Adolescents are considered as a risk group for malnutrition because of their increasing needs of nutrients and energy for adequate growth and development that vary with age(Reference Ovesen, Andersen and Jakobsen1–Reference Moreno and Koletzko3). Specifically different levels of vitamin D deficiency at these early ages could be considered a risk factor for osteomalacia(Reference Bischoff-Ferrari4–Reference Scharla, Scheidt-Nave and Leidig6), impaired cognitive function and concentration problems(Reference Annweiler, Allali and Allain7), hyperactivity(Reference Elmadfa, Godina-Zarfl and Dichtl8) and immune system deficiency(Reference Bischoff-Ferrari4). Inadequate vitamin D levels have also been related to other diseases such as diabetes, multiple sclerosis and cancer(Reference Summerbell, Waters and Edmunds9–Reference Rolland-Cachera, Bellisle and Deheeger11). One of the most important applications of vitamin D assessment in adolescence is related to bone health(Reference Rizzoli, Bianchi and Garabédian12) and reaching an optimal peak bone mass in adulthood(Reference Lamberg-Allardt and Viljakainen13, Reference Cranney, Weiler and O'Donnell14). The main sources of vitamin D are food intake and subcutaneous skin synthesis, under UV light (290–315 nm) exposure. However, due to the geographical situation of our continent, vitamin D synthesis may not compensate for a low nutritional intake(Reference Scharla15). Subclinical vitamin D deficiency could remain undetected as it is not routinely screened for in these population groups. The main circulating vitamin D metabolite, 25-hydroxycholecalciferol (25(OH)D), has been proposed as the best indicator of vitamin D status, because it represents not only the amount consumed through diet and supplements but also the subcutaneous synthesis(Reference Scharla15–18).
Scientific knowledge about vitamin D status in the period of adolescence in both developed and developing countries is still scarce. Several studies on the status of vitamin D in European adolescents have been carried out in the last decade, but only a few have used a significant number of subjects. As we have recently reviewed(Reference Valtueña, Breidenassel and Folle19), comparison of the data is not always possible due to the use of different age ranges, different methods, different ways of presenting them in the different studies and a lack of consensus on cut-off levels. Proposed deficient and sufficient 25(OH)D vitamin concentrations vary from 20 to 100 nmol/l depending on the studies(Reference Guillemant, Le and Maria20–Reference Gregory, Lowe and Bates22). While there are no universally accepted blood 25(OH)D thresholds to define adequacy in adolescents, the following set has been proposed: concentrations of 25(OH)D less than 75 nmol/l as insufficient, concentrations less than 50 nmol/l as deficient and severe vitamin D deficiency when values are less than 27·5 nmol/l(Reference Ross, Manson and Abrams23). The proposed reference value for insufficiency in children (<75 nmol/l) has been extrapolated from adult data(18).
One of the main aims of the Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study was to provide, for the first time, comparable data about micronutrient status in European adolescents(Reference Moreno, de Henauw and Gonzalez-Gross24). The main objective of the present study was to describe vitamin D status in adolescents and to analyse vitamin plasma concentrations by sex, age and weight status, thus contributing to establishing reference values that are not available for the adolescent population(Reference Bonofiglio, Maggiolini and Marsico25, Reference Lambert, Agostoni and Elmadfa26).
Subjects and methods
Subjects, recruitment and study design
The HELENA cross-sectional study was a multi-centre cross-sectional study aiming to obtain reliable and comparable data from a random sample of 3000 European adolescents aged between 12·5 and 17·49 years on a broad battery of nutrition and health-related parameters(Reference Moreno, de Henauw and Gonzalez-Gross24, Reference De Henauw, Gottrand and De Bourdeaudhuij27). Subjects were recruited by a school-based, multi-step, stratified random and cluster sampling selection. Criteria for city selection included geographic balance and the presence of an experienced research group. The sample size was calculated to establish distributions of relevant study variables. Exclusion criteria were limited to subjects who were not able to speak the local language, who were participating simultaneously in another clinical trial, who were aged < 12·5 or >17·5 years and who had suffered from acute infection 1 week before the visit. Exclusion from the study was decided a posteriori, without the knowledge of the affected subjects, to avoid non-desirable situations, and so whole classes were included. A complete description of the design and implementation of the study has been published elsewhere(Reference Moreno, de Henauw and Gonzalez-Gross24).
