The vitamin A equivalency of β-carotene is defined as the amount of β-carotene which is required in the diet to produce 1 μg retinol in the body. According to the current views, 6 μg(1, 2) or 12 μg(3) of β-carotene in a mixed diet are equivalent to 1 μg dietary retinol. For supplemental β-carotene in oil, the current views are that 3·3 μg(1, 2) or 2 μg(3) of β-carotene are equivalent to 1 μg retinol.
Data concerning the vitamin A equivalency of β-carotene from various dietary sources are inconsistent. The way β-carotene is incorporated in dietary matrices (plant foods or dissolved in oil) influences the degree of absorption. Uptake into the enterocyte is the critical step in the bioconversion of β-carotene, since it is generally assumed that 2 μg β-carotene in the enterocyte is equivalent to 1 μg retinol in the body(2, 3). In order to quantify how much β-carotene enters the enterocyte, stable-isotope techniques have been developed in the last decade(Reference van Lieshout, West and van Breemen4, Reference Burri and Clifford5). However, these techniques and the studies in which they have been applied have reported conflicting results(Reference Wang, Yin and Zhoa6–Reference Edwards, Nguyen and You9).
Absorption studies performed with healthy subjects with an ileostomy have the advantage of excluding the possible effect of bacterial degradation or even synthesis in the large bowel of the nutrient under study, allowing accurate measurement of its apparent fractional absorption.
Unfortunately, absorption studies alone cannot quantify the vitamin A equivalency of β-carotene. Therefore in the present investigation, the extrinsic labelling technique and the classical oral–faecal balance technique were combined. In addition to the administered [13C10]retinol, [13C5]retinol, formed by central cleavage of administered [13C10]β-carotene, was measured in serum(Reference van Lieshout, West and Muhilal10, Reference Van Loo-Bouwman, West and van Breemen11). The efficiency of absorption of retinol is generally expected to be over 90 % in healthy subjects(2, 3).
The aim of the present 14 d cross-over diet-controlled study was to quantify the vitamin A equivalency of β-carotene and to measure the apparent β-carotene absorption in healthy adults with an ileostomy with an adequate vitamin A status. Subjects were given two types of controlled Western diets: an ‘oil diet’ which contained mainly supplemental β-carotene in oil as the source of β-carotene and a ‘mixed diet’ which contained mainly β-carotene from vegetables and fruits.
Subjects and methods
Recruitment of subjects
Subjects with an ileostomy aged 23–75 years from four hospitals in the surroundings of Nijmegen in The Netherlands were selected for participation. Their general health status was checked in their medical dossiers. The screening examination included a health and lifestyle questionnaire, a FFQ(Reference Feunekes, van Staveren and de Vries12), weight (precise to 0·1 kg) and height measurement (precise to 0·5 cm), and haematological analyses of blood, liver enzymes, creatinine, alkaline phosphatase and cholesterol. Exclusion criteria were as follows: routine clinical chemistry abnormalities, chronic diseases, ileal resection of>15 cm, medication suspected of interfering with fat-soluble-vitamin absorption, pregnancy, BMI < 18 or>30 kg/m2, abnormal dietary pattern, excessive alcohol consumption (>40 g/d), and consumption of carotenoids, vitamin, or mineral supplements 6 weeks before or during the study. A total of eighteen volunteers participated in the screening and were selected to form two groups, which were matched for sex, age, BMI and habitual energy intake. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Committee on Research Involving Human Subjects, Region Arnhem-Nijmegen, The Netherlands. Written informed consent was obtained from all subjects.
