Olive oil is the primary source of fat in the Mediterranean diet (MedDiet), which is associated with a low mortality for CVD(Reference Estruch, Ros and Salas-Salvadó1). In spite of this, data concerning olive oil consumption and primary end points for CVD, as well as for total mortality, have only been provided recently. Results of large EPIC (European Prospective Investigation into Cancer and Nutrition) cohorts have shown an inverse relationship between olive oil consumption and CHD mortality and incidence(Reference Buckland, Mayén and Agudo2–Reference Bendinelli, Masala and Saieva4). Also, results of the Three-City Study have shown an inverse relationship between olive oil consumption and stroke risk in women(Reference Samieri, Féart and Proust-Lima5). Recent results of the PREDIMED (Prevention by Mediterranean Diet) study have shown that consumption of extra-virgin olive oil (VOO), within the frame of the MedDiet, reduces the risk of atrial fibrillation in elder, high cardiovascular risk individuals(Reference Martínez-Gonzalez, Toledo and Arós6). A large body of knowledge exists providing evidence of the benefits of olive oil consumption on secondary end points for CVD. Olive oil consumption has proven to promote benefits on lipid profile, insulin sensitivity, lipid and DNA oxidation, inflammation, endothelial function, thrombotic factors and blood pressure(Reference López-Miranda, Pérez-Jiménez and Ros7) (Fig. 1). Due to this, on November 2004, the Federal Drug Administration of the USA permitted a claim on olive oil labels concerning: ‘the benefits on the risk of CHD of eating about two tablespoons (23 g) of olive oil daily, due to the monounsaturated fat (MUFA) in olive oil’. To achieve this possible benefit, olive oil is to replace a similar amount of saturated fat and not increase the total number of calories you eat in a day’(8).
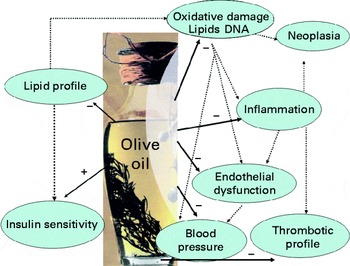
Fig. 1 Benefits of olive oil consumption on secondary end points for CVD. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
The major components of olive oil are fatty acids, of which the MUFA oleic acid represents from 55 to 83 % of the total fatty acids, PUFA from 4 to 20 % and SFA from 8 to 14 %. The minor components of olive oil constitute from 1 to 2 % of the total content of an olive oil. They are classified into two types: (1) the unsaponifiable fraction that could be extracted with solvents after the saponification of the oil and contains squalene and other triterpenes, sterols, tocopherols and pigments; (2) the soluble fraction that includes the phenolic compounds, which are the most studied and best-known components in terms of their benefits for health(Reference Covas, Ruiz-Gutiérrez and de la Torre9). The content of the minor components of an olive oil varies depending on the cultivar, climate, ripeness of the olives at harvesting and the processing system used to produce the olive oil. Olive oils currently present in the market are extra-virgin, virgin, olive oil (EU regulation 2568/1991) or pomace olive oil(Reference Gimeno, Castellote and Lamuela-Raventós10). VOO are obtained from the fruit of the olive tree solely by mechanical or other physical means under conditions that do not lead to alteration in the oil. They have not undergone any treatment other than washing, decantation, centrifugation or filtration. Oils obtained using solvents, adjuvant, having a chemical or biochemical action, re-esterification process or any mixture with oils of other kinds are excluded from this category(11). Extra-VOO are VOO with a free acidity, expressed as g of oleic acid/100 g of olive oil, less than 0·8 g. VOO with an acidity greater than or equal to 3·3 (International Olive Oil Council Regulation/T.15/NC.n3.Rev2.Nov24, 2006) or greater than 2 in Europe (European Regulation No. 1513/0) are submitted to a refining process in which some components, mainly phenolic compounds, and to a lesser degree squalene, are lost(Reference Owen, Mier and Giacosa12). By mixing virgin and refined olive oil, an ordinary olive oil (olive oil; EU regulation 2568/1991) is produced and marketed. After VOO production, the rest of the olive drupe and seed are submitted to a refining process, resulting in pomace olive oil, to which a certain quantity of VOO is added before marketing.
And, the key question is: What of the olive oils is the best when concerning nutritional purposes? With the data available at present, the response is extra-VOO (or extra-virgin olive oil) rich in phenolic compounds. In this review, we summarised the key work that has provided evidence of the benefits of VOO consumption over other types of edible oils, even olive oils. A literature review had been carried out in MEDLINE until August 2014. We searched for clinical studies assessing the effect of either acute (single dose) or sustained doses of VOO on the risk factors for CVD. Cohort studies that aimed to assess the relationship between olive oil consumption and end point for CVD were also included. Concerning the clinical trials, we refer to only randomised, controlled human studies, which are capable of providing the evidence of Level I(Reference Woolf, Battista and Anderson13). According to Evidence-Based Medicine, the evidence of Level I is that required to perform nutritional recommendations at population level. Other types of studies including those performed in cellular or animal models were beyond the scope of the present review and thus were not included in the present review.
Virgin olive oil, lipoproteins and lipoprotein oxidation
The results of a meta-analysis of fourteen studies have shown that replacement of SFA by oils enriched in MUFA v. PUFA had similar effects on total-, LDL- and HDL-cholesterol. The PUFA-enriched oil had a slight TAG-lowering effect, and there was an increase in HDL-cholesterol after MUFA consumption in some studies(Reference Gardner and Kraemer14). However, despite the fact that no great differences in lipoprotein content exist between the consumption of MUFA and PUFA, there is an issue in which consumption of MUFA prevails over the consumption of PUFA: the lipoprotein oxidation. Oleate-rich LDL are less susceptible against oxidation than linoleate-rich particles(Reference Reaven, Parthasarathy and Grasse15). This is due to the fact that PUFA are the key substrate for lipid peroxidation whose propagation chain is going on via the double bonds of the fatty acid(Reference Gutteridge16). From fourteen studies comparing the resistance of LDL to oxidation, only two studies have shown that MUFA-rich diets did not promote a higher resistance of LDL to oxidation than PUFA-rich diets(Reference Lapointe, Couillard and Lemieux17).
Oxidation of the lipid part, or directly of the apoB, of the LDL leads to a change in the lipoprotein conformation by which the LDL is better able to enter into the monocyte/macrophage system of the arterial wall and develop the atherosclerotic process(Reference Witzum18, Reference Steinberg, Parthasarathy and Carew19). Oxidised LDL has been shown to be independently associated with 10-year coronary artery disease events in general population, and improved the reclassification capacity of Framingham-derived coronary artery disease risk functions(Reference Gómez, Vila and Elosua20). In addition, oxidation of HDL reduces the HDL functionality by impairing cholesterol efflux from macrophages(Reference Girona, LaVille and Solà21). Oleic acid consumption has been shown to reduce in vivo HDL oxidation in humans(Reference Solà, La Ville and Richard22). Phenolic compounds present in VOO have proven to be protective against LDL oxidation. On November 2011, the European Food Safety Authority released a claim concerning the benefits of daily ingestion of olive oil rich in phenolic compounds, such as VOO. The Panel considers that, in order to bear the claim, 5 mg of hydroxytyrosol (OHTyr) and its derivatives (e.g. oleuropein complex and tyrosol (Tyr)) in olive oil should be consumed daily. These quantities, if provided by moderate amounts of olive oil, can be easily consumed in the context of a balanced diet(23). Here, we revise the main studies that supported this European Food Safety Authority claim concerning the benefits of polyphenol-rich olive oils.
Bioavailability of olive oil phenolