained a feeder and a nipple waterer to allow piglets ad libitum access to feed and water. There were six replicate pens for each treatment. Diets were formulated to meet or exceed requirements as suggested by the National Research Council (2012) (Table 1). The crude protein, Ca and total P contents in diet were analysed according to the method of Association of Official Analytical Chemists( 15 ). After 19 d feeding with control or 5 %WPC diets, the two challenged groups (the LPS group and the LPS+WPC group) were intraperitoneally injected with Escherichia coli LPS (E. coli serotype 055: B5; Sigma Chemical) at 100 μg/kg body weight (BW), and the unchallenged group was injected with the same amount of 0·9 % NaCl solution. The dose of LPS was chosen to cause acute intestinal injury in weaned pigs according to Liu et al. ( Reference Liu, Huang and Hou 16 – Reference Pi, Liu and Shi 18 ).
Table 1 Ingredients and chemical composition of the weaned diets on an as-fed basis
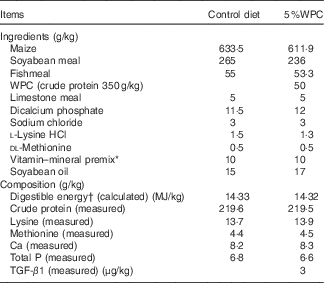
WPC, whey protein concentrate; TGF-β1, transforming growth factor-β1.
* Provided per kilogram of diet: vitamin A, 3·3mg; vitamin E, 33·4mg; vitamin D3, 0·015mg; vitamin K3, 1·5 mg; biotin, 0·10 mg; riboflavin, 8·0 mg; thiamine, 2·0 mg; niacin, 30 mg; pantothenic acid, 20 mg; pyridoxine, 3·0 mg; folic acid, 0·6 mg; vitamin B12, 0·04 mg; choline, 800 mg; Cu (CuSO4·5H2O), 16 mg; Fe (FeSO4), 125 mg; Zn (ZnSO4), 100 mg; Mn (MnSO4·H2O), 15 mg; Se (Na2SeO3), 0·3 mg; I (KI), 0·2 mg.
† Digestible energy was calculated from data provided by Feed Database in China (2011).
Sample collection
Four hours following the injection of LPS or saline, piglets were killed under anaesthesia with an intravenous injection of sodium pentobarbital (40 mg/kg BW). The reason for the choice of measurement at one point (4 h) after LPS injection was because previous studies have demonstrated that, within 3–6 h after injection, LPS caused acute intestinal morphological damage and a breakdown in intestinal barrier function in rats, mice and pigs( Reference Liu, Huang and Hou 16 – Reference Ewaschuk, Endersby and Thiel 22 ). Jejunum is one of the most susceptible intestinal segment to damage from endotoxins (LPS)( Reference Wallace, Steel and Whittle 23 ). Segments of the mid-jejunum were harvested immediately after the animals were killed and prepared for Ussing chamber studies. Adjacent specimens were fixed in buffered 10 % formalin until morphological measurements. Mucosal scrapings from the remaining jejunum samples were collected, rapidly frozen in liquid N2 and stored at −80°C.
Intestinal morphology and barrier function
After fixation, the intestinal segments were dehydrated, embedded in paraffin, sectioned (5 µm) and stained with haematoxylin and eosin( Reference Hu, Xiao and Luan 24 ). Villus height and crypt depth were measured in three intestinal cross-sections per animal with at least ten well-oriented crypt–villus units for each cross-section using image analysis (Leica Imaging Systems Limited) and averaged for each sample. The transepithelial electrical resistance (TER) and mucosal-to-serosal permeability to 4-kDa FITC dextran (fluorescein isothiocyanate dextran 4 kDa (FD4); Sigma-Aldrich) were determined in vitro in a Ussing chamber system, according to the procedures outlined by Moeser et al. ( Reference Moeser, Ryan and Nighot 25 ). In brief, segments of the jejunum were stripped from the seromuscular layer in oxygenated (95 % O2/5 % CO2) Ringer’s solution and then mounted in the Easy Mount Ussing chamber system with a multi-channel voltage-current clamp (model VCC MC6; Physiologic Instruments). Tissues were bathed on the serosal and mucosal sides with 5 ml Ringer’s solution. The serosal bathing solution contained 10 mm glucose, which was osmotically balanced on the mucosal side with 10 mm mannitol. Bathing solutions were oxygenated (95 % O2–5 % CO2) and circulated in water-jacketed reservoirs maintained at 37°C. The clamps were connected to Acquire and Analyze software (Physiologic Instruments) for automatic data collection. After a 30-min equilibration period on the Ussing chambers, TER (Ω cm2) was recorded at 15-min intervals over a 2-h period and then averaged to derive the TER values for a given pig. FD4 was added on the mucosal side at a final concentration of 0·375 mg/ml. Mucosal-to-serosal flux of FD4 (μg/cm2 per h) was monitored from the serosal side at 30-min intervals for 120 min. The concentrations of FD4 in the serosal side were measured using a fluorescence microplate reader (FLx800; Bio-Tek Instruments Inc.). The flux over the 2-h period was calculated.