Study design
The study was designed as a cross-over intervention with two controlled diets in eighteen healthy ileostomy subjects. Each subject followed both diets for 2 weeks each. The subjects consumed capsules each day for 4 weeks during both diets. The capsules contained a mean of 190 μg/d [13C10]β-carotene and 195 μg/d [13C10]retinyl palmitate in oil relative to their daily energy intake. The extrinsic dual-isotope-labelling technique is based on attaining a plateau of isotopic enrichment of β-carotene and retinol in serum during prolonged daily intake of capsules containing low doses of labelled β-carotene and labelled retinol(Reference van Lieshout, West and Muhilal10, Reference Van Loo-Bouwman, West and van Breemen11). The plateau has been reported to be reached by day 21(Reference van Lieshout, West and Muhilal10, Reference Van Loo-Bouwman, West and van Breemen11), and in preliminary investigations for the present study, it was shown that the plateau was reached by day 14. On days 0, 1, 14, 15, 27 and 28 fasting blood samples of 13 ml were obtained, and then kept in the dark at 4°C for 30 min before being centrifuged at 3000 rpm for 10 min at 4°C to separate cells from serum. Serum was stored at − 80°C until analysis. Fasting was defined as not consuming any food or energy-containing drinks for 12 h before the blood sampling. On days 13 and 14 and also on days 27 and 28, complete 48 h ileostomy effluent (faeces) was collected at home, stored on dry ice in plastic containers, and then transported to the − 80°C freezer. The concentrations of carotenoids and retinol in duplicate diets, serum and in faeces were measured by HPLC, and the isotopic enrichments of serum and faecal retinol with [13C5]retinol and [13C10]retinol and of β-carotene with [13C10]β-carotene were measured by using liquid chromatography–MS (LC–MS).
Diets and compliance
One diet contained vegetables low in β-carotene content with supplemental β-carotene in salad dressing oil (‘oil diet’) and the other diet contained vegetables high in β-carotene content (‘mixed diet’). The ratio of β-carotene provided by fruits to vegetables was 1 to 2·2 in the ‘oil diet’ and 1 to 8·4 in the ‘mixed diet’ as estimated from the Dutch food database(13). Low-β-carotene fruits included a mix of orange, apple, kiwi and banana. Menus were designed for ten levels of energy intake ranging from 7 to 16 MJ/d. The subjects were allocated to an energy intake level close to their habitual energy intake, which was estimated from a FFQ(Reference Feunekes, van Staveren and de Vries12). Both diets were designed according to the Dutch guidelines for a healthy diet(14) and provided 90 % of energy intake. All food was weighed out for each subject. The remaining 10 % of energy had to be chosen from a list of low-fat food items, which did not contain carotenoids or retinol, and which had to be recorded in a diary. The diaries were inspected twice weekly. Body weight was recorded twice weekly and energy intake was adjusted, when necessary, to limit changes in weight to less than 2 kg. The food had to be reheated in a microwave oven at home. With each hot meal, a salad with salad dressing was provided. The salad dressing for the ‘oil diet’ was supplemented with synthetic β-carotene (all-trans β-carotene, 30 % suspension in vegetable oil; Hoffmann-La Roche, Basle, Switzerland). The margarine (Unilever, Rotterdam, The Netherlands) was not supplemented with retinol or β-carotene. Subjects kept a diary for monitoring compliance to the diet, to the intake of the capsules, to fasting instructions, and to ileostomy effluent collection. In the diary, illnesses, medication and the daily choice of low-fat food items had to be recorded.
Diets were homogenised, extracted and analysed in duplicate by HPLC as described previously(Reference Van Loo-Bouwman, West and van Breemen11).
Chemical analysis of retinol and carotenoids in serum and in faeces
Retinol and carotenoids in serum were analysed using the HPLC method with absorbance detection as described previously(Reference Van Loo-Bouwman, West and van Breemen11). Within- and between-run CV for the quantitative analysis of retinol in serum were 1·1 and 3·8 % and were 6·1 and 9·2 % for the quantitative analysis of β-carotene.
Ileostomy effluent of 48 h from each subject were pooled, homogenised and weighed. Sample preparation was carried out in duplicate and has been described previously(Reference Van Loo-Bouwman, West and van Breemen11). The recovery of β-carotene was 86 (sd 6) % (n 3) which was measured by spiking faeces samples with β-carotene. Within- and between-run CV for the chemical analysis of β-carotene in faeces were 3·9 and 6·2 %.
Capsule administration and measurement of isotopic enrichment in serum and in faeces
Three capsules were prepared to meet the proportion of labelled compounds to the daily level of energy intake. The capsules contained 65·1, 82·8 or 98·0 μg [12,13,14,15,20,12′,13′,14′,15′,20′-13C10]β-carotene and 61·7, 83·2 or 102·1 μg [8,9,10,11,12,13,14,15,19,20-13C10]retinyl palmitate in oil (analysed values). The preparation of the capsules and β-carotene analyses were performed as described previously(Reference Van Loo-Bouwman, West and van Breemen11). The [13C10]β-carotene and [13C10]retinyl palmitate were synthesised at ARC Laboratories (Apeldoorn, The Netherlands) as described previously(Reference Lugtenburg, Creemers and Verhoeven15). The ratios of unlabelled retinol, [13C5]retinol, [13C10]retinol, unlabelled β-carotene and [13C10]β-carotene in serum and in faeces were measured using LC–atmosphere pressure chemical ionisation–MS assay (LC-APCI-MS)(Reference van Breemen, Nikolic and Xu16, Reference Zhu, Wang and Pang17). β-Carotene and [13C10]β-carotene were monitored in negative ion mode at m/z 536 and m/z 546, respectively(Reference Zhu, Wang and Pang17). Retinol, [13C5]retinol and [13C10]retinol were measured as [MH-H2O]+ ions in positive ion mode at m/z 269, m/z 274 and m/z 279, respectively(Reference Zhu, Wang and Pang17, Reference Wang, Xu and van Lieshout18).
Calculations
The isotopic enrichment levels of β-carotene were calculated as the signal measured by LC–MS at m/z 546 divided by the total signal at m/z 536 and 546. The isotopic enrichment levels of retinol were calculated as the signal at m/z 274 (or 279) divided by the total signal at m/z 269, 274 and 279. The vitamin A equivalency of β-carotene in oil relative to that of retinol in oil was calculated for each subject as the inverse ratio of the dose-corrected ratio of [13C5]retinol to [13C10]retinol in serum (by wt as compared with 1 μg retinol) as follows(Reference van Lieshout, West and Wang19):
![1/(Enrichment\,of\,retinol\,in\,serum\,with\,[^{13}C_{5}]retinol/enrichment\,of\,retinol\,in\,serum\,with\,[^{13}C_{10}]retinol)\times (dose\,of\,[^{13}C_{10}]retinol/dose\,of\,[^{13}C_{10}]\beta - carotene).](https://static.cambridge.org/binary/version/id/urn:cambridge.org:id:binary:20160920223829458-0503:S0007114509993849:S0007114509993849_eqnU1.gif?pub-status=live)
For each diet, the apparent absorption (%) of total β-carotene (both labelled and unlabelled) was calculated for each subject by subtracting the amount of β-carotene in faeces (48 h) from the amount consumed (48 h) and dividing the difference by the amount consumed multiplied by 100. A necessary condition to use this calculation is to standardise strictly the daily nutrient intake during the 2-week intervention study.
Design and statistical analysis
A cross-over design was chosen to eliminate between-subject variation. A carry-over effect was unlikely, because in preliminary investigations, it was shown that isotopic enrichment levels were under the detection limit after 7 d after intervention. Based on previous data(Reference Van Loo-Bouwman, West and van Breemen11), a sample size of seventeen subjects had a 80 % power to detect a difference in vitamin A equivalency between treatments of 0·27 with a significance level (α) of 0·05 (two-sided). Data are shown as means and 95 % CI or standard deviations (in the case of descriptive measures). Data of serum concentrations and enrichments were averaged for days 0 and 1, for days 14 and 15, and for days 27 and 28 for each subject. To evaluate differences in baseline blood values between the two groups, t tests for independent samples were performed. ANOVA was used to test order effects. Because the order of the two diets did not significantly contribute to the model (P = 0·23), serum retinol and serum β-carotene concentrations at baseline and after each diet were compared between the two dietary treatments with a paired t test. To test whether the percentages of apparent absorption were significantly different between both diets, paired t tests were performed. All tests were two-sided, and P values < 0·05 were considered significant. The computer package SPSS 12.0 (SPSS Inc., Chicago, IL, USA) and Microsoft Office Excel 2003 (Microsoft Corp., Redmond, WA, USA) were used for all statistical calculations and data handling.
Results
The baseline characteristics of the seventeen subjects who completed the study are shown in Table 1. One individual dropped out because of not following the diet. No significant differences were observed between both groups with respect to haematological analyses of blood, liver enzymes, creatinine, alkaline phosphatase and cholesterol. The reported compliance to the diets was very good: 99·4 % of the capsules and 97 % of the diet. Inspection of the diaries did not reveal any deviations from the protocol, which could have affected the results.
Table 1 Characteristics of the seventeen ileostomy subjects at baseline*
(Mean values and standard deviations)

* Groups 1 and 2 were matched for sex, age, BMI and habitual energy intake. There were no differences between the groups (two-tailed t tests for independent samples). Medication that is suspected of interfering with fat-soluble-vitamin absorption was not allowed and not used.
The daily energy and nutrient contents of the diets are given in Table 2, and the dietary sources of β-carotene in the diets are divided into the salad dressing oil and the vegetables and fruits. Table 3 shows the serum concentrations of retinol and provitamin A carotenoids of the subjects during the study. There were no significant differences between the groups in terms of serum retinol and serum β-carotene concentrations after each diet period compared with baseline. No significant post-intervention inter-diet differences for either retinol or β-carotene concentrations in serum were found.
Table 2 Total daily intake of energy, macronutrients, fibres, retinol and provitamin A carotenoids of two controlled diets during the 4-week cross-over intervention study*
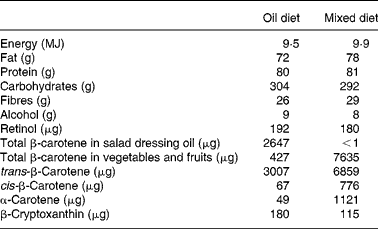
* Results are based on the analysis of typical 11 MJ duplicate diets and the calculated composition(13) of the consumed free items, which did not contain carotenoids or retinol.
Table 3 Serum concentrations (μmol/l) of retinol and provitamin A carotenoids of two consecutive days of collecting fasting blood samples at baseline and after 2 weeks of controlled diets*
(Mean values and standard deviations)
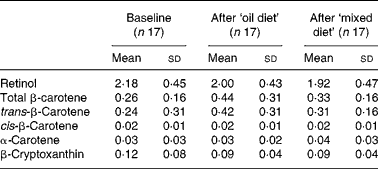
* There were no differences between the groups at baseline, after the ‘oil diet’, and after the ‘mixed diet’ and no differences between the measurement time points (paired t tests). Group 1 (n 9) followed for 2 weeks the ‘oil diet’ diet and consecutively for 2 weeks the ‘mixed diet’ and group 2 (n 8) followed for 2 weeks the ‘mixed diet’ and consecutively for 2 weeks the ‘oil diet’.
The isotopic enrichments of retinol and β-carotene in serum are shown in Table 4. For each subject, the vitamin A equivalency of [13C10]β-carotene in oil was calculated. The mean vitamin A equivalency of [13C10]β-carotene in oil was 3·71 (95 % CI 2·79, 4·63) μg (CV 49 %) in response to the ‘oil diet’ and 3·56 (95 % CI 2·86, 4·26) μg (CV 40 %) in response to the ‘mixed diet’.
Table 4 Vitamin A equivalency of [13C10]β-carotene in oil after 2 weeks of controlled diets*
(Mean values and 95 % confidence intervals)

* The ‘oil diet’ contained vegetables low in β-carotene content supplemented with synthetic β-carotene in salad dressing oil and the ‘mixed diet’ contained vegetables high in β-carotene content. Each subject followed both diets for 2 weeks in a cross-over design. All 4 weeks, each subject daily consumed capsules with [13C10]β-carotene and [13C10]retinol in oil.
† AEq,C is the amount of β-carotene that has the same vitamin A activity as 1 μg retinol and was calculated by 1/((E5,sR/E10,sC) × (AR,cap × 286/AC,cap × 537)).
