Coronavirus disease 2019 (Covid-19) is an infectious disorder caused by the severe acute respiratory syndrome coronavirus (SARS-CoV)-2 that emerged from the Chinese city of Wuhan at the end of 2019 and has since then relentlessly spread across the globe(Reference Huang, Wang and Li1). The Covid-19 pandemic is causing a worldwide medical and socio-economic crisis of unprecedented proportions in modern times. The vast majority of individuals who contract SARS-CoV-2 have mild to moderate symptoms. However, a significant minority develops respiratory failure due to pneumonia and/or acute respiratory distress syndrome(Reference Huang, Wang and Li1).
Particularly, SARS-CoV-2-infected individuals suffering from certain premorbid conditions, such as hypertension, diabetes, CVD and obesity, are at increased risk of complicated disease course(Reference Huang, Wang and Li1). Although these conditions are also associated with poor outcomes due to other infectious diseases, precisely why these groups have high morbidity and mortality is currently unknown. We made the observation that these disorders are associated with elastic fibre pathologies as well as vitamin K insufficiency.
Thromboembolism and generalised microvascular thrombosis are also prevalent in severe Covid-19(Reference Tang, Li and Wang2,Reference Cui, Chen and Li3) . The mechanisms leading from pulmonary infection to systemic coagulopathy in Covid-19 have not yet been entirely elucidated. It has previously been shown that severe vitamin K deficiency in critically ill patients can be misdiagnosed as disseminated intravascular coagulation(Reference Alperin4). Given the importance of vitamin K-dependent proteins in coagulation as well as elastic fibre metabolism, we recently hypothesised that vitamin K is implicated in Covid-19 pathogenesis and could represent the missing link between pulmonary damage and thrombogenicity.
Vitamin K metabolism
Vitamin K is a monofunctional nutrient from a biochemical perspective as its only well-described function is facilitating γ-carboxylation. However, it can be regarded as pleiotropic because it activates proteins with distinct, opposing and not yet fully unravelled functions.
Vitamin K catalyses the carboxylation reaction that transforms glutamic acid into γ-carboxyglutamic (Gla) residues and is well known as an activator of hepatic procoagulant factors II (prothrombin), VII, IX and X. However, vitamin K also activates anticoagulant proteins C and S as well as a number of extrahepatic proteins not involved in blood coagulation.
Endothelial protein S
Contrary to other vitamin K-dependent procoagulant factors and protein C, which are almost exclusively hepatic proteins, about 50 % of anticoagulant protein S is produced outside the liver(Reference Burstyn-Cohen, Heeb and Lemke5). This part of protein S is mainly synthesised in endothelial cells and thought to play an important role in the local prevention of thrombosis(Reference Burstyn-Cohen, Heeb and Lemke5–Reference Stern, Brett and Harris7). Endothelium-produced protein S has the ability to associate with the cell surface and promote procoagulant factor V inactivation in the presence of activated protein C(Reference Stern, Brett and Harris7).
Matrix Gla protein
Vitamin K-dependent matrix Gla protein (MGP) has been extensively studied as an inhibitor of vascular mineralisation(Reference Schurgers, Uitto and Reutelingsperger8); however, its role in the pulmonary compartment seems to be comparable(Reference Price, Buckley and Williamson9). Besides preventing soft tissue calcification, MGP also protects against elastic fibre degradation. This was demonstrated in MGP knockout mice, which developed severely mineralised as well as fragmented elastic fibres(Reference Luo, Ducy and McKee10).
Elastic fibres are critical components in the extracellular matrix of dynamic tissues(Reference Mithieux and Weiss11). They provide deformability to lungs and arteries, which facilitates respiration and circulation(Reference Mithieux and Weiss11). Initial elastic fibre development is almost exclusively restricted to the perinatal period(Reference Mithieux and Weiss11). Elastic fibre degradation and repair, however, are continuous processes(Reference Mithieux and Weiss11). The balance between the two is delicate and of vital importance for cardiovascular and pulmonary health(Reference Liu, Zhao and Gao12). The rate of proteolytic elastic fibre degradation increases during ageing(Reference Rabinovich, Miller and Wrobel13). This age-related acceleration of elastolysis is enhanced in certain pulmonary conditions such as chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis(Reference Rabinovich, Miller and Wrobel13,Reference Huang, Bolton and Miller14) .
Affinity of elastic fibres for Ca is high(Reference Rucker15). Critically, elastic fibre calcification and proteolytic degradation processes are closely related. Partially degraded elastic fibres are more negatively charged, attracting positively charged Ca(Reference Rucker15). As elastic fibre Ca content increases, the synthesis of matrix metalloproteinases, proteolytic enzymes that degrade elastin fibres, is also up-regulated(Reference Lee, Basalyga and Simionescu16). Peri-arterial application of Ca on rat abdominal aortas induces both calcification and proteolytic degradation of elastic fibres(Reference Basalyga, Simionescu and Xiong17). Subdermal implantation of elastin in rats results in significant calcification, but local application of a protease inhibitor attenuates this mineralisation(Reference Vyavahare, Jones and Tallapragada18,Reference Bailey, Xiao and Ogle19) . MGP plays a critical role in the protection of elastic tissues against mineralisation(Reference Luo, Ducy and McKee10), most likely because other proteins that inhibit calcification (e.g. fetuin-A) are too large to enter the lumen of the fibres(Reference Schurgers, Uitto and Reutelingsperger8,Reference Price, Toroian and Lim20) .