In the same manner as described earlier, a subsample of 1006 adolescents was selected for blood sampling in the ten HELENA cities in nine European countries: Athens (Greece), Dortmund (Germany), Ghent (Belgium), Heraklion (Greece), Lille (France), Pecs (Hungary), Rome (Italy), Stockholm (Sweden), Vienna (Austria) and Zaragoza (Spain). The protocol was approved by the corresponding human research review committees of Bonn (Dortmund), Lille, Rome, Zaragoza, Athens, Heraklion, Pecs, Ghent, Stockholm and Vienna. The study was performed following the ethical guidelines of the Declaration of Helsinki 1964 (revision of Edinburgh 2000), Convention of Oviedo (1997), the Good Clinical Practice, and the legislation about clinical research in human subjects in each of the participating countries. Informed written consent was obtained from subjects and parents or guardians. A complete description of ethical issues and good clinical practice within the HELENA cross-sectional study has been published elsewhere(Reference Beghin, Castera and Manios28).
Specimen collection and biochemical analyses
A specific handling, transport and traceability system for biological samples was developed for the HELENA study and has already been described by González-Gross et al. (Reference González-Gross, Breidenassel and Gómez29). Blood samples were obtained between October 2006 and June 2007, and in October 2007 (see Annex). A blood-sampling calendar was developed to coordinate the fieldwork between the centres and the central laboratory at the University of Bonn (Institut für Ernährungs- und Lebensmittelwissenschaften (IEL); Bonn, Germany). The blood sampling date depended on local fieldwork planning, the agreement of the school, and availability and capacity of the lab at IEL. Fasting blood samples were collected by venepuncture at school between 08.00 and 10.00 hours. For the measurement of vitamin D, blood was collected in EDTA tubes and transported at room temperature to the central laboratory at IEL within 24 h. There it was centrifuged at 3500 rpm for 15 min at 4°C and the supernatant was stored at − 80°C until assayed. The samples were kept stable for 24 h at room temperature (CV: 4·3 %).
Serum concentration of 25(OH)D is considered to be the most reliable measure of overall vitamin D status and thus can be used to determine whether a subject is vitamin D sufficient. Plasma 25(OH)D was analysed by ELISA using a kit (OCTEIA 25(OH)D) from Immunodiagnostic System (Frankfurt am Main, Germany) and measured with a Sunrise™ Photometer by TECAN (Männedorf, Germany). The IDS OCTEIA 25(OH)D kit is an enzyme immunoassay intended for the quantitative determination of 25(OH)D and other hydroxylated metabolites in human serum or plasma. Results are used in conjunction with other clinical and laboratory data to assist the clinician in the assessment of vitamin D sufficiency. The sensitivity of this method is 5 nmol/l 25(OH)D and the variation is less than 6 %. The mean recovery of 25(OH)D is 101 %. The CV for the method was less than 1 %.
Physical examination
The adolescents had their height and weight measured by trained researchers in a standardised way: weight was recorded to the nearest 0·1 kg, using an electronic scale (type SECA 861; SECA, Hamburg, Germany) and height was recorded to the nearest 0·1 cm, using a telescopic height measuring instrument (type SECA 225). The BMI was calculated from their measured height and weight (BMI = weight divided by height squared, (kg/m2)). International age- and sex-specific cut-off points(Reference Markou, Mylonas and Theodoropoulou30, Reference Misra, Aggarwal and Miller31) were used to assess BMI category (underweight/normal weight/overweight/obese). The complete description of the anthropometric measurements of the study has been published elsewhere(Reference Nagy, Vicente-Rodriguez and Manios32). A physician classified the adolescents into one of the five maturation stages described by Tanner & Whitehouse(Reference Tanner and Whitehouse33).