Oral–faecal balance data demonstrate an apparent fractional absorption of β-carotene of 1·9-fold higher from the ‘oil diet’ (30 %) than from the ‘mixed diet’(16 %) (Table 5). With the oral–faecal balance data and the generally assumed bioconversion of 50 % for absorbed β-carotene(2, 3), the vitamin A equivalency of the unlabelled β-carotene was estimated as 6·7:1 (1/(0·30 × 0·5)) for the ‘oil diet’ and as 12·5:1 (1/(0·16 × 0·5)) for the ‘mixed diet’. Neither labelled retinol nor unlabelled retinol was detected in faeces.
Table 5 Apparent absorption of total β-carotene (labelled and unlabelled) from 48 h after 2 weeks of controlled diets†
(Mean values and standard deviations)
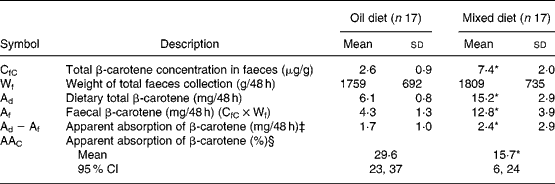
* Mean value was significantly different from that on the oil diet (P < 0·001; paired t test).
† The ‘oil diet’ contained vegetables low in β-carotene content supplemented with synthetic β-carotene in salad dressing oil and the ‘mixed diet’ contained vegetables high in β-carotene content. Each subject followed both diets for 2 weeks in a cross-over design. Daily for 4 weeks, each subject consumed capsules with labelled [13C10]β-carotene in oil.
‡ Two subjects had a negative oral–faecal total β-carotene balance for the ‘mixed diet’.
§ AAC was calculated by oral–faecal balance (48 h) as follows: (Ad − Af)/Ad × 100.
Discussion
Vitamin A equivalency of [13C10]β-carotene in oil using data obtained with a dual-isotope dilution technique
Our data in these healthy adults with an ileostomy show that the average vitamin A equivalency of extrinsic labelled β-carotene in oil is 3·6:1 regardless of dietary matrices differences. This finding is consistent with the vitamin A equivalency of β-carotene of 3·3:1 of FAO/WHO(1, 2). The vitamin A equivalencies of β-carotene were similar for both diets, mainly because the enrichment of retinol in serum with [13C5]retinol and with [13C10]retinol were similar for both diets (Table 4). It appears that labelled β-carotene added to the diet in oily capsules did not exchange with β-carotene in plant cells in the intestinal lumen. As a consequence, the assumption of this method, that labelled and unlabelled β-carotene fully mix, should be rejected. This isotopic technique cannot show that the dietary matrix affects the vitamin A equivalency of β-carotene; however, the oral–faecal balance technique does show that the dietary matrix affects the vitamin A equivalency of dietary β-carotene. This indicates that the extrinsic dual-isotope dilution technique (adding a tracer in oil capsules to the diet) with the current calculation is not suitable to investigate the absorption of β-carotene from plant matrices. Thus, the measured vitamin A equivalency of labelled β-carotene of 3·6:1 represented the highest feasible vitamin A equivalency, because the β-carotene was delivered to the intestine in the most optimal form, a solution in oil in a capsule, which dissolved in the stomach.
A preceding study with twenty-four healthy adults in The Netherlands showed a very similar vitamin A equivalency of β-carotene to retinol of 3·4:1(Reference Van Loo-Bouwman, West and van Breemen11), which is comparable with the equivalency in the present study. This indicates that the vitamin A equivalency of β-carotene in healthy ileostomy subjects does not differ from that of healthy adults with an intact gastrointestinal tract.
Two other studies in Indonesian children(Reference van Lieshout, West and Muhilal10, Reference van Lieshout, West and Wang19) with the same isotope technique showed vitamin A equivalencies of β-carotene to retinol of 2·7:1 and 2·4:1, respectively. These studies in a developing country were not diet-controlled, and the children did not have an optimal vitamin A status. Therefore, the present results are consistent with the expectation that the vitamin A equivalency of β-carotene could be lower in those with lower needs(1).