Vitamin K recycling
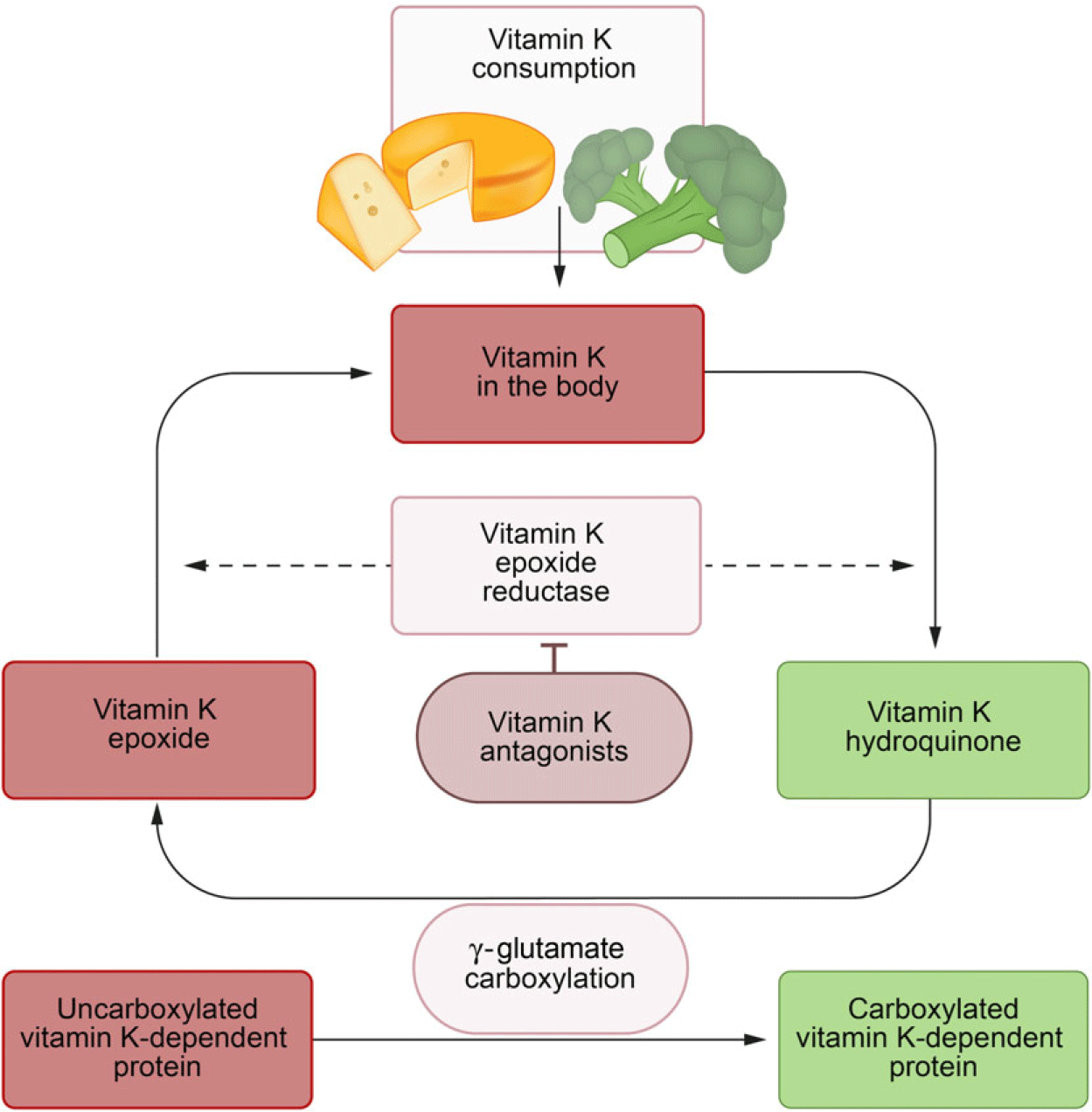
Storage capacity of vitamin K is limited, and therefore, its metabolism must be very efficient. After being oxidised during the carboxylation reaction, vitamin K is reactivated repeatedly by the enzyme vitamin K epoxide reductase in the vitamin K cycle (Fig. 1)(Reference Shearer and Newman21). Nevertheless, insufficiency may develop within days of poor intake, particularly in pathological states of increased vitamin K utilisation(Reference Alperin4,Reference Price, Buckley and Williamson9,Reference Usui, Tanimura and Nishimura22) .

Fig. 1. Vitamin K cycle. The vitamin K cycle and the effects of vitamin K antagonists. ![]() , Active;
, Active; ![]() , inactive.
, inactive.
Triage-based distribution
The triage theory posits that during times of scarcity, micronutrients are reserved for use in processes that form the greatest threat to short-term survival if not properly executed(Reference Ames23). This implies that in case of vitamin K insufficiency, the vitamin is preferentially transported to the liver for the activation of the above-mentioned procoagulant factors at the expense of extrahepatic vitamin K-dependent proteins such as MGP (Fig. 2).