Statistical analysis
25(OH)D showed a normal histogram distribution. Descriptive statistics were performed and values are shown as mean, standard deviation, percentile, median, minimum and maximum. For this study, vitamin D status was classified into four groups (vitamin D sufficiency/optimal levels ≥ 75 nmol/l; insufficiency 50–75 nmol/l; deficiency 27·5–49·99 nmol/l and severe deficiency < 27·5 nmol/l) following international guidelines(18, Reference Malabanan, Veronikis and Holick34, Reference Bischoff-Ferrari, Giovannucci and Willett35). The differences between sex, age groups and BMI groups were analysed using one-way ANOVA. All the analyses were adjusted by a weighting factor to balance the sample according to the age and sex distribution of the theoretical sample, to guarantee representation of each of the stratified groups.
To provide percentile value curves for European adolescents, we analysed vitamin D data by maximum penalised likelihood using the least mean square statistical method for boys and girls separately(Reference Cole and Green36, Reference Cole, Freeman and Preece37). We derived smoothed centile charts using the least mean square method. This estimates the measurement centiles in terms of three age–sex-specific cubic spline curves: the L curve (Box–Cox power to remove skewness), M curve (median) and S curve (CV). For the construction of the percentile curves, data were imported into the LmsChartMaker software (version 2.3; by Tim Cole and Huiqi Pan, Harlow Healthcare, South Shields, Tyne and Wear, UK) and the L, M and S curves were estimated. The rest of the data were analysed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Descriptive characteristics and mean vitamin D concentrations of the study sample by age and sex are shown in Tables 1 and 2. Girls had slightly higher mean concentrations than boys. Prevalence rates of vitamin D status according to the aforementioned sufficient–deficient classification are shown in Fig. 1. Considering the cut-off set for adults at 75 nmol/l, approximately 80 % of the sample was below the optimal levels. A slightly higher percentage of females (22·2 %) had sufficient 25(OH)D concentrations compared to males (15·1 %). Regarding 25(OH)D deficiency ( < 27·5 nmol/l), an equal and high proportion of males and females revealed this status (15 %).
Table 1 Descriptive characteristics of participants
(Mean values, standard deviations and number of participants)

25(OH)D, 25-hydroxycholecalciferol.
Table 2 25-Hydroxycholecalciferol concentrations by age and sex in European adolescents (nmol/l)
(Mean values, standard deviations, number of participants and percentiles)

* Mean values were significantly different between the 13 years age group and the rest of the age groups (P < 0·05).
† Four age groups: 13 years, age between 12·5 and 13·99 years; 14 years, age between 14 and 14·99 years; 15 years, age between 15 and 15·99 years; 16 years, age between 16 and 17·49 years.

Fig. 1 25-Hydroxycholecalciferol (25(OH)D) status classification.
There is a tendency of increasing 25(OH)D concentrations with increasing age for the whole group (P < 0·001), which is only significant in girls when the sample is split by sex (P < 0·05). Percentile distribution by age and sex for the whole sample is shown in Table 2. 25(OH)D sufficiency (>75 nmol/l) is reached at lower percentiles with increasing age. That means that at increasing ages there are fewer subjects with insufficient 25(OH)D levels. Regarding deficiency, the fifth percentile of 25(OH)D in both males and females is close to the level of < 27·5 nmol/l for all ages.
Fig. 2 shows smoothed centile curves (P5, P25, P50, P75, P95) for 25(OH)D levels studied by age and sex. Concentrations were similar in boys and girls, although in boys, first a decrease and after the age of 14 years an increase is observed. In girls, the curves seem to indicate that the decrease comes before the age of 13 years, because at age 13 years a slightly progressive increase with age with a similar slope to that of the boys was observed. In both boys and girls, the trend to higher 25(OH)D levels is seen for those at P75 and P95, whereas at the other lower levels there is a trend to stability.

Fig. 2 Smoothed (least mean square method) centile curves (from the bottom to the top: P5, P25, P50, P75, P95) of 25-hydroxycholecalciferol (25(OH)D) plasma concentrations (nmol/l) in (a) males and (b) females.