In 2005, two studies were published that used intrinsic labelling of vegetables to quantify the vitamin A equivalency of β-carotene(Reference Novotny, Kurilich and Britz20, Reference Tang, Qin and Dolnikowski21). Novotny et al. (Reference Novotny, Kurilich and Britz20) produced complete 13C-labelled kale and calculated 28 d serum areas under the curve but did not estimate the vitamin A equivalency of β-carotene. Tang et al. (Reference Tang, Qin and Dolnikowski21) produced two 2H-labelled vegetables and showed a vitamin A equivalency of β-carotene from spinach of 21:1 and of β-carotene from carrot of 15:1 calculated from 35 d serum areas under the curve, which are lower than we found. However, our isotope technique measured the plateau at 2 weeks after a diet with a mix of vegetables and fruits.
Strengths and limitations of the extrinsic labelling technique
The advantage of intrinsic labelling is that labelled β-carotene is contained within the food matrix instead of being added to or co-administered with food. However, intrinsic labelling has some significant limitations; these specially produced vegetables are very expensive, few subjects have been able to participate in the published studies and the vitamin A equivalency of β-carotene was determined after only a single meal. Furthermore, the vitamin A equivalency of β-carotene determined using intrinsic labelling might vary depending upon the plant and how it is prepared. Therefore, while the intrinsic labelling technique could provide reliable data for a few individuals eating a specific vegetable, it cannot provide data for a large population consuming a varied diet. Our extrinsic labelling technique can measure the vitamin A equivalency of the labelled β-carotene in oil capsules accurately but not the effect of dietary matrices.
Estimated vitamin A equivalency of dietary β-carotene using data obtained with the oral–faecal balance technique
Using data obtained with the oral–faecal balance technique, we observed that supplemental β-carotene in the ‘oil diet’ is an approximately 1·9 times better source of β-carotene (and thus vitamin A) than β-carotene in a ‘mixed diet’. We cannot exclude the possibility that the lower apparent absorption in the ‘mixed diet’ is attributable to the higher absolute amount of β-carotene ingested. The ‘oil diet’ was representative of a diet high in β-carotene-poor vegetables and fruits with consumption of food items fortified with β-carotene and/or β-carotene supplements, such as regularly consumed in industrialised societies. The ‘oil diet’, which still contained about 15 % β-carotene from vegetables and fruits (Table 2), had an estimated vitamin A equivalency of β-carotene of 6·7:1.
According to the current mixed-diet guideline of the US Institute of Medicine(3), the vitamin A equivalency of β-carotene is 12:1, which is similar to our estimation for the ‘mixed diet’ (12·5:1). In the present study, the ‘mixed diet’ represented a healthy diet with amounts of cooked vegetables and fruits high in β-carotene content and including all necessary nutrients and fibres for optimal health.
When using the oral–faecal balance technique, the apparent absorption of β-carotene in healthy adults with an intact large bowel will probably always be overestimated because of bacterial degradation of β-carotene in the large bowel. The apparent absorption of β-carotene from both diets in these seventeen ileostomy subjects approaches apparent absorption of β-carotene in healthy adults, even with the shorter gastrointestinal transit time in the small intestine resulting from the surgical removal of the ileocaecal valve. Because of this fast transit, the collected ileostomy effluent over 48 h is representative for the dietary intake during this period, which is in contrast to adults with an intact gastrointestinal tract because of a variable residence time in the large bowel. Bacterial degradation in the ileostomy bag might still occur; however, the subjects were instructed to empty the bag into a box containing dry ice to optimise the integrity of β-carotene in the effluent.
Three studies have been published involving ileostomy subjects that all used mass balance over 24 h for measurement of apparent absorption of β-carotene. Faulks et al. (Reference Faulks, Hart and Wilson22) showed an apparent absorption of 90 % (range 74–97 %) in five ileostomy subjects after the consumption of 13C-labelled β-carotene in oil (dose 10 mg). Faulks et al. (Reference Faulks, Hart and Brett23) showed an apparent absorption of β-carotene of 25 % (range 4–41 %) in seven ileostomy subjects after the consumption of spinach meals (β-carotene intake 10 mg). Livny et al. (Reference Livny, Reifen and Levy24) showed an apparent absorption of β-carotene of 65 (sd 7) % from cooked carrot meals and 41 % ( ± 7) from raw carrot meals in eight ileostomy subjects (β-carotene intake 15 mg). In the present study, in which seventeen ileostomy subjects participated, the daily intake of β-carotene was either 3·1 or 7·6 mg, and ileostomy effluent collections were over 48 h. Our data showed an apparent absorption of β-carotene of 30 % (range 11–53 %) in the ‘oil diet’ and 16 % (range 5–42 %) in the ‘mixed diet’. In each study, the inter-individual apparent absorption of β-carotene was highly variable. Nevertheless, these results indicate clearly that the vitamin A equivalency of β-carotene from various dietary matrices is dependent on the release of β-carotene from vegetable foods. A factor of 1·9 between the intestinal absorption of β-carotene dissolved in oil and β-carotene in vegetable foods appears to be realistic.