Transforming growth factor-β1 content in whey protein concentrate by ELISA measurements
The TGF-β1 content in WPC was determined by ELISA according to the manufacturer’s protocol (R&D Systems)( Reference Visser, Lammers and Hoogendijk 37 ). TGF-β1 content was also assayed in the control group.
Protein expression analysis by Western blot
The Western blot analysis was performed according to the procedures outlined by Hu et al. ( Reference Hu, Xiao and Luan 24 ). In brief, after electrophoresis, the proteins were transferred to polyvinylidene difluoride membranes (Millipore). The membranes were incubated overnight at 4°C with primary Antibody (Ab) and then with the secondary Ab for 120 min at room temperature. The primary Ab (occludin, claudin-1, zonula occludens-1 (ZO-1), TGF-β1, Smad2, phospho-Smad2, p38, phospho-p38, JNK, phospho-JNK (p-JNK), ERK, phospho-ERK 1/2 (p-ERK), β-actin rabbit mAb) were purchased from Santa Cruz Technology Inc. The secondary Ab was HRP-conjugated anti-rabbit antibody (Cell Signaling Technology). Western blot was detected using an enhanced chemiluminescence detection kit (Amersham), photographed by a ChemiScope 3400 (Clinx Science Instruments) and analysed using Quantity One software. β-Actin was used as an internal control, which exhibited no difference among each group. The relative abundance of each target protein was expressed as target protein: β-actin protein ratio or ratio of phosphorylated protein:total protein. The protein expression of all samples was expressed as fold changes, calculated relative to the control group.
mRNA expression analysis by real-time PCR
The mRNA levels of TNF-α, IL-1β, IL-6, IL-8 and TGF-β receptors, as well as their downstream signal Smads (2,3,4,7), were determined by real-time PCR, as described by Liu et al.
(
Reference Liu, Chen and Odle
17
). In brief, total RNA was extracted from jejunal mucosa using TRIzol reagent (Invitrogen) following the manufacturer’s guidelines. The purity and concentration of all RNA samples were measured using a NanoDrop spectrophotometer (ND-2000; NanoDrop Technologies). Reverse transcription using the PrimeScript RT reagent kit (Takara Biotechnology) was carried out following the manufacturer’s instructions. Quantitative analysis of PCR was carried out on a StepOne Plus real-time PCR system (Applied Biosystems) using SYBR Green Master mix (Promega) according to the manufacturer’s instructions. The primers used are given in Table 2. Gene-specific amplification was determined by melting curve analysis and agarose gel electrophoresis. The
![]() $2^{{{\minus}\Delta \Delta C_{t} }} $
method was used to analyse the relative changes in each target gene expression. The change (Δ) in C
t
values in each group was compared with the C
t
value of glyceraldehyde 3-phosphate dehydrogenase (GAPHH) (ΔC
t
). Subsequently, ΔΔC
t
was computed for each target gene from the treatment groups by subtracting the averaged ΔC
t
for the control group. The final fold differences were computed as
$2^{{{\minus}\Delta \Delta C_{t} }} $
method was used to analyse the relative changes in each target gene expression. The change (Δ) in C
t
values in each group was compared with the C
t
value of glyceraldehyde 3-phosphate dehydrogenase (GAPHH) (ΔC
t
). Subsequently, ΔΔC
t
was computed for each target gene from the treatment groups by subtracting the averaged ΔC
t
for the control group. The final fold differences were computed as
![]() $2^{{{\minus}\Delta \Delta C_{t} }} $
for each target gene. The results showed that GAPDH exhibited no difference between the three groups.
$2^{{{\minus}\Delta \Delta C_{t} }} $
for each target gene. The results showed that GAPDH exhibited no difference between the three groups.
