It is well established that prolonged and severe clinical vitamin D deficiency (represented as serum 25-hydroxyvitamin D (25(OH)D)) concentrations < 10–25 nmol/l) leads to rickets in children and osteomalacia in adults(1). Currently in the UK, a plasma level of 25 nmol/l 25(OH)D is used as the lower threshold for vitamin D status(1). There is also a growing body of evidence to suggest that less severe degrees of deficiency, or sub-clinical deficiency (serum 25(OH)D concentrations < 50 nmol/l), may be associated with increased risk of a wide range of chronic diseases, including tuberculosis, rheumatoid arthritis, multiple sclerosis, inflammatory bowel diseases, CVD, hypertension and certain cancers(Reference Zittermann2, Reference Holick3). With this in mind, it is of concern that a high prevalence of sub-clinical deficiency has been reported in adults from many countries (see reviews(Reference McKenna4–Reference Lips, Hosking, Lippuner, Norquist, Wehren, Maalouf, Ragi-Eis and Chandler7)).
Until recently, it had been assumed that children and adolescents were not at risk of low vitamin D status because of their outdoor activities and dietary intake(Reference Holick3). However, a number of recent studies in adolescents have revealed a high prevalence of vitamin D insufficiency in Europe(Reference Lehtonen-Veromaa, Mottonen, Nuotio, Irjala, Leino and Viikari8–Reference Ginty, Cavadini and Michaud15), the USA(Reference Looker, Dawson-Hughes, Calvo, Gunter and Sahyoun16–Reference Sullivan, Rosen, Halteman, Chen and Holick19) and elsewhere (the Lebanon(Reference El-Hajj Fuleihan, Nabulsi and Choucair20), New Zealand(Reference Skeaff and Green21, Reference Rockell, Green and Skeaff22), Tasmania(Reference Jones, Blizzard, Riley, Parameswaran, Greenaway and Dwyer23)), especially during the winter months. Several of the afore-mentioned studies have also provided evidence of a possible adverse effect of low vitamin D status for adolescent bone growth and strength(Reference Lehtonen-Veromaa, Mottonen, Nuotio, Irjala, Leino and Viikari8, Reference Outila, Karkkainen and Lamberg-Allardt11, Reference Cheng, Tylavsky and Kroger12). Holick in his recent review of childhood vitamin D deficiency highlights evidence that living at latitudes above 35°N for the first 10 years of life increases risk of multiple sclerosis by 100 %, as well as increasing risk of several other autoimmune diseases(Reference Holick24). Thus, failure to address low vitamin D status among adolescents could have serious long-term implications for public health.
Despite concerns about the high prevalence of low vitamin D status in adolescents, relatively few studies have investigated the potential determinants of vitamin D status in this life-stage group. A small number of studies of non-nationally representative samples of adolescent girls in Europe and USA have shown that season, sun exposure, ethnicity and race, as well as body weight, are determinants of vitamin D status(Reference Outila, Karkkainen and Lamberg-Allardt11, Reference Andersen, Molgaard and Skovgaard13, Reference Harkness and Cromer18). Rockell et al. reported that ethnicity, season and, to lesser degrees, obesity and gender were significant predictors of year-round vitamin D status in a nationally representative sample of New Zealand children and adolescents(Reference Rockell, Green and Skeaff22). Knowledge of such determinants is of importance for the development of public health strategies for prevention of low vitamin D status. Despite our relatively northerly latitude (51–55°N), there are almost no data on vitamin D status and its determinants among adolescents in Ireland. Therefore, we examined vitamin D status and its determinants during winter and summer in a representative sample of adolescents in Northern Ireland, a region with a high prevalence of diseases associated with low vitamin D status, including CVD(Reference Yarnell25), multiple sclerosis(Reference McDonnell and Hawkins26) and colon cancer(Reference Kee, Collins and Patterson27).