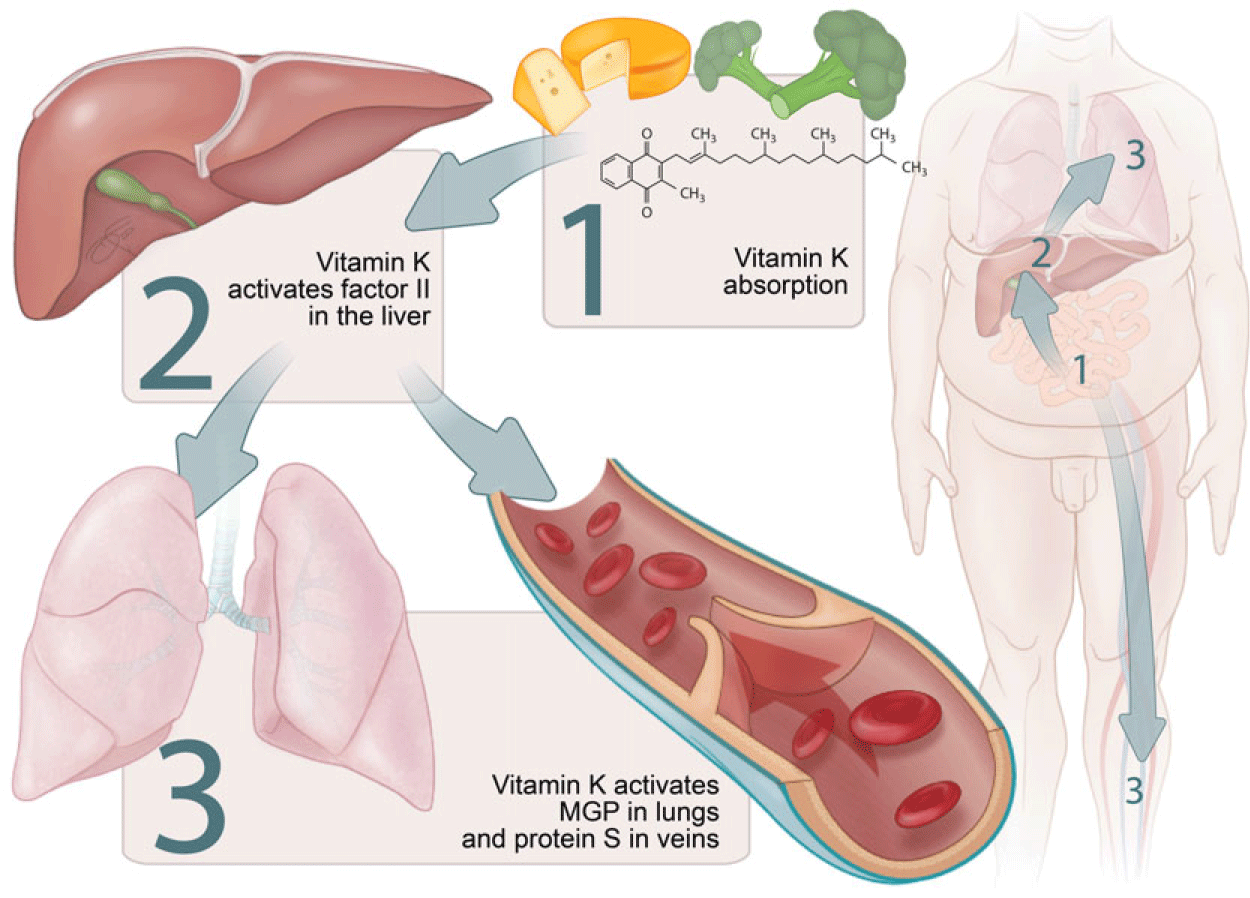
Fig. 2. Micronutrient triage theory with regard to vitamin K. Particularly vitamin K1 is preferentially transported to the liver. This implies that the grade of carboxylation in a state of vitamin K deficiency is usually higher for hepatic procoagulant factors, such as factor II, than for endothelial protein S as well as for pulmonary matrix Gla protein (MGP).
This was demonstrated in women between 60 and 80 years old who consumed a vitamin K1-deficient diet for 28 d. Undercarboxylated osteocalcin, a vitamin K-dependent bone protein, increased almost immediately, whereas undercarboxylated factor II increased more slowly(Reference Booth, Martini and Peterson24). Moreover, when patients using vitamin K antagonists (VKA) as anticoagulants steadily increased their dietary intake of vitamin K1, a significant decrease in undercarboxylated factor II was seen at 150 μg/d, while a significant decrease in undercarboxylated osteocalcin was only seen at an intake of 300 μg/d(Reference Schurgers, Shearer and Hamulyák25).
Similar to osteocalcin and MGP, vitamin K insufficiency would result in deficient activation of endothelial protein S before causing a decrease in carboxylated procoagulant factors (Fig. 2)(Reference McCann and Ames26). This could explain the seemingly paradoxical increase of thrombosis risk in the first week of treatment with VKA(Reference Azoulay, Dell’Aniello and Simon27).