Table 2 GenBank accession numbers, sequences of forward and reverse primers and fragment sizes used for real-time PCR

TβRI, transforming growth factor-β receptor I; TβRII, transforming growth factor-β receptor II; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Statistical analysis
Data were analysed using the SAS statistical package (SAS Institute), with each animal considered an experimental unit. Results were statistically analysed by one-way ANOVA. Differences between the means were tested using Duncan’s multiple range tests. Differences were considered significant at P<0·05.
Results
Content of transforming growth factor-β1 in whey protein concentrate and whey protein concentrate diet and control diet
WPC contains abundant TGF-β1 as much as 0·06 ng/mg. The content of TGF-β1 in the WPC diet was 3 μg/kg. We checked for the presence of TGF-β1 in the control diet but we obtained negative results. The reason may be that the TGF-β1 content in the control diet was too low to detect.
Effects of dietary whey protein concentrate on growth performance
Throughout the 19-d trial (pre-challenge), there were no differences in initial (9·5 (sd 0·6) kg) and final BW (19·25 (sd 1·2) kg), daily gain (513 (sd 32) g) (P=0·741), daily feed intake (823 (sd 61) g) (P=0·362) and the gain:feed ratio (0·62 (sd 0·07)) (P=0·641) between the WPC and control groups.
Effects of dietary whey protein concentrate on the intestinal mucosal morphology and barrier function of piglets after lipopolysaccharide challenge
Table 3 shows the jejunum morphology and barrier function of piglets. Compared with the control group, the pigs challenged with LPS had shorter (P<0·05) villus height and lower villus height:crypt depth ratio in the jejunum. LPS challenge also increased the FD4 flux and lowered (P<0·05) TER. However, dietary WPC significantly prevented the LPS-induced decrease (P<0·05) in villus height:crypt depth ratio and TER and limited the LPS-induced decrease in villous height and the LPS-induced increase in FD4 flux.
Table 3 Effects of dietary whey protein concentrate (WPC) on the jejunum morphology and barrier function of pigletsFootnote * (Mean values with their standard errors; n 6 pigs)

LPS, lipopolysaccharide; FD4, mucosal:serosal flux of fluorescein isothiocyanate dextran (4 kDa); TER, transepithelial electrical resistance.
a,b,c Mean values within a row with superscript letters were significantly different (P<0·05)
* Control (non-challenged control), piglets receiving a control diet and injected with 0·9 % sterile saline; LPS (LPS-challenged control), piglets receiving the same control diet and injected with Escherichia coli LPS; LPS+WPC (LPS challenged+5 %WPC), piglets receiving a 5 %WPC diet and injected with LPS.
Effects of dietary whey protein concentrate on the tight-junction proteins in the jejunal mucosa of piglets after lipopolysaccharide challenge
Fig. 1 shows the protein expressions of occludin, claudin-1 and ZO-1 in the jejunal mucosa of piglets. Compared with the control group, LPS challenge decreased (P<0·05) protein expressions of occludin, claudin-1 and ZO-1. Dietary WPC prevented the LPS-induced decrease (P<0·05) in occludin, claudin-1 and ZO-1 protein expressions.

Fig. 1 Effects of dietary whey protein concentrate (WPC) on protein expressions of occludin, claudin-1 and zonula occludens-1 (ZO-1) in jejunal mucosa of piglets. (A) Representative blots of occludin, claudin, ZO-1 and β-actin in the jejunal mucosa of piglets. (B) Relative tight-junction protein expressions in the jejunal mucosa of piglets. Values are means (n 6), and standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different (P<0·05). The control sample was used as the reference sample. The protein expression of all samples was expressed as fold changes, calculated relative to the control group. ![]() , Control;
, Control; ![]() , lipopolysaccharide (LPS);
, lipopolysaccharide (LPS); ![]() , LPS+WPC. Control (non-challenged control), piglets receiving a control diet and injected with 0·9 % sterile saline; LPS (LPS-challenged control), piglets receiving the same control diet and injected with Escherichia coli LPS; LPS+WPC (LPS challenged+5 %WPC), piglets receiving a 5 %WPC diet and injected with LPS.
, LPS+WPC. Control (non-challenged control), piglets receiving a control diet and injected with 0·9 % sterile saline; LPS (LPS-challenged control), piglets receiving the same control diet and injected with Escherichia coli LPS; LPS+WPC (LPS challenged+5 %WPC), piglets receiving a 5 %WPC diet and injected with LPS.