Subjects and methods
Design
The Young Hearts 2000 (YH2000) survey is the second in a series of cross-sectional studies examining a representative sample of adolescents in Northern Ireland. The primary aim of YH2000 was to identify the prevalence of risk factors for CHD in adolescents aged 12 and 15 years. Details of subject recruitment, inclusion and exclusion criteria, response rate and ethical approval have been described elsewhere(Reference McGartland, Robson, Murray, Cran, Savage, Watkins, Rooney and Boreham28–Reference Watkins, Murray, McCarron, Boreham, Cran, Young, McGartland, Robson and Savage30). During the months of January, February, March, April, May, June, September, October, November and December, 11 %, 16 %, 16 %, 6 %, 2 %, 7 %, 10 %, 11 %, 11 % and 11 % of the group were sampled respectively. None of the subjects was sampled during July or August owing to summer vacation. Given that Northern Ireland is at a latitude where UVB intensity is insufficient to promote dermal synthesis of vitamin D between November to March(Reference Webb, Kline and Holick31), we defined winter as November to March, and summer as April to October. Complete records were available for 1015 adolescents who had provided a blood sample and for whom data on pubertal status, anthropometry, habitual physical activity and food intakes were also available.
Anthropometry, lifestyle and dietary data
Standing height and body weight were measured as described previously(Reference McGartland, Robson, Murray, Cran, Savage, Watkins, Rooney and Boreham29). Pubertal status of each subject was assessed by a paediatrician using visual signs such as non-genital secondary hair growth, vocal timbre, body habitus, general muscular development and overall breast development in girls. Lifestyle data and physical activity data were obtained from questionnaires, as described previously(Reference McGartland, Robson, Murray, Cran, Savage, Watkins, Rooney and Boreham29, Reference Pereira, FitzGerald, Gregg, Joswiak, Ryan, Suminski, Utter and Zmuda32). Dietary data were collected by a nutritionist-administered 7 d diet history method(Reference van Staveren, de Boer and Burema33). Energy and nutrient intakes were calculated using a computer program (WISP; Tinuviel Software, Warrington, UK) based on McCance and Widdowson's Composition of Foods (Reference Holland, Welch, Unwin, Buss, Paul and Southgate34).
Collection and preparation of samples
Blood was collected by venipuncture into a vacutainer tube with no additive and processed to serum, which was immediately stored at − 80°C until required for analysis.
Experimental techniques
Serum 25-hydroxyvitamin D
25(OH)D concentrations were measured in serum samples using ELISA (OCTEIA® 25-Hydroxy Vitamin D; Immuno Diagnostic Systems, Ltd., Boldon, UK). The intra- and inter-assay CV for the ELISA method was 5·9 % and 6·6 %, respectively. The quality and accuracy of serum 25(OH)D analysis in our laboratory is assured on an ongoing basis by participation in the Vitamin D External Quality Assessment Scheme (Charing Cross Hospital, London, UK).
Statistical analysis
Statistical analysis of the data was conducted using SPSS© for WindowsTM Version 12.0 (SPSS, Inc., Chicago, IL, USA). Serum 25(OH)D concentrations and dietary vitamin D intakes were not normally distributed and, therefore, values were logarithmically (natural log) transformed prior to statistical analysis, to achieve near-normal distributions. Differences in age, height, weight, BMI, physical activity, serum 25(OH)D and dietary vitamin D and Ca between the genders and age-groupings were examined using unpaired Student's t tests. Tests for independence were used to compare demographic variables such as age-grouping, gender, season of sampling and pubertal status between those included in current analysis (n 1015) and the complete YH2000 dataset (n 2017). Differences in proportion of vitamin D deficiency/inadequacy between genders and age-groupings were assessed by χ2 tests. Two-way ANOVA was used to investigate gender–season interactions on serum 25(OH)D concentrations. Tests for independence were used to compare serum 25(OH)D concentrations between boys and girls during winter and summer, separately. Multiple logistic regression was used to investigate predictors of risk of vitamin D inadequacy (serum 25 (OH)D < 50 nmol/l), whilst adjusting for possible confounding factors. The following categorical variables were included: season (coded as: 0, winter, November–March; 1, summer, April–October); gender (coded as: 0, female; 1, male); pubertal status (coded as: 0, pre-puberty; 1, post-puberty); vitamin D intake above or below the median (coded as: 0, greater than median; 1, less than median); Ca intake above or below the median (coded as: 0, greater than median; 1, less than median); physical activity level above or below the median (coded as: 0, greater than median; 1, less than median). The continuous numerical variable BMI (kg/m2) was also included. Multiple linear regression analysis was performed to identify independent predictors of serum 25(OH)D. The following categorical variables were included: season (coded as: 0, winter, November–March; 1, summer, April–October); gender (coded as: 0, female; 1, male); age-grouping (coded as: 0, aged 12 years; 1, aged 15 years); pubertal status (coded as 0, pre-puberty; 1, post-puberty). The continuous numerical variables, BMI (kg/m2), vitamin D intake (μg/d), Ca intake (mg/d) and physical activity score were also included. P values < 0·05 were considered statistically significant.