When analysing the data according to BMI, a non-significant and progressive decrease of 25(OH)D concentrations with increasing BMI is observed, the lowest levels being observed in obese adolescents (equivalent to BMI >30 kg/m2; Table 3). The highest mean levels were for boys in the underweight group and for girls in the optimal weight group (66·6 (sd 28·9) and 61·1 (sd 23·5) nmol/l, respectively). Most of the adolescents had optimal weight status (BMI 20–25 kg/m2).
Table 3 25-Hydroxycholecalciferol concentrations by BMI (kg/m2) in European adolescents (nmol/l)
(Mean values, standard deviations, number of participants, minimum and maximum values)
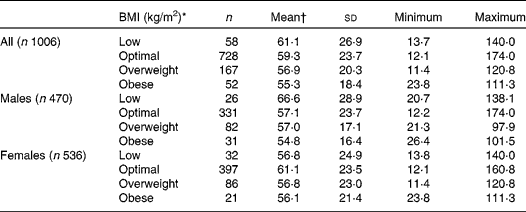
* BMI category calculated using polynomial from Cole et al. (Reference Cole and Green36, Reference Cole, Freeman and Preece37). Four BMI groups: low ( < 18·5 in adults), optimal (18·5–25 in adults), overweight (25–30 in adults), obese (>30 in adults).
† Mean values were not significantly different.
Table 4 shows 25(OH)D levels by study centre, for the whole group split by sex. The highest levels were obtained in Rome, Athens, Vienna and Zaragoza, and the lowest levels were found in Dortmund, Heraklion and Ghent for the whole group, where the sampling procedure went on for most of the academic year. In none of the cities were mean levels above the proposed cut-off of 75 nmol/l. Girls had higher mean levels in all cities except for Athens, Pecs and Lille. Deficient levels ( < 50 nmol/l) were highest in Dortmund (62·9 % of the population) and Ghent (53·3 %), and lowest in Athens (25·7 %) and Rome (26·4 %) (data not shown).
Table 4 Mean 25-hydroxycholecalciferol (25(OH)D) concentrations (nmol/l) in the Healthy Lifestyle in Europe by Nutrition in Adolescence cities
(Mean values, standard deviations, minimum and maximum values)

Discussion
Since the publication of the results of the SENECA (Survey in Europe on Nutrition and the Elderly; a Concerted Action) study(Reference Van der Wielen, de Groot and van Staveren38), where unexpectedly only 3·5 % of the analysed European elderly presented optimum 25(OH)D levels (>60 nmol/l), public health authorities have been concerned about the widespread 25(OH)D deficiency in the European population. To the best of our knowledge, the data obtained in the framework of the HELENA study are the first to aim at establishing descriptive 25(OH)D status in adolescents at a European level. According to the Institute of Medicine report 2011, vitamin D intake for bone health should correspond to a serum 25(OH)D level of at least 20 ng/ml (50 nmol/l)(Reference Ross, Manson and Abrams23). Our study results showed that approximately 40 % of the subjects had deficient levels lower than 50 nmol/l, 15 % with levels less than 27·5 nmol/l. None of the subjects had levels less than 10 nmol/l, which, according to the literature, elevates the risk for osteomalacia and rickets(Reference Cranney, Weiler and O'Donnell14, Reference Carter, Carter and Gunter39–Reference Carter, Carter and Jones42). The HELENA percentile distribution is in agreement with data coming from other studies(Reference Guillemant, Le and Maria20, Reference Moreno, de Henauw and Gonzalez-Gross24, Reference De Henauw, Gottrand and De Bourdeaudhuij27, Reference Beghin, Castera and Manios28, Reference Lehtonen-Veromaa, Möttönen and Irjala43, Reference Hill, Cotter and Mitchell44). When analysing the percentile distribution of 25(OH)D we observed that the fifth percentile of 25(OH)D in both males and females, stratified by age, is close to the level of < 27·5 nmol/l for all ages. A general hypovitaminosis problem in adolescence varying from 13 to 72 % has already been postulated in studies performed in several European countries(Reference Guillemant, Le and Maria20, Reference Moreno, de Henauw and Gonzalez-Gross24, Reference De Henauw, Gottrand and De Bourdeaudhuij27, Reference