Although the biological function of vitamins K1 and K2 is similar, there are differences with regard to bioavailability and tissue distribution. Half-life times of most K2 vitamins are longer than that of K1, and vitamin K2 may have more extrahepatic potential than K1 (Reference Akbulut, Pavlic and Petsophonsakul28). Vitamin K1 is found in green vegetables such as broccoli, spinach and kale. Certain bacteria have the ability to produce vitamin K2. It is therefore present in fermented food products such as cheese, curd and sauerkraut as well as in certain fishes.
Assessment of vitamin K status
Measuring circulating levels of the two naturally occurring forms of vitamin K – vitamin K1 (phylloquinone) and K2 (the group of menaquinones) – is technically feasible(Reference Shea and Booth29). However, the value of such measurements for assessing general vitamin K status is limited. Quantification of vitamin K-dependent proteins that have not been carboxylated, on the other hand, is a valuable method reflecting the combined functional deficit of vitamin K1 and K2 (Reference Shea and Booth29). Determination of desphospho-uncarboxylated (dp-uc; i.e. inactive) MGP levels and the ratio between uncarboxylated and carboxylated osteocalcin are validated assays of extrahepatic vitamin K status(Reference Shea and Booth29).
High dp-ucMGP reflects low vitamin K status and vice versa. Although increasing vitamin K consumption decreases dp-ucMGP(Reference Brandenburg, Reinartz and Kaesler30–Reference Knapen, Drummen and Smit32), its levels are not simply a biomarker of vitamin K intake but depend on other factors as well. Circulating dp-ucMGP concentration can best be regarded as a reflection of the total extrahepatic vitamin K deficit, that is, the amount of vitamin K that is needed to carboxylate all the uncarboxylated vitamin K-dependent proteins in the body(Reference Janssen and Vermeer33).
Hepatic vitamin K status is usually quantified by measuring levels of protein induced by vitamin K absence (PIVKA)-II (i.e. uncarboxylated prothrombin)(Reference Booth, Martini and Peterson24).
Vitamin K metabolism in Coronavirus disease 2019
Extrahepatic vitamin K status is severely reduced in Covid-19 patients, reflected by elevated dp-ucMGP levels(Reference Dofferhoff, Piscaer and Schurgers34). Reasons for this could include premorbid low vitamin K status in combination with accelerated utilisation during infection.
Vitamin K status in co-morbidities associated with poor Coronavirus disease 2019 outcomes
dp-ucMGP levels are elevated in various diseases that are associated with elastic fibre calcification and degradation such as diabetes(Reference Griffin, Islam and Wall35), hypertension(Reference Chirinos, Sardana and Syed36), CVD(Reference Mayer, Seidlerová and Bruthans37), chronic kidney disease(Reference Griffin, Islam and Wall35,Reference Schurgers, Barreto and Barreto38) and obesity(Reference Jespersen, Møllehave and Thuesen39). It is possible that reduced vitamin K intake increases the risk of developing these conditions(Reference Chen, Sheng and Zhang40). However, increased vitamin K demand due to enhanced utilisation may be another important cause of high dp-ucMGP(Reference Parker, Ix and Cranenburg41). Partially degraded and mineralised elastic fibres – which are prevalent in diabetic, hypertensive, renal and cardiovascular patients – are more vulnerable to further proteolysis and calcification(Reference Basalyga, Simionescu and Xiong17,Reference Umeda, Aikawa and Libby42) . This increases the need for MGP synthesis to protect elastic fibres(Reference Price, Buckley and Williamson9), draining vitamin K stores for MGP carboxylation and leading to higher dp-ucMGP levels.
In vascular diseases(Reference Chen, Sheng and Zhang40), as well as in the general population(Reference Liu, Gu and Thijs43,Reference Riphagen, Keyzer and Drummen44) , increased dp-ucMGP levels associate with higher all-cause mortality. Vitamin K supplementation reduces dp-ucMGP levels and has a favourable effect on progression of clinically relevant endpoints, including aortic valve calcification, arterial stiffness and bone loss(Reference Brandenburg, Reinartz and Kaesler30–Reference Knapen, Drummen and Smit32).
Vitamin K insufficiency in the pathogenesis of Coronavirus disease 2019
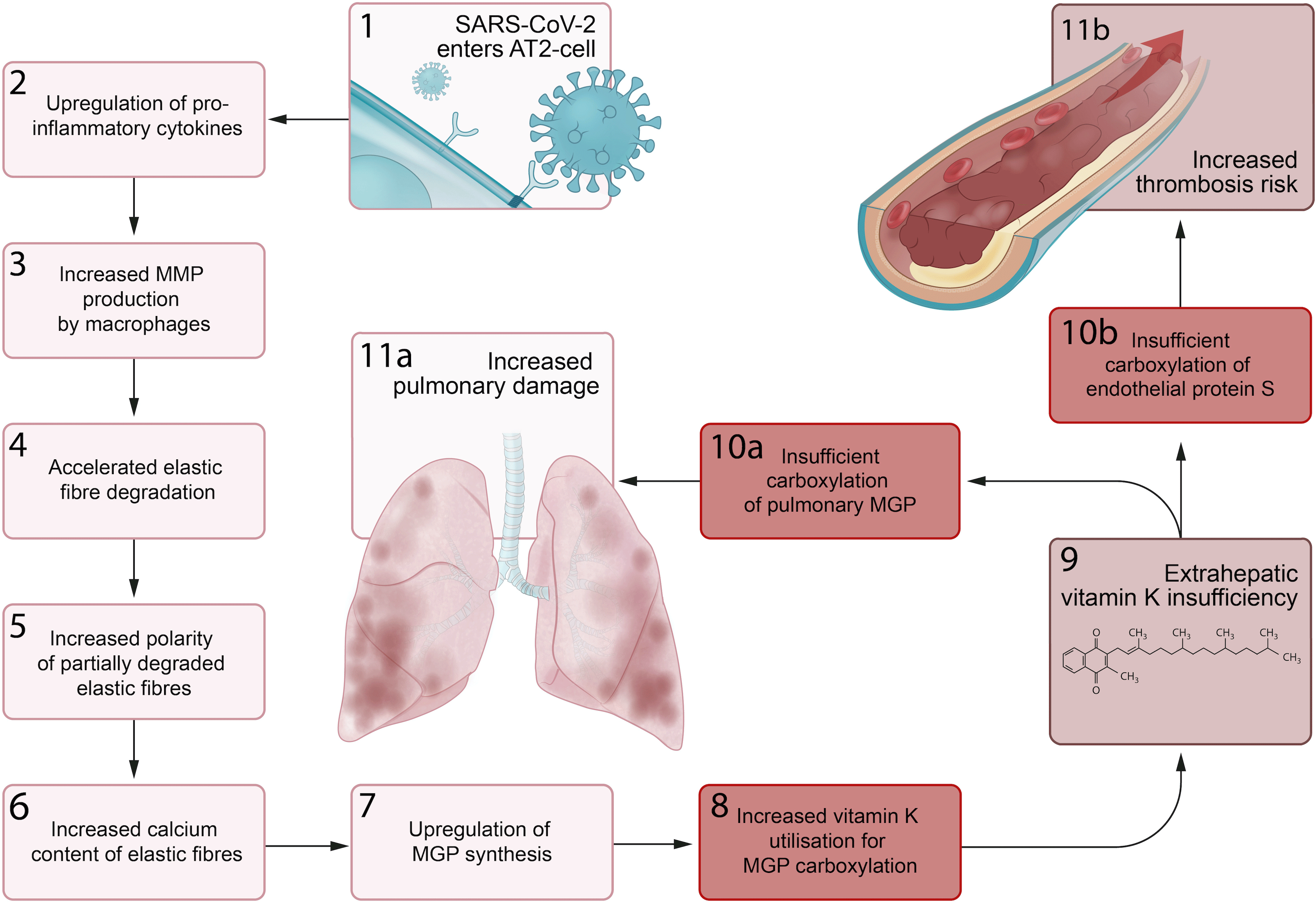
We propose a series of sequential pathological steps occurring in response to SARS-CoV-2 infection that are responsible for up-regulation of MGP expression and extrahepatic vitamin K depletion, leading to pulmonary damage and thrombosis in Covid-19 (Fig. 3).
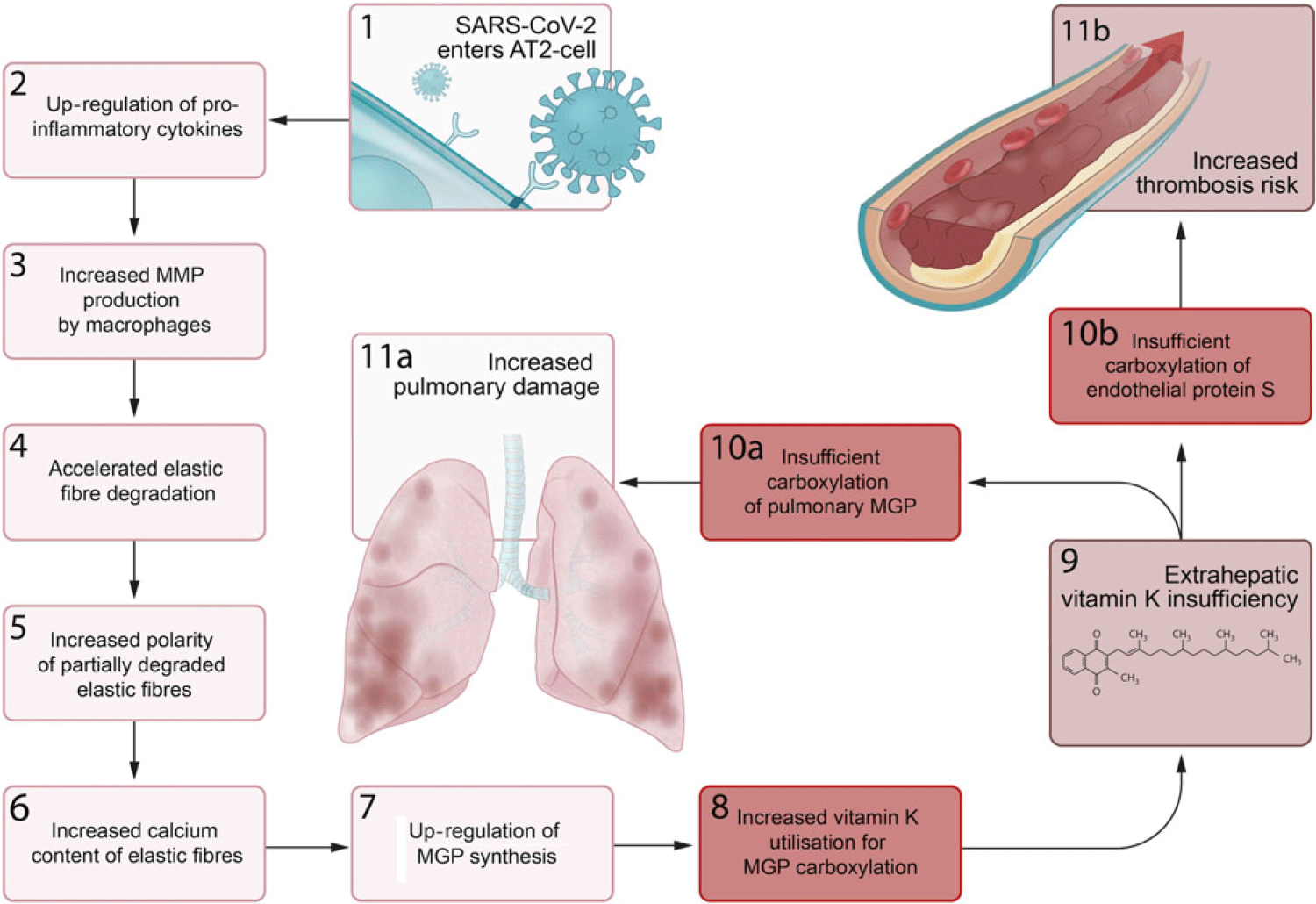
Fig. 3. Proposed sequential steps linking severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pneumonia to vitamin K insufficiency, pulmonary damage and thrombogenecity. (1) SARS-CoV-2 enters alveolar type II (ATII) cells. (2) Infected AT2 cells response by up-regulating synthesis of proinflammatory cytokines, including IL-6. (3) This leads to an increase in the number and activation of alveolar macrophages (4) that produce matrix metalloproteinases (MMP), which accelerates degradation of elastic fibres. (5) The increased polarity of partially degraded elastic fibres (6) enhances their affinity for calcium and leads to increased elastic fibre calcium content. (7) Matrix Gla protein (MGP) synthesis is up-regulated in an attempt to protect elastic fibres from calcification and degradation, (8) and the need for vitamin K to carboxylate additional MGP increases. (9) This increased utilisation of vitamin K may induce extrahepatic vitamin K insufficiency, (10a) leading to insufficient carboxylation of pulmonary MGP and (11a) increased pulmonary damage. (10b) The second consequence of extrahepatic vitamin K insufficiency is decreased carboxylation of endothelial protein S, (11b) which increases thrombosis risk.
Infection primarily begins through the inhalation of SARS-CoV-2-containing aerosols. The virus’ so-called ‘spike proteins’ have the ability of binding to angiotensin-converting enzyme 2 receptors, which enables viral entry into human cells, including alveolar type II cells. These proteins are also the most immunogenic part of the virus(Reference Wan, Shang and Graham45).