Results
Baseline characteristics of adolescents
Characteristics of the adolescents included in the current analysis (n 1015) were compared with those of all participants in the YH2000 study (n 2017), which was a representative sample of adolescents in Northern Ireland (Table 1). No significant differences were evident between these two groups (Table 1).
Table 1 Characteristics of Northern Ireland Young Hearts 2000 Project participants entire cohort and those in the current analysis of vitamin D status§
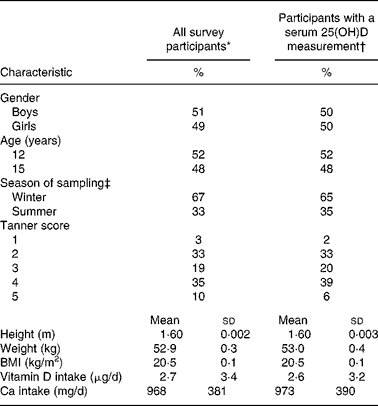
25(OH)D, 25-hydroxyvitamin D.
* In the Young Hearts Project, a participant was defined as an adolescent who completed a diet history (n 2017).
† (n 1015).
‡ Winter was defined as months November to March and summer was defined as months April to October.
§ For details of subjects and procedures, see Subjects and methods.
Vitamin D status and vitamin D intakes
The mean, standard deviation and distribution of serum 25(OH)D concentrations throughout the year in the sample of 1015 adolescents, and in each age and gender group, separately, are shown in Table 2. While all girls had a slightly lower mean year-round serum 25(OH)D concentration compared with boys (P = 0·054), a difference between the genders was significant in 15 year-olds (P = 0·018) but not in 12 year-olds (P = 0·81). Two-way ANOVA showed that serum 25(OH)D concentration in the entire group was significantly affected by season (mean 56·7 (sd 24·3) nmol/l and 78·1 (sd 27·1) nmol/l, for winter (n 657) and summer (n 358), respectively; P < 0·0001) but not gender (P>0·05; data in Table 2); there was, however, a significant interaction between these two factors (P < 0·001). While all girls (n 326) had a significantly lower mean wintertime serum 25(OH)D concentration compared with all boys (n 331) (mean 52·3 (sd 22·6) nmol/l and 61·1 (sd 25·1) nmol/l, respectively; P < 0·001), all girls (n 184) had significantly higher mean summertime serum 25(OH)D concentrations compared with all boys (n 174) (mean 81·5 (sd 23·9) nmol/l and 74·7 (sd 29·7) nmol/l, respectively; P = 0·002).
Table 2 Serum 25-hydroxyvitamin D (S-25(OH)D) concentrations throughout the year in healthy adolescents in Northern Ireland†
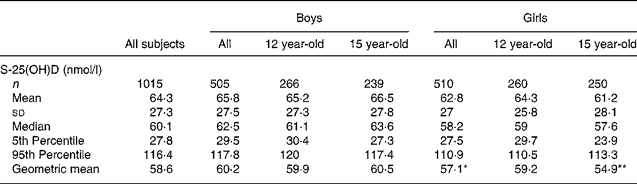
* v. all boys (P ≤ 0·05).
** v. 15 year-old boys (P = 0·018).
† For details of subjects and procedures, see Subjects and methods.
The mean, standard deviation, median and 5th and 95th percentiles of vitamin D and Ca intakes (from food and supplements) were 2·6, 3·2, 1·7, 0·6 and 10·5 μg and 973, 390, 907, 467 and 1676 mg/d, respectively. Vitamin D and Ca intakes were significantly higher (P < 0·0001) in boys than girls (3·0 v. 2·2 μg/d (vitamin D); 1089 v. 858 mg/d (Ca)). There were no significant differences in vitamin D or Ca intakes between 12 and 15 year-olds (data not shown). Mean physical activity score was significantly higher (P < 0·05) in boys than girls (30 v. 18, respectively). In addition, mean physical activity score was significantly higher (P < 0·001) in boys than girls during summertime (data not shown).