Beghin, Castera and Manios28, Reference Lehtonen-Veromaa, Möttönen and Irjala43, Reference Hill, Cotter and Mitchell44), the USA and Canada(Reference Yetley45–Reference Langlois, Greene-Finestone and Little49). In a recent study published by Dong et al. (Reference Dong, Pollock and Stallmann-Jorgensen47), the overall prevalence of vitamin D insufficiency and deficiency in US children and adolescents was 56·4 and 28·8 %, respectively. All together, the high levels of vitamin D deficiency found in the present and other studies should be treated with caution. Regarding our percentile distribution, the median value of a 25(OH)D concentration in our European adolescents is close to 60 nmol/l, much lower than the optimal levels proposed of 75 nmol/l. Following Lanham-New et al. (Reference Lanham-New, Buttriss and Miles50), any discussion of an ‘optimal’ serum 25(OH)D concentration needs to define ‘optimal’ with care since it is important to consider the normal distribution of requirements and the vitamin D needs for a wide range of outcomes. In addition, in the Rank Forum on Vitamin D, 2009, there was also some uncertainty about the strength of evidence for the need to aim for substantially higher concentrations (25(OH)D) concentrations >75 nmol/l(Reference Lanham-New, Buttriss and Miles50).
Analysing vitamin D status by age, we have observed a steady increase in 25(OH)D concentrations with increasing age but which is only significant in girls. This is not in line with other published data. Koenig & Elmadfa(Reference Koenig and Elmadfa51) found a decrease in 25(OH)D serum concentrations in Austrian adolescents up to 14 years of age and a slow increase between the ages of 15 and 19 years. Similar findings were observed by Gregory et al. (Reference Gregory, Lowe and Bates22), with a significant reduction in 25(OH)D serum concentrations according to increasing age in adolescence. Dong et al. (Reference Dong, Pollock and Stallmann-Jorgensen47) concluded in their study that plasma 25(OH)D levels were not associated with age (P = 0·460). Conversely, Bonofiglio et al. (Reference Bonofiglio, Maggiolini and Marsico25) found higher 25(OH)D serum concentrations in post-menarcheal girls when compared with pre-menarcheal girls. These higher concentrations were explained as an increase in the binding protein of vitamin D because of higher oestrogen levels caused by menarche. This could also be the explanation for the differences observed in the centile curves in Fig. 2, as Tanner stages differ and girls are on average 2 years in advance within the maturation process. In the US study by Yetley(Reference Yetley45) deficiency percentage also increased with increasing age (1 % for infants and children aged < 11 years, 5 % for adolescents aged 12–19 years and 6 % for adults aged < 20 years).
Several reports have observed a relationship between BMI and vitamin D concentrations(Reference Souberbielle, Friedlander and Kahan52). An inverse, but not significant, relationship between 25(OH)D and BMI was found in the HELENA sample (Table 3). In the literature there are discrepancies regarding this issue. While several studies reported a significant and inverse relationship(Reference Kaur, Hyder and Poston46, Reference Smotkin-Tangorra, Purushothaman and Gupta53, Reference Lenders, Feldman and Von Scheven54), others did not find any associations of BMI and/or fat mass with 25(OH)D levels in the paediatric population(Reference Jago, Harrell and McMurray55, Reference Weng, Shults and Leonard56). This may be attributed, in part, to BMI-based categorisation of obesity and the variations associated with growth and development. There are also studies reporting that obesity is associated with decreased bioavailability of dietary and cutaneously synthesised vitamin D. This may be secondary to the sequestration of vitamin D into a larger pool of adipose tissue(Reference Holick57).