Effects of dietary whey protein concentrate on pro-inflammatory cytokine mRNA expressions in the jejunal mucosa of piglets after lipopolysaccharide challenge
Table 4 shows the pro-inflammatory cytokine mRNA expressions in the jejunal mucosa of piglets. Compared with the control group, piglets challenged with LPS had higher (P<0·05) mRNA expressions of TNF-α, IL-1β, IL-6 and IL-8 levels in the jejunal mucosa. Dietary WPC prevented (P<0·05) the LPS-induced increase in mRNA expressions of IL-6, IL-8 and IL-1β and limited the LPS-induced increase in the mRNA expression of TNF-α.
Table 4 Effects of dietary whey protein concentrate (WPC) on cytokine mRNA levels on the jejunal mucosa of pigletsFootnote * (Mean values with their standard errors; n 6 pigs)
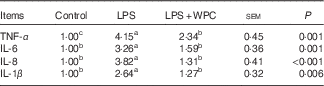
LPS, lipopolysaccharide.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* Control (non-challenged control), piglets receiving a control diet and injected with 0·9 % sterile saline; LPS (LPS-challenged control), piglets receiving the same control diet and injected with Escherichia coli LPS; LPS+WPC (LPS challenged+5 %WPC), piglets receiving a 5 %WPC diet and injected with LPS. The
![]() $2^{{{\minus}\Delta \Delta C_{t} }} $
method was used to analyse the relative expression (fold changes), calculated relative to the values in samples from the control pigs.
$2^{{{\minus}\Delta \Delta C_{t} }} $
method was used to analyse the relative expression (fold changes), calculated relative to the values in samples from the control pigs.
Effects of dietary whey protein concentrate on transforming growth factor-β1 expression and its canonical Smad signalling pathway in the jejunal mucosa of piglets after lipopolysaccharide challenge
Fig. 2 and 3 present the protein expressions of TGF-β1 and Smad2 in the jejunal mucosa of piglets. Piglets challenged with LPS did not differ (P>0·05) from the control group regarding TGF-β1 protein expression and Smad2 activation. Dietary supplementation with WPC increased (P<0·05) the expression of TGF-β1 and phosphorylated-Smad2 compared with the LPS group.

Fig. 2 Effects of dietary whey protein concentrate (WPC) on protein expression of transforming growth factor-β1 (TGF-β1) in the jejunal mucosa of piglets. (A) Representative blots of TGF-β1 expression and β-actin. (B) Relative TGF-β1 protein expression in the jejunal mucosa of piglets. a,bMean values with unlike letters were significantly different (P<0·05). Values are means (n 6), and standard deviations represented by vertical bars. The control sample was used as the reference sample. The protein expression of all samples was expressed as fold changes, calculated relative to the control group. Control (non-challenged control), piglets receiving a control diet and injected with 0·9 % sterile saline; lipopolysaccharide (LPS) (LPS-challenged control), piglets receiving the same control diet and injected with Escherichia coli LPS; LPS+WPC (LPS challenged+5 %WPC), piglets receiving a 5 %WPC diet and injected with LPS.

Fig. 3 Effects of dietary whey protein concentrate (WPC) on Smad2 activation in the jejunal mucosa of piglets. (A) Representative blots from one of the six pigs. (B) Values calculated as the ratio of the phosphorylation level (p-Smad2):total level of Smad2. The control sample was used as the reference sample. The protein expression of all samples was expressed as fold changes, calculated relative to the control group. Values are means (n 6), with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different (P<0.05). Control (non-challenged control), piglets receiving a control diet and injected with 0·9% sterile saline; LPS (lipopolysaccharide-challenged control), piglets receiving the same control diet and injected with Escherichia coli LPS; LPS+WPC (LPS challenged +5% WPC), piglets receiving a 5% WPC diet and injected with LPS.
Table 5 shows the mRNA expressions of canonical Smad signals in the jejunal mucosa of piglets. Compared with the control group, LPS challenge did not (P>0·05) activated Smad signals as indicated by no significant increase in the mRNA expressions of TGFβ type I receptors (TβRI), TGFβ type II receptors (TβRII), Smad2, Smad3, Smad4 and Smad7. Supplementation with WPC significantly improved (P<0·05) the Smad4 and Smad7 mRNA expressions, whereas it did not change the mRNA expressions of TβRI, TβRII, Smad2 and Smad3 in the jejunal mucosa of piglets when compared with the LPS group.