Although Covid-19 is a communicable disease, morbidity and mortality are mainly attributable to immunological rather than direct infectious complications. SARS-CoV-2 has the ability to put the immune system of infected individuals into overdrive resulting in a hyperinflammatory pulmonary state(Reference Mehta, McAuley and Brown46). Data from SARS infections suggest that the synthesis of proinflammatory cytokines begins in infected alveolar type II cells(Reference He, Ding and Zhang47). Increased levels of IL-6 and TNF-α are associated with poor Covid-19 outcome(Reference Qin, Zhou and Hu48). Autopsy studies consistently implicate infiltrating lymphocytes and macrophages as further drivers of pulmonary inflammation during Covid-19(Reference Carsana, Sonzogni and Nasr49,Reference Beigmohammadi, Jahanbin and Safaei50) . Importantly, a specific subset of macrophages appears in the lungs of Covid-19 patients(Reference Liao, Liu and Yuan51), which has previously been demonstrated to produce matrix metalloproteinases-9 in patients with idiopathic pulmonary fibrosis(Reference Morse, Tabib and Sembrat52).
Upon SARS-CoV-2 infection, elastic fibre breakdown accelerates compared with degradation rates in age-matched controls(Reference Dofferhoff, Piscaer and Schurgers34). High pulmonary concentrations of matrix metalloproteinases-9 or other proteases produced either by infiltrating or locally proliferating macrophages could be an explanation for this(Reference Liao, Liu and Yuan51,Reference Morse, Tabib and Sembrat52) . A significant correlation between dp-ucMGP and the rate of elastic fibre degradation was observed in Covid-19 patients(Reference Dofferhoff, Piscaer and Schurgers34). We suspect that accelerated elastic fibre degradation due to enhanced proteolytic activity in SARS-CoV-2-infected lungs increases elastic fibre vulnerability to Ca, leading to an up-regulation of MGP synthesis and depletion of extrahepatic vitamin K stores. This shortage impairs MGP activation, presumably causing further elastic fibre damage, and elevation of circulating dp-ucMGP. The rate of elastic fibre degradation associates with poor outcome in patients with SARS-CoV-2 pneumonia(Reference Dofferhoff, Piscaer and Schurgers34), as it does in other pulmonary diseases such as chronic obstructive pulmonary disease, cystic fibrosis and bronchiectasis(Reference Rabinovich, Miller and Wrobel13,Reference Downey, Martin and Dempster53,Reference Huang, Kuzmanova and Dicker54) .