As we have reviewed recently(Reference Valtueña, Breidenassel and Folle19), both geographical and seasonal differences can be appreciated throughout Europe when analysing independent studies. Owing to the complex methodology and the multiple objectives of the HELENA study, no specific calendar for vitamin D sampling could be established, which would have contributed to getting a more in-depth appreciation of these aspects. Although not assessed in the study, dietary vitamin D intake and personal UV exposure habits may partly explain geographical differences in vitamin D status. Nevertheless, our study data are similar to those published by others(Reference Guillemant, Le and Maria20, Reference Moreno, de Henauw and Gonzalez-Gross24, Reference De Henauw, Gottrand and De Bourdeaudhuij27, Reference Beghin, Castera and Manios28, Reference Lehtonen-Veromaa, Möttönen and Irjala43, Reference Hill, Cotter and Mitchell44), as the highest concentrations were observed in Rome, Athens and Zaragoza, and the lowest concentrations in Dortmund, Gent and Lille. The low mean concentrations observed in Heraklion could be due to seasonal influences. Because of local logistics, blood sampling in Heraklion was performed only in February and March, two winter months, while in the other centres, with the exception of Athens, blood sampling was distributed throughout the school year (see Annex). The low concentrations obtained in Heraklion could indicate a risk during the winter months even in the Mediterranean countries. This is in accordance with some researchers who have already emphasised the need to supplement vitamin D due to low 25(OH)D concentrations(Reference Serra-Majem58, Reference Pilz, Tomaschitz and Drechsler59), especially during winter months. The high mean concentrations observed in Vienna need further analysis, which is out of the scope of this article. All in all, the detected geographical differences make it difficult to give common recommendations to improve vitamin D status in adolescents at the European level.
Increasing mean 25(OH)D blood levels up to 40 ng/ml would have a positive impact on reducing the direct and indirect economic burden of disease(Reference Grant, Cross and Garland60).
Apart from the aforementioned limitation, the HELENA study has several strengths. The sampling procedure and the strict standardisation of the fieldwork among the countries involved in the study avoided to a great extent the kind of confounding bias due to inconsistent protocols and different laboratory methods, which makes comparing results from isolated studies difficult. The main contribution of the present data is, for the first time, to give a global overview of adolescent vitamin D status in Europe. In the absence of reference values and specific cut-off points for this age group, percentile distribution as presented can be used in clinics and further research. It is important to remember that current blood concentrations of vitamins in the adolescent population do not necessary mean that these concentrations are the most adequate ones from the biological point of view. For a future study, serum parathyroid hormone concentrations should be included as in children and adolescents, the relationship between serum 25(OH)D and parathyroid hormone is less clear(Reference Hill, McCabe and McCabe61). Owing to the complex and enormous amount of variables analysed in the HELENA project, parathyroid hormone could not be assessed. Considering the cut-offs used, deficiencies have been observed. Apart from an insufficiency, this could indicate that vitamin D concentrations in adolescents may be different from those of adults, making it necessary to establish general cut-offs for this micronutrient concentration in blood for the adolescence period.
In conclusion, our data give descriptive information about vitamin D status in European adolescents. Age, sex and weight status seem to have an influence on blood concentrations and should be taken into account. Our study results, with the limitations described earlier, indicate that vitamin D deficiency is a highly prevalent condition in European adolescents and needs to be addressed by public health authorities.
Disclosure
The content of this paper reflects only the authors' view and the rest of HELENA study members are not responsible for it. The writing group takes sole responsibility for the content of this article.
Acknowledgements
The HELENA study has taken place with the financial support of the European Community Sixth RTD Framework Programme (contract FOOD-CT-2005-007034). The content of this article reflects only the authors' views and the European Community is not liable for any use that may be made of the information contained therein. Additional support was received from the Spanish Ministry of Education (AGL2007-29784-E/ALI; AP-2005-3827) and Universidad Politécnica de Madrid (CH/018/2008). Many thanks to Adelheid Schuch for her contribution to laboratory work, to Laura Barrios for statistical assistance and to Diane Schofield for final English review of the manuscript. None of the authors had any conflict of interests. Contributions of each author: M. G.-G., L. A. M., M. K., S. D. H., F. G., A. K., Y. M., M. F. and P. S. designed the HELENA study and conducted research (recruitment of adolescents, collection of data, and blood sampling; J. V., C. B., E. A. conducted research and performed blood analysis; M. G.-G. and J. V. performed statistical analysis; M. G.-G., J. V., C. B., L. A. M., M. K. and P. S. wrote the paper. M. G.-G. and P. S. had primary responsibility for final content. All authors read and approved the final manuscript.