Table 5 Effects of dietary whey protein concentrate (WPC) on mRNA expressions of smad signals in the jejunal mucosa of pigletsFootnote * (Mean values with their standard errors; n 6 pigs)
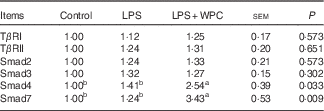
LPS, lipopolysaccharide; TβRI, transforming growth factor-β receptor I; TβRII, transforming growth factor-β receptor II.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* Control (non-challenged control), piglets receiving a control diet and injected with 0·9 % sterile saline; lipopolysaccharide (LPS) (LPS-challenged control), piglets receiving the same control diet and injected with Escherichia coli LPS; LPS+WPC (LPS challenged+5 %WPC), piglets receiving a 5 %WPC diet and injected with LPS. The
![]() $2^{{{\minus}\Delta \Delta C_{t} }} $
method was used to analyse the relative expression (fold changes), calculated relative to the values in samples from the control pigs.
$2^{{{\minus}\Delta \Delta C_{t} }} $
method was used to analyse the relative expression (fold changes), calculated relative to the values in samples from the control pigs.
Effects of dietary whey protein concentrate on mitogen-activated protein kinase signalling pathways of piglets in the jejunal mucosa after lipopolysaccharide challenge
There was no difference in the total protein levels of the MAPK between groups (data not shown). Fig. 4 shows the activation of the MAPK signalling pathways in jejunal mucosa of piglets. Compared with the control group, LPS challenge significantly increased (P<0·05) the ratios of the phosphorylated forms to the total levels of JNK and p38 (p-JNK:JNK and p-p38:p38). Supplementation with WPC significantly decreased (P<0·05) the ratios of the phosphorylated forms to the total levels of JNK and p38 (p-JNK:JNK and p-p38:p38), whereas WPC significantly increased (P<0·05) the ratio of the phosphorylated forms to the total levels of ERK (p-ERK:ERK) compared with the LPS group.

Fig. 4 Effects of whey protein concentrate (WPC) on mitogen-activated protein kinases (MAPK) signal pathways in the jejunal mucosa of piglets. The three MAPK are the c-Jun NH2-terminal kinase (JNK), p38 MAPK and extracellular regulated kinases (ERK 1/2). (A) Representative blots from one of the six pigs. (B) Values calculated as the ratios of their phosphorylation levels (phospho-JNK:phospho-p38:phospho-ERK (p-JNK:p-p38:p-ERK)) to the total levels of MAPK. The values in samples from the control were used as the reference sample. The protein expression of all samples was expressed as fold changes, calculated relative to the values from the control group. Values are means (n 6), with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different (P<0·05). ![]() , Control;
, Control; ![]() , lipopolysaccharide (LPS);
, lipopolysaccharide (LPS); ![]() , LPS+WPC. Control (non-challenged control), piglets receiving a control diet and injected with 0·9% sterile saline; LPS (LPS-challenged control), piglets receiving the same control diet and injected with Escherichia coli LPS; LPS+WPC (LPS challenged + 5 % WPC), piglets receiving a 5% WPC diet and injected with LPS. JNK, c-jun N-terminal kinase; p-JNK, phospho-JNK; ERK, extracellular regulated kinases; p-ERK, phospho-ERK.
, LPS+WPC. Control (non-challenged control), piglets receiving a control diet and injected with 0·9% sterile saline; LPS (LPS-challenged control), piglets receiving the same control diet and injected with Escherichia coli LPS; LPS+WPC (LPS challenged + 5 % WPC), piglets receiving a 5% WPC diet and injected with LPS. JNK, c-jun N-terminal kinase; p-JNK, phospho-JNK; ERK, extracellular regulated kinases; p-ERK, phospho-ERK.