Conditions associated with chronic elastic fibre pathology, including diabetes, hypertension and CVD, are also related to worse prognosis of SARS-CoV-2 infection. Recent data demonstrated that Covid-19 patients with poor outcomes had increased thoracic aortic and coronary artery calcification on computed tomography scan, though these analyses lost significance after correction for age and sex(Reference Dofferhoff, Piscaer and Schurgers34). Nevertheless, pre-existing elastic fibre damage predisposes to enhanced proteolytic degradation during inflammation(Reference Umeda, Aikawa and Libby42), potentially explaining the increased severity of Covid-19 in those populations.
We further theorise that vitamin K depletion also has a key effect on another characteristic disease manifestation of Covid-19. Though dp-ucMGP is elevated in Covid-19, hepatic procoagulant vitamin K status, quantified by measuring PIVKA-II, was hardly affected(Reference Dofferhoff, Piscaer and Schurgers34). According to the micronutrient triage theory(Reference Ames23), the preferential activation of hepatic over extrahepatic proteins and the fact that about 50 % of protein S synthesis occurs in endothelial cells imply that the uncarboxylated protein S fraction would also be increased(Reference Fair, Marlar and Levin6). This could increase the risk of thrombosis. Consumption of clotting factors during thrombosis puts a further burden on vitamin K stores by increasing demand for activation of newly synthesised coagulation factors to replace used ones(Reference Iba and Levy55). With preference given to the carboxylation of procoagulant factors, progressive depletion of active endothelial protein S increasingly skews the balance towards coagulation.
Interaction between vitamins D and K
Vitamin D is both endogenously produced in the skin and exogenously acquired from food. Intake of vitamins D and K is correlated due to their co-presence in various food sources. Both vitamin D and K deficiencies are prevalent around the world. Contrary to vitamin K, however, assessment of vitamin D status and propagation of vitamin D supplementation are widespread.
A meta-analysis conducted prior to the emergence of SARS-CoV-2 demonstrated that daily or weekly vitamin D supplementation reduced the risk of acute respiratory tract infection(Reference Martineau, Jolliffe and Hooper56). The role of vitamin D in susceptibility to SARS-CoV-2 infection has been assessed by various groups, and to date, results appear to be conflicting(Reference Hastie, Mackay and Ho57–Reference D’Avolio, Avataneo and Manca59). Studies evaluating the modulatory role of vitamin D on disease severity in Covid-19 have not yet been reported. Vitamin D has anti-inflammatory and anti-proteolytic properties(Reference Janssens, Decramer and Mathieu60–Reference Heulens, Korf and Cielen62), which may potentially be favourable in Covid-19. Increasing vitamin D intake is generally regarded to be safe(Reference Mitchell63), although clinical data are limited. Due to tight hormonal regulation, serum Ca levels are hardly and at most transiently increased even after high-dose vitamin D administration(Reference Niederhoffer, Bobryshev and Lartaud-Idjouadiene64). However, short-term hypercalcaemia may induce deposition of Ca on elastic fibres, which is not necessarily released from fibres after normalisation of systemic Ca levels(Reference Niederhoffer, Bobryshev and Lartaud-Idjouadiene64). High-dose vitamin D administration in rats depletes extrahepatic vitamin K stores by strongly up-regulating MGP synthesis leading to acceleration of elastic fibre calcification and degradation(Reference Price, Buckley and Williamson9,Reference Niederhoffer, Bobryshev and Lartaud-Idjouadiene64) . Vitamin D administration in a state of vitamin K deficiency may thereby endanger pulmonary and vascular health. There is also human data that raised these concerns. Vitamin D supplementation was associated with premature mortality in vitamin K-insufficient stable kidney transplant recipients(Reference van Ballegooijen, Beulens and Keyzer65). It may therefore be prudent to first supplement vitamin K in invariably vitamin K-insufficient Covid-19 hospitalised patients and to start vitamin D supplementation in those who are vitamin D-deficient only when extrahepatic vitamin K status has been restored(Reference Dofferhoff, Piscaer and Schurgers34).
Furthermore, vitamin K might be a useful additive to vitamin D because there is some evidence that it can act as an anti-inflammatory agent by suppressing NF-κB signal transduction. It may also exert a protective effect against oxidative stress by blocking the generation of reactive oxygen species(Reference Simes, Viegas and Araújo66).
Vitamin K antagonists
Although progressively substituted by direct oral anticoagulants, VKA remain important drugs for the prevention of venous and arterial thrombosis. VKA exert their antithrombotic function through inhibition of vitamin K 2,3-epoxide reductase complex 1, thereby interrupting the vitamin K cycle and inducing vitamin K deficiency (Fig. 1). This obstructs carboxylation of hepatic procoagulant factors, which delays blood clotting.