Prevalence of vitamin D deficiency and inadequacy
Cumulative percentages of adolescents with serum 25(OH)D concentrations ranging from < 10 nmol/l to < 120 nmol/l, which have been variably suggested as cut-off values for defining vitamin D deficiency to insufficiency(1, Reference Zittermann2, Reference Holick3), are shown in Fig. 1. Stratification of the adolescents by sampling period showed that the proportion of subjects with serum 25(OH)D concentrations < 50 nmol/l (the cut-off value that defines sub-clinical deficiency) was 46 % during winter and 17 % during summer. During winter, 38 % of boys and 55 % of girls had serum 25(OH)D < 50 nmol/l (P < 0·001).

Fig. 1 Cumulative percentage of Northern Ireland adolescents (n 1015) with serum 25-hydroxyvitamin D (25(OH)D) between 10 and 120 nmol/l. For details of subjects and procedures, see Subjects and methods.
Mean daily vitamin D intakes in subjects with serum 25(OH)D concentrations < 25 and ≥ 25 nmol/l were 1·5 and 2·6 μg/d, respectively (P = 0·003). Mean daily vitamin D intakes in subjects with serum 25(OH)D concentrations < 50 and ≥ 50 nmol/l were 2·1 and 2·9 μg/d, respectively (P < 0·001).
Determinants of serum 25-hydroxyvitamin D levels
Potential determinants of serum 25(OH)D levels throughout the year were assessed using multiple linear regression analysis. Ca intake, gender and physical activity score were non-significant (P>0·05) determinants of serum 25(OH)D levels. Being sampled during the sunnier half of the year (i.e. summer to autumn) (β 0·370; CI 0·318, 0·.423; P < 0·0001), vitamin D intake (β 0·083; CI 0·050, 0·115; P < 0·0001) and increasing BMI (β 0·008; CI 0·001, 0·015; P = 0·035) were positively associated with serum 25(OH)D levels. Limiting the analysis to only those subjects sampled during winter showed that vitamin D intake (β 0·998; CI 0·478, 1·518; P < 0·0001) and gender (β 6·041; CI 2·162, 9·919; P = 0·002) were the only two significant determinants of serum 25(OH)D levels.
Predictors of low vitamin D status
Multiple logistic regression analysis was used to identify the factors contributing to serum 25(OH)D concentrations < 50 nmol/l (Table 3). Winter sampling time and low vitamin D intake were significant predictors of low vitamin D status (Table 3). Ca intake, gender, age, BMI, pubertal status and physical activity were not significant predictors of low vitamin D status (data not shown). For those subjects sampled during winter, a vitamin D intake below the median of 1·7 μg/d (OR 1·589 (95 % CI 1·131, 2·232); P = 0·008) and being female (OR 1·460 (95 % CI 1·017, 2·097); P = 0·04) were significant predictors of low vitamin D status, after adjusting for possible confounders. On the other hand, limiting the analysis to only those subjects sampled during summer did not reveal any significant predictors of low vitamin D status among those tested.
Table 3 Multiple logistic regression analysis of predictors of serum 25(OH) D values <50 nmol/l in 1015 Northern Ireland adolescents*

* For details of subjects and procedures, see Subjects and methods.
Discussion
It is clearly recognised that serum/plasma 25(OH)D levels below 12·5 nmol/l can result in bone diseases, such as rickets in children and osteomalacia in adults(Reference Scharla35). There is also evidence that circulating 25(OH)D levels below 20–25 nmol/l may result in rickets and osteomalacia in the longer term(1, Reference Basha, Rao, Han and Parfitt36). In the present study, 2·9 % of a representative sample of 12 and 15 year-old adolescents from Northern Ireland had serum 25(OH)D concentrations < 25 nmol/l throughout the year. Only three other studies to date have used a nationally representative sample. Data from the UK NDNSReference Gregory, Lowe, Bates, Prentice, Jackson, Smithers, Wenlock and Farron9 showed that 11–16 % of 11–18 year-old adolescents in England, Wales and Scotland had serum 25(OH)D < 25 nmol/l throughout the year. One fundamental difference between the NDNS sample and the current cohort is the homogeneity of the YH2000 sample in terms of race; the overwhelming majority of the adolescents in the current sample were white as at the time of sampling, ethnic diversity within the population of Northern Ireland was almost non-existent. In contrast, the NDNS included a representative sample from across Great Britain, which has a more ethnically diverse population profile. Skin colour is an important predictor of vitamin D status. The efficiency of synthesis of cholecalciferol is negatively related to the extent of skin pigmentation; thus, the lower prevalence of vitamin D deficiency observed in the YH2000 cohort than in the NDNS is most likely related to differences in the capacity for vitamin D synthesis. In the US-based National Health and Nutrition Examination Survey study, ≤ 1 % of 12–19 year-olds had serum 25(OH)D < 25 nmol/l(Reference Looker, Dawson-Hughes, Calvo, Gunter and Sahyoun16), although this is likely to be an underestimate because vitamin D status was measured in northern United States during summer and in southern United States during winter. A study in New Zealand found that 4 % of adolescents aged 11–14 years had serum 25(OH)D < 17·5 nmol/l(Reference Rockell, Green and Skeaff22).