Discussion
WPC is a protein-enriched powder derived from whey of cow’s milk, which is wildly used in the food industry as a functional and nutritional ingredient. It contains not only essential nutrients but also a number of natural bioactive substances such as α-lactalbumin, LF, lactoperoxidase, lysozyme, EGF and TGF that have many beneficial effects on animal health( Reference Marshall 3 , Reference Verhasselt, Milcent and Cazareth 26 , Reference Hsieh, Hernández-Ledesma and Fernández-Tomé 27 ). Substantial evidence has shown that WPC exerts beneficial effects on a wide variety of gastrointestinal disorders such as inflammatory bowel disease and necrotising enterocolitis in animal models and clinical trials( Reference Sprong, Schonewille and van der Meer 1 , Reference Playford, Macdonald and Johnson 2 ). On the basis of this, we investigated the protective effect of supplementation with 5 %WPC on intestinal morphology and barrier function after a 4-h E. coli LPS challenge using a piglet model. LPS-induced intestinal injury in piglets is one of the well-established animal models for studying infant nutrition and gastrointestinal physiology( Reference Liu, Chen and Odle 17 , Reference Hou, Wang and Zhang 28 ). In agreement with earlier reports( Reference Liu, Chen and Odle 17 , Reference Hou, Wang and Zhang 28 ), the present study showed that LPS challenge decreased jejunal villus height and villus height:crypt depth ratio at 4 h after LPS challenge, which suggests that LPS caused acute intestinal mucosal damage. However, supplementation with 5 %WPC ameliorated LPS-induced intestinal injury by increased jejunal villus height and villus height:crypt depth ratio, which indicated that WPC improved intestinal morphology after damage. Similarly, Li et al. ( Reference Li, Mette and Jiang 29 ) found that feeding WPC pigs had greater villus heights for preterm pigs. Enteral supplementation with colostrum has also been reported to increase villus growth in newborn pigs( Reference Penttila, van Sprie and Zhang 7 , Reference Mei, Zhang and Wang 30 ). The gut is a target during endotoxin challenge. LPS could excessively activate inflammatory response and induce nitric oxide synthase production and lipid peroxidation, which finally led to local intestinal damage such as morphological damage and a breakdown in intestinal barrier function( Reference Liu, Chen and Odle 17 , Reference Mercer, Smith and Cross 19 , Reference Touchette, Carroll and Allee 21 ). We conjectured that endotoxin-induced intestinal epithelial injury may be a result of oxygen-related free radical damage and inflammatory response. When exposed to LPS, the villi were frequently noted to have epithelial vacuolisation, lifting, sloughing and focal necrosis, which would lead to villi shortening as a result. Our study also demonstrated that LPS can damage the intestinal morphology and barrier integrity. WPC contains abundant bioactive substances such as anti-inflammatory cytokines and antioxidative factors (e.g. TGF-β, EGF, LF) that can counteract the effects of LPS, thus protecting the intestinal morphology and barrier integrity( Reference Hering, Andres and Fromm 4 , Reference Ozawa, Miyata and Nishimura 31 – Reference Xu, Liu and Xu 35 ). In the present experiment, consistent with improved intestinal morphology, WPC improved intestinal barrier function, indicated by increased TER and decreased FD4 flux in the jejunum of piglets compared with the LPS group. Our data were supported by the results of Hering et al. ( Reference Hering, Andres and Fromm 4 ), who reported that WPC protects against interferon-γ-induced barrier impairment by increased TER and decreased fluorescein permeability in HT-29/B6 cells. Moreover, WPC also reduced intestinal permeability in preterm pigs( Reference Li, Mette and Jiang 29 ). To our knowledge, the present study is the first to determine the effects of dietary supplementation with WPC on the attenuation of LPS-induced intestinal injury in weaned piglets.
The tight-junction (TJ) is a major cellular component for maintenance of tissue integrity and barrier function. It has a complex molecular composition that forms the continuous intercellular barrier against external agents in the intestine. The integral membrane components of TJ include claudins, occludins and ZO( Reference Hu, Xiao and Luan 24 , Reference Suzuki 36 ). In our present study, consistent with the improved intestinal morphology and barrier function, WPC prevented the LPS-induced decrease in claudin-1, occludin and ZO-1 expressions. Our observations were supported by Hering et al. ( Reference Hering, Andres and Fromm 4 ) and Visser et al. ( Reference Visser, Lammers and Hoogendijk 37 ), who reported that milk protein components improved intestinal barrier function, in part, by altering the expressions and functions of claudins. In our present study, WPC may have partially improved the intestinal barrier function via improving the expressions of intestinal TJ proteins.
Cytokines also participate in the regulation of the intestinal barrier integrity( Reference Al-Sadi, Boivin and Ma 38 ). Over-production of pro-inflammatory cytokines has a negative influence on gut integrity and epithelial function( Reference Pié, Lallès and Blazy 39 ). The TJ barrier disruptive actions of TNF-α have been well established( Reference Ma, Boivin and Ye 40 ). It has been reported that interferon-γ-induced reductions in epithelial barrier function are linked to decreases in the expressions of TJ proteins such as occludin and ZO-1( Reference Blikslager, Moeser and Gookin 41 ). In the present study, the expressions of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6 and IL-8 were elevated in the jejunal mucosa of piglets subjected to LPS challenge. Consistent with the improved intestinal integrity by WPC supplementation, WPC decreased the TNF-α, IL-1β, IL-6 and IL-8 gene expressions compared with the LPS-challenged group. In line with our findings, Sprong et al. ( Reference Sprong, Schonewille and van der Meer 1 ) reported that cheese whey protein protected rats against mild dextran sulphate Na-induced colitis by inhibiting the expressions of inflammatory cytokines. Whey product has demonstrated a number of anti-inflammatory effects including decreased cytokine release in rodent models after exposure to LPS( Reference Beaulieu, Girard and Dupont 42 ). It is possible that the protective effects of WPC supplementation on intestinal integrity were closely related to reducing the expressions of intestinal pro-inflammatory cytokines. According to the above results, we speculate that WPC may exert beneficial effects in intestinal damage by enhancing intestinal integrity and barrier function.