Helena Study Group
Co-ordinator: Luis A. Moreno.
Core Group members: Luis A. Moreno, Fréderic Gottrand, Stefaan De Henauw, Marcela González-Gross, Chantal Gilbert.
Steering Committee: Anthony Kafatos (President), Luis A. Moreno, Christian Libersa, Stefaan De Henauw, Jackie Sáchez, Fréderic Gottrand, Mathilde Kesting, Michael Sjostrom, Dénes Molnár, Marcela González-Gross, Jean Dallongeville, Chantal Gilbert, Gunnar Hall, Lea Maes, Luca Scalfi.
Project Manager: Pilar Meléndez.
1. Universidad de Zaragoza (Spain): Luis A. Moreno, Jesús Fleta, José A. Casajús, Gerardo Rodríguez, Concepción Tomás, María I. Mesana, Germán Vicente-Rodríguez, Adoración Villarroya, Carlos M. Gil, Ignacio Ara, Juan Revenga, Carmen Lachen, Juan Fernández Alvira, Gloria Bueno, Aurora Lázaro, Olga Bueno, Juan F. León, Jesús Mª Garagorri, Manuel Bueno, Juan Pablo Rey López, Iris Iglesia, Paula Velasco, Silvia Bel.
2. Consejo Superior de Investigaciones Científicas (Spain): Ascensión Marcos, Julia Wärnberg, Esther Nova, Sonia Gómez, Esperanza Ligia Díaz, Javier Romeo, Ana Veses, Mari Angeles Puertollano, Belén Zapatera, Tamara Pozo.
3. Université de Lille 2 (France): Laurent Beghin, Christian Libersa, Frédéric Gottrand, Catalina Iliescu, Juliana Von Berlepsch.
4. Research Institute of Child Nutrition Dortmund, Rheinische Friedrich-Wilhelms-Universität Bonn (Germany): Mathilde Kersting, Wolfgang Sichert-Hellert, Ellen Koeppen.
5. Pécsi Tudományegyetem (University of Pécs) (Hungary): Dénes Molnar, Eva Erhardt, Katalin Csernus, Katalin Török, Szilvia Bokor, Mrs. Angster, Enikö Nagy, Orsolya Kovács, Judit Repásy.
6. University of Crete School of Medicine (Greece): Anthony Kafatos, Caroline Codrington, María Plada, Angeliki Papadaki, Katerina Sarri, Anna Viskadourou, Christos Hatzis, Michael Kiriakakis, George Tsibinos, Constantine Vardavas Manolis Sbokos, Eva Protoyeraki, Maria Fasoulaki.
7. Institut für Ernährungs- und Lebensmittelwissenschaften –Ernährungphysiologie. Rheinische Friedrich Wilhelms Universität (Germany): Peter Stehle, Klaus Pietrzik, Marcela González-Gross, Christina Breidenassel, Andre Spinneker, Jasmin Al-Tahan, Miriam Segoviano, Anke Berchtold, Christine Bierschbach, Erika Blatzheim, Adelheid Schuch, Petra Pickert.
8. University of Granada (Spain): Manuel J. Castillo, Ángel Gutiérrez, Francisco B. Ortega, Jonatan R Ruiz, Enrique G. Artero, Vanesa España-Romero, David Jiménez-Pavón, Palma Chillón.