Circulating 25(OH)D concentrations greater than 25 nmol/l but less then 50 nmol/l reflect sub-clinical vitamin D deficiency, which is associated with elevated parathyroid hormone with consequences for bone turnover rates, at least in adults(Reference Lips37). Furthermore, serum 25(OH)D concentration < 50 nmol/l has also been linked to the development of various chronic diseases (e.g. hypertension, CVD, diabetes mellitus, as well as some inflammatory and autoimmune diseases and some forms of cancer).(Reference Zittermann2, Reference Holick3) Over one-third (36 %) of adolescents in the present study had 25(OH)D concentrations < 50 nmol/l across the year and the prevalence of vitamin D inadequacy, or low vitamin D status, was much higher (48–52 %) in subjects sampled during winter (November–March). These findings are broadly in line with those of the three previously mentioned studies of nationally representative samples of adolescents(Reference Gregory, Lowe, Bates, Prentice, Jackson, Smithers, Wenlock and Farron9, Reference Looker, Dawson-Hughes, Calvo, Gunter and Sahyoun16, Reference Rockell, Green and Skeaff22) and in keeping with several studies that investigated the prevalence of vitamin D inadequacy in adolescents in Europe(Reference Lehtonen-Veromaa, Mottonen, Nuotio, Irjala, Leino and Viikari8, Reference Guillemant, Le, Maria, Allemandou, Peres and Guillemant10–Reference Das, Crocombe, McGrath, Berry and Mughal14), the USA(Reference Looker, Dawson-Hughes, Calvo, Gunter and Sahyoun16–Reference Sullivan, Rosen, Halteman, Chen and Holick19) and elsewhere(Reference El-Hajj Fuleihan, Nabulsi and Choucair20, Reference Skeaff and Green21, Reference Jones, Blizzard, Riley, Parameswaran, Greenaway and Dwyer23).
In the current study, girls had lower wintertime mean serum 25(OH)D levels than boys, although girls had higher levels during summertime. The gender difference during summertime might relate to a higher proportion of girls (72 %) being sampled in late summer (September/October; when vitamin D status would be at its peak) compared with only 43 % of boys being sampled during that time. Those sampled in earlier summer (April–June) would be expected to have lower vitamin D status. There was an increased proportion of girls with low ( < 50 nmol/l) 25(OH)D levels throughout the year (39 % v. 32 %, for girls and boys, respectively) as well as in winter only (55 % v. 38 %, for girls and boys, respectively). While not evident in the UK-based NDNS study of adolescents(Reference Gregory, Lowe, Bates, Prentice, Jackson, Smithers, Wenlock and Farron9), this lower vitamin D status among girls has also been reported in the national studies of children and adolescents in the USA(Reference Looker, Dawson-Hughes, Calvo, Gunter and Sahyoun16) and New Zealand(Reference Rockell, Green and Skeaff22). The reason for this apparent gender difference is unclear. However, in the current study, girls had lower vitamin D intakes than boys. It is also interesting to note that subjects with vitamin D deficiency and insufficiency had significantly lower vitamin D intakes compared with those in vitamin D-replete subjects. Multiple linear regression analysis of subjects sampled in winter showed that being female and low vitamin D intake were the only two significant determinants of serum 25(OH)D levels. Rockell et al. suggested that the difference in vitamin D status between the genders was acting as a surrogate marker for sunlight exposure through an association with physical activity(Reference Rockell, Green and Skeaff22). In the present study, physical activity was significantly higher in boys than girls, even during summertime. Physical activity was not a determinant of serum 25(OH)D levels in these adolescents. The physical activity score, however, included activity that is both indoor- and outdoor-based.