WPC contains abundant TGF-β1( Reference Penttila 5 ), but whether TGF-β1 plays a role in these protection processes is not clear. TGF-β1 is of particular interest as it has known effects in a remarkable number of biological processes including epithelial cell growth and differentiation( Reference van’t Land, Meijer and Frerichs 11 ), restitution of intestinal epithelium after injury( Reference Xiao, Song and Jiao 12 , Reference McKaig, Hughes and Tighe 43 ) and immune regulation( Reference Penttila 5 ). TGF-β has been reported to play an important role in the post-weaning adaptation process in the intestine of pigs( Reference Mei and Xu 9 , Reference Xiao, Song and Jiao 12 ). In our present experiment, dietary supplementation with 5 %WPC increased the TGF-β1 protein expression in the jejunum mucosa of piglets compared with the LPS-challenged pigs. Similarly, Andújar et al. ( Reference Andújar, Ríos and Giner 44 ) found that TGF-β1 positively regulates gastrointestinal ulcer healing. TGF-β was also reported to provide protection against dextran sodium sulphate-induced colitis and LPS-induced endotoxaemia/shock in rodents( Reference Hahm, Im and Parks 45 , Reference McCartney-Francis, Jin and Wahl 46 ), and it is likely that the increased TGF-β1 concentration by supplementation with WPC is directly responsible for the protection. Therefore, our hypothesis is that the beneficial role exerted by WPC in intestinal barrier protection may be partially influenced by TGF-β1 in the intestinal mucosa. We speculated that the increased expression of TGF-β1 may due to the TGF-β1 in WPC, as TGF-β1 in the diet can be absorbed by the intestine in vivo and TGF-β retains biological activity when given as a supplement in infant formula( Reference Ozawa, Miyata and Nishimura 31 , Reference Ando, Hatsushika and Wako 47 , Reference Penttila, Flesch and McCue 48 ). It is possible that the higher presence of TGF-β1 is from dietary WPC by intestinal absorption.
To determine whether the TGF-β1 signalling pathway is involved in WPC exerting beneficial effects on LPS-induced intestinal injury, we next evaluated the canonical downstream substrate of TGF-β signal. The canonical TGF-β signalling pathway is mediated by Smad family proteins( Reference Weiss and Attisano 13 ). When TGF-β ligands reach the membrane of target cells, they bind directly to TβRII, which leads to the recruitment of TβRI, and TβRII then trans-phosphorylates TβRI, enabling the TβRI kinase domain to act on cytoplasmic Smad proteins, and thereby propel downstream signalling actions. Smad 2 and 3 are receptor-regulated Smad. Following stimulation by TGF-β, Smad2 and Smad3 become phosphorylated. Phosphorylated Smad2 and Smad3 can complex with Smad4 (the common mediator Smad) and then translocate to the nucleus and regulate gene expression( Reference Weiss and Attisano 13 ). In the present study, we observed that supplementation with WPC promoted phosphorylated-Smad2 expression and mRNA expressions of Smad4 and Smad7 in the jejunal mucosa compared with the LPS-challenged pigs fed the control diet, which indicates that canonical Smad signalling pathways were activated. In line with our study, a recent study has revealed that oral administration of TGF-β1 protects the immature gut from injury via Smad protein-dependent signalling pathways( Reference Shiou, Yu and Guo 49 ). Ozawa et al. ( Reference Ozawa, Miyata and Nishimura 31 ) have also found that TGF-β in cows’ milk provided protection against experimental colitis and endotoxaemia by inducing Smad2 phosphorylation and the transcription of the TGF-β/Smad target genes TGF-β itself and Smad7 in vitro. It is also reported that TGF-β1 enhancement of epithelial barrier function was mediated partly by Smad2/3 signalling pathways( Reference Howe, Reardon and Wang 50 ). Consequently, in the present study, we speculated that the WPC may have protected the intestinal epithelial barrier from LPS-induced intestinal injury partially through TGF-β1 canonical Smad pathways directly or indirectly.