9. Istituto Nazionale di Ricerca per gli Alimenti e la Nutrizione (Italy): Davide Arcella, Elena Azzini, Emma Barrison, Noemi Bevilacqua, Pasquale Buonocore, Giovina Catasta, Laura Censi, Donatella Ciarapica, Paola D'Acapito, Marika Ferrari, Myriam Galfo, Cinzia Le Donne, Catherine Leclercq, Giuseppe Maiani, Beatrice Mauro, Lorenza Mistura, Antonella Pasquali, Raffaela Piccinelli, Angela Polito, Raffaella Spada, Stefania Sette, Maria Zaccaria.
10. University of Napoli “Federico II” Dept of Food Science (Italy): Luca Scalfi, Paola Vitaglione, Concetta Montagnese.
11. Ghent University (Belgium): Ilse De Bourdeaudhuij, Stefaan De Henauw, Inge Huybrechts, Tineke De Vriendt, Lea Maes, Christophe Matthys, Carine Vereecken, Mieke de Maeyer, Charlene Ottevaere.
12. Medical University of Vienna (Austria): Kurt Widhalm, Katharina Phillipp, Sabine Dietrich, Birgit Kubelka Marion Boriss-Riedl.
13. Harokopio University (Greece): Yannis Manios, Eva Grammatikaki, Zoi Bouloubasi, Tina Louisa Cook, Sofia Eleutheriou, Orsalia Consta, George Moschonis, Ioanna Katsaroli, George Kraniou, Stalo Papoutsou, Despoina Keke, Ioanna Petraki, Elena Bellou, Sofia Tanagra, Kostalenia Kallianoti, Dionysia Argyropoulou, Katerina Kondaki, Stamatoula Tsikrika, Christos Karaiskos.
14. Institut Pasteur de Lille (France): Jean Dallongeville, Aline Meirhaeghe.
15. Karolinska Institutet (Sweden): Michael Sjöstrom, Patrick Bergman, María Hagströmer, Lena Hallström, Mårten Hallberg, Eric Poortvliet, Julia Wärnberg, Nico Rizzo, Linda Beckman, Anita Hurtig Wennlöf, Emma Patterson, Lydia Kwak, Lars Cernerud, Per Tillgren, Stefaan Sörensen.
16. Asociación de Investigación de la Industria Agroalimentaria (Spain): Jackie Sánchez-Molero, Elena Picó, Maite Navarro, Blanca Viadel, José Enrique Carreres, Gema Merino, Rosa Sanjuán, María Lorente, María José Sánchez, Sara Castelló.
17. Campden & Chorleywood Food Research Association (United Kingdom): Chantal Gilbert, Sarah Thomas, Elaine Allchurch, Peter Burguess.
18. SIK - Institutet foer Livsmedel och Bioteknik (Sweden): Gunnar Hall, Annika Astrom, Anna Sverkén, Agneta Broberg.
19. Meurice Recherche & Development asbl (Belgium): Annick Masson, Claire Lehoux, Pascal Brabant, Philippe Pate, Laurence Fontaine.
20. Campden & Chorleywood Food Development Institute (Hungary): Andras Sebok, Tunde Kuti, Adrienn Hegyi.
21. Productos Aditivos SA (Spain): Cristina Maldonado, Ana Llorente.
22. Cárnicas Serrano SL (Spain): Emilio García.
23. Cederroth International AB (Sweden): Holger von Fircks, Marianne Lilja Hallberg, Maria Messerer.
24. Lantmännen Food R&D (Sweden): Mats Larsson, Helena Fredriksson, Viola Adamsson, Ingmar Börjesson.
25. European Food Information Council (Belgium): Laura Fernández, Laura Smillie, Josephine Wills.
26. Universidad Politécnica de Madrid (Spain): Marcela González-Gross, Jara Valtueña, David Jiménez-Pavón, Ulrike Albers, Raquel Pedrero, Agustín Meléndez, Pedro J. Benito, Juan José Gómez Lorente, David Cañada, Alejandro Urzanqui, Juan Carlos Ortiz, Francisco Fuentes, Rosa María Torres, Paloma Navarro.
Annex Blood sampling calendar: number of subjects (age range) per city, during the sampling period