To explore the reasons for the high prevalence of low vitamin D status in the adolescents in Northern Ireland, we investigated the potential predictors of vitamin D inadequacy (serum 25(OH)D < 50 nmol/l). Not surprisingly, season was the major determinant of vitamin D status, a finding shown in many studies of adults(Reference Hill, O'Brien, Lamberg-Allardt, Jakobsen, Kiely, Flynn and Cashman38, Reference Jacques, Felson, Tucker, Mahnken, Wilson, Rosenberg and Rush39), and in the New Zealand study of determinants of low vitamin D status in children and adolescents(Reference Rockell, Green and Skeaff22). Potentially of concern was our observation that 13 % of adolescents living in Northern Ireland had low vitamin D status during summertime, at a time when vitamin D synthesis and status would be expected to be optimum(Reference McKenna4). As season was such a major predictor, we repeated the analysis in the subgroups sampled during winter- and summertime separately. During winter, low vitamin D intake (less than 1·7 μg/d) and being female were significant predictors of having a serum 25(OH)D value of < 50 nmol/l.
There is no dietary recommendation for vitamin D for adolescents in the UK, as there is an assumption that sun exposure will provide for adequate status during summer and allow for stores to be laid down to support vitamin D status in winter1. This lack of a dietary recommendation appears unwise when 12 % and 47 % of UK adolescents (aged 11–18 years) have plasma 25(OH)D concentrations that are reflective of serious and mild deficiency, respectively(Reference Gregory, Lowe, Bates, Prentice, Jackson, Smithers, Wenlock and Farron9). In contrast, the US Institute of Medicine recommends 5 μg/d for adolescents(40), an intake level that only 10 % of the current sample managed to achieve. These low intakes are in line with similar reports from several countries(Reference Lehtonen-Veromaa, Mottonen, Nuotio, Irjala, Leino and Viikari8, Reference Gregory, Lowe, Bates, Prentice, Jackson, Smithers, Wenlock and Farron9, Reference Andersen, Molgaard and Skovgaard13, Reference Ginty, Cavadini and Michaud15, Reference Samuelson, Bratteby, Enghardt and Hedgren41, Reference Salamoun, Kizirian and Tannous42). For example, data from the NDNS show that the mean daily intake of vitamin D for adolescents (aged 11–18 years) in the UK is 2·6 μg(Reference Gregory, Lowe, Bates, Prentice, Jackson, Smithers, Wenlock and Farron9). For individuals living in Ireland, the UK and elsewhere in Europe and North America at latitudes above 37°N, low intakes of vitamin D may take on increased importance during wintertime when sunlight is of insufficient intensity to stimulate dermal vitamin D synthesis.
We did not identify any significant physiological or lifestyle predictors of low vitamin D status during summer, indicating that UVB exposure, which was not directly assessed in the current study, was the overriding determinant of vitamin D status at this time. Despite being by far the most efficacious method of improving vitamin D status(Reference Holick3), recommendations for increased UVB exposure are fraught with public health difficulties in relation to evidence for increased risk of skin cancer. Thus, a dietary strategy (including supplemental and/or fortification sources of vitamin D) appears a more acceptable mode of improving vitamin D status of the population, including adolescents. More research is needed to define the levels of intakes required and the best strategies to achieve these intakes on a population basis. The lack of an international consensus on cut-off levels for vitamin D deficiency/insufficiency is certainly a limitation in terms of better defining dietary requirements. While there are several reasons for this uncertainty(Reference Dawson-Hughes, Heaney, Holick, Lips, Meunier and Vieth43), it is further complicated by methodological issues surrounding analysis of serum 25(OH)D levels. Different methods of analysis can produce different serum 25(OH)D levels, as illustrated recently in round robin analyses of the Vitamin D External Quality Assessment Scheme samples(Reference Carter, Carter, Jones and Berry44–Reference Carter, Jones and Berry46). In addition, significant variation between laboratories using the same analytical technique can exist due to operator bias. For these reasons, participation of laboratories in the Vitamin D External Quality Assessment Scheme is highly recommended, as well as the future inclusion of standard reference materials for serum 25(OH)D, which are currently being developed in the USA.
In conclusion, there was a high prevalence of low vitamin D status among adolescents living in Northern Ireland at latitudes of 54–55°N, most marked during the winter months. This seems to be a consequence of the failure of a low dietary intake of vitamin D to compensate for the lack of dermal synthesis during this period. Increased emphasis needs to be given to exploring strategies for improving vitamin D status in adolescents.
Acknowledgements
This project was financially supported by the Higher Education Authority under their Strand 1: North/South Programme for Collaborative Research and the Northern Ireland Department of Health, Social Services and Public Safety. None of the authors has a conflict of interest.