Apart from canonical Smad pathways, MAPK has been reported to be involved in TGF-β actions( Reference Derynck and Zhang 14 ). The three primary MAPK signalling pathways are the ERK 1/2, p38 and JNK. It has been demonstrated that MAPK become activated when stimulated by LPS( Reference Chen, Yang and Liu 51 ). Moreover, a recent study showed that weaning stress activates p38, JNK and ERK 1/2 signalling pathways in the intestine, which may be an important mechanism of weaning-associated enteric disorders of piglets( Reference Hu, Xiao and Luan 24 ). Thus far, there are a few reports investigating the effects of WPC on MAPK signalling pathways( Reference Rusu, Drouin and Pouliot 52 ). In the present study, we observed an increase in the phospho-p38 and p-JNK in the jejunum of LPS-challenged pigs and we demonstrated, for the first time, that dietary WPC decreased the relative protein levels of phosphorylated p38 and JNK, while increasing p-ERK 1/2 protein levels, indicating that WPC inhibited the JNK and p38 signalling pathways while activating ERK 1/2 signalling in LPS-challenged pigs. In general, ERK delivers a survival signal, whereas JNK and p38 are associated with the induction of cell apoptosis under stressful conditions( Reference Benhar, Dalyot and Engelberg 53 , Reference Ku, Lee and Jeong 54 ). Cell apoptosis can disrupt intestinal mucosal integrity( Reference Zhu, Xu and Zhu 55 ). Activation of ERK pathways and inhibition of p38 and JNK pathways also improved intestinal barrier function in weaned pigs( Reference Song, Xiao and Ke 56 ). The ERK 1/2 cascade can be activated by growth factors and preferentially regulates cell growth and differentiation. There is evidence that activation of the ERK 1/2 signalling is linked to the TGF-β1-induced modulation of TJ permeability and wound closure( Reference Song, Xiao and Ke 56 , Reference Ma, Misawa and Yamaguchi 57 ). TGF-β has been shown to attenuate IL-1β-induced pro-inflammatory cytokine production in immature human intestinal epithelia cells by inhibiting ERK signalling( Reference Rautava, Nanthakumar and Dubert-Ferrandon 58 ). In our study, it is possible that the protective effects of WPC on intestinal integrity after exposure to LPS are also related to activation of ERK 1/2 and inhibition of JNK and p38 MAPK signalling pathways.
Other bioactive substances than TGF-β1 in WPC may be implied in the effects of WPC. EGF is another important growth factor in WPC that has protective barrier effects on intestinal epithelia and has often been related to effects on cell proliferation and/or epithelial restitution, which could indirectly or secondarily affect the TJ( Reference Khailova, Dvorak and Arganbright 59 ). Accumulating evidence has shown that EGF/EGF receptor signal improves healing of the gastrointestinal tract and enhances gut integrity and intestinal barrier function( Reference Clark, Doelle and Halpern 32 , Reference Haedo, Gonzalez and Mas 60 , Reference Tarnawski, Stachura and Durbin 61 ). In addition, LF is a multifunctional glycoprotein present at high concentrations in milk that exerts antibacterial, immune-modulating and anti-inflammatory effects on intestinal health( Reference Kuhara, Tanaka and Yamauchi 33 ). Studies have found that LF could directly induce enterocyte growth and proliferation and improve gut barrier function( Reference Buccigrossi, de Marco and Bruzzese 34 , Reference Wu, Chen and Wu 62 ). However, it is still unclear whether these constituents of WPC are the crucial biological factors that provide beneficial effects.
In summary, the present study demonstrated that dietary supplementation with WPC attenuates LPS-induced intestinal injury via improving mucosal barrier function, alleviating intestinal inflammation and influencing TGF-β1, Smad and MAPK signalling pathways.
Acknowledgements
This research was jointly supported by the National Natural Science Foundation of China (31472103), the Special Fund for Agroscientific Research in Public Interest (201403047).
The authors’ contributions are as follows: C. H. and X. H. contributed to the study design; K. X. collected and analysed the data and wrote the paper; L. J., S. C and Z. S. participated in data collection; C. H. and X. H. had the primary responsibility for the final content. All the authors read and approved the final version of the manuscript.
The authors declare that they have no conflicts of interest.