Remarkably, it has been reported that within the epicentre of the Covid-19 outbreak in the UK, the OR of having a supra-therapeutic anticoagulation with VKA (i.e. international normalised ratio > 8·0) was 6·3 around the lockdown date compared with the same period in the year before(Reference Speed, Patel and Byrne67). Root cause analysis suggested that at least 50 % of these elevations were related to Covid-19(Reference Speed, Patel and Byrne67). Although the majority of possible/confirmed Covid-19 cases had used antibiotics which may influence INR(Reference Clark and Burns68), we speculate that enhanced pulmonary vitamin K utilisation during SARS-CoV-2 pneumonia could also disturb the narrow therapeutic balance between VKA dosage and vitamin K intake levels.
VKA use in idiopathic pulmonary fibrosis patients associates with reduced survival(Reference Noth, Anstrom and Calvert69), and it has been suggested that this effect of VKA may be very acute(Reference Alagha, Secq and Pahus70). There are reasons to suspect that vitamin K-dependent MGP activation is already compromised in both animals and humans with fibrotic lung disease and that this is further compromised by VKA administration(Reference Hardie, Korfhagen and Sartor71,Reference Booth, Hadley and Cornett72) . Other potential mechanisms by which VKA could exacerbate lung fibrosis may be via preventing anticoagulant protein C and S activation, which both have antifibrotic properties(Reference Urawa, Kobayashi and D’Alessandro-Gabazza73,Reference Lin, von der Thüsen and Isermann74) . Through these mechanisms, VKA may also have an unfavourable effect on pneumonia severity in Covid-19 patients; however, this has not yet been evaluated.
VKA may also have potentially favourable effects on disease course of Covid-19 by prevention of thrombosis, as has been shown for heparins(Reference Tang, Bai and Chen75). However, considering the consequences of vitamin K insufficiency for pulmonary disease(Reference Noth, Anstrom and Calvert69,Reference Alagha, Secq and Pahus70) , it may be worthwhile to conduct a study comparing the risk of severe Covid-19 in patients on VKA with those using other classes of anticoagulant medications, provided the availability of a sufficiently large cohort to correct for confounding factors.
Future perspective
There is a need for further experimental evidence to link vitamin K deficiency with the pathology of Covid-19 and determine whether vitamin K supplementation has a place in treatment protocols.
First, there is need for lung-specific data. The current data on vitamin K status in Covid-19 are confined to measurement of circulating parameters, and we were unable to distinguish pulmonary from systemic elastic fibre degradation(Reference Dofferhoff, Piscaer and Schurgers34). Autopsy studies performed on Covid-19 patients could shed light on the presence of carboxylated and uncarboxylated vitamin K-dependent proteins at sites of SARS-CoV-2-related lung disease. This could give support to our hypothesis of increased pulmonary MGP expression and enhanced vitamin K utilisation. Animal models may also be used to elucidate the effect of vitamin K insufficiency, administration and antagonism specifically on pathologies of pulmonary elastic fibres.
Second, it is important to confirm that protein S activity is decreased during vitamin K insufficiency in Covid-19. This could be explored by measuring protein S activity, but this method has potential confounders(Reference Marlar and Gausman76). An alternative would be to quantify undercarboxylated protein S either with targeted antibodies or using liquid chromatography-tandem MS; however, to our knowledge, such assays have yet to be developed.
Finally, there is need for human intervention studies to determine whether vitamin K supplementation has a place in the prevention and treatment of severe Covid-19. Clinical trials assessing vitamin K administration in hospitalised populations are needed to evaluate both safety and efficacy. The safety of even high doses of vitamin K has been established in healthy persons(77) but remains to be assessed in severely ill Covid-19 patients. The potential role of vitamin K supplementation to prevent development of severe Covid-19 in subjects who have not yet contracted SARS-CoV-2, but are at risk for the infection, is also very relevant to assess.
In conclusion, the potential role of vitamin K supplementation to prevent the development and progression of severe Covid-19 remains largely unexplored. We would argue that the impact of the current crisis warrants thorough evaluation of the therapeutic potential of vitamin K in Covid-19 pathogenesis for two key reasons. Unlike other treatment strategies currently under development for Covid-19 such as dexamethasone, vitamin K does not have any known unfavourable effects in those who do not use VKA. Furthermore, it is relatively simple and inexpensive to manufacture contrary to other therapies like remdesivir or convalescent plasma. Taken together this means that effectiveness can be rapidly and cheaply evaluated in clinical trials and easily implemented if proven successful.
Acknowledgements
This work was funded by Kappa Bioscience AS, a manufacturer of vitamin K2 (MK-7).
R. J. developed the theory. R. J., M. P. J. V. and J. W. wrote the first draft of the manuscript. A. S. M. D., C. V. and W. J. critically revised the manuscript.
R. J. discloses application of a patent on vitamin K in Covid-19. R. J. and J. W. have a scientific collaboration with Kappa Bioscience AS, a manufacturer of vitamin K2 (MK-7). M. P. J. V., A. S. M. D., C. V. and W. J. declare no competing interests.