An earlier study of twenty-four healthy adults with similar diets showed a factor of 2·9 between apparent absorption of β-carotene in oil and β-carotene in vegetable foods(Reference Van Loo-Bouwman, West and van Breemen11). The difference can be explained by intra-individual variation in faecal β-carotene and a much higher degree of bacterial degradation in the adults with an intact gastrointestinal tract.
Serum β-carotene concentrations
The serum β-carotene concentrations in these ileostomy subjects were three times lower than in the healthy adults in the previous study(Reference Van Loo-Bouwman, West and van Breemen11). Also serum α-carotene and serum β-cryptoxanthin concentrations in the ileostomy subjects were strikingly low. It should be noted that the serum retinol concentrations in these subjects were much greater than 1·07 μmol/l, which excludes vitamin A deficiency.
An explanation of the low serum β-carotene concentrations at baseline in these ileostomy subjects could be the somewhat lower absorption of fat because of faster transit compared with subjects with an intact gastrointestinal tract and the relatively low consumption of vegetables and fruits because of the possibility of temporary blockage of the ileostomy. Based on the FFQ, which were completed before the present study to assess their daily habitual energy intake, these ileostomy subjects consume a mean of 2·7 servings of vegetables and 1·1 pieces of fruit. Their daily fibre consumption was 3·5 g lower than the habitual fibre consumption of the average Dutch adult (mean of 18·1 v. 22·5 g, taking into account the age group and sex)(25). During the present study, the subjects consumed about 28 g fibre (Table 2), and indeed, their serum β-carotene concentrations increased compared with baseline by consumption of more vegetable foods than their habitual intake. The influence of serum β-carotene concentrations (which also decrease with ageing) on the vitamin A equivalency of β-carotene should be assessed in future research.
Conclusion
Our oral–faecal balance data of seventeen healthy ileostomy subjects consuming a Western diet showed that the apparent absorption from supplemental β-carotene in oil was approximately 1·9-fold higher than of β-carotene from vegetables and fruits. Our isotopic data showed that the vitamin A equivalency of [13C10]β-carotene in oil was 3·6:1 regardless of a high amount of unlabelled β-carotene either in an oil or vegetable matrix.
Acknowledgements
We kindly thank the volunteers who participated in the present study; Marjo Peters, Rianne Verhof, Ellen Geerards and Ellen Rasmussen of the Division of Dietetics for their advice and support; Marco Waals and all other kitchen staff members in the hospital for their cooperation; Pieter Versloot and Tineke van Roekel-Jansen of the Division of Human Nutrition for their help; Karin Bourgonjen, Janneke Dopheide, Celine Brattinga, Marlies Regelink and Lillian van Nispen for their assistance during the intervention study; and all other individuals who contributed to this research. The present study was conducted under ClinicalTrials.gov identifier NCT00128804. The present study was financially supported by the Dutch Dairy Association.
C. A. vL.-B., T. H. J. N. and C. E. W. designed the study and H. D., E. S., P. J. M. H., F. G. M. R., and G. S. provided significant advice and consultation. R. B. vB. and D. Z. performed the enrichment analyses and P. J. M. H. was responsible for the carotenoid analyses. H. D. and E. S. coordinated the preparation and distribution of the diets. C. A. vL.-B. was in charge of the data collection and analysed the data. T. H. J. N., F. G. M. R., and G. S. assisted with the calculations. C. A. v L.-B. wrote the manuscript and all authors, except C. E. W. (deceased), provided a critical review of the manuscript.
None of the authors had a personal or financial conflict of interest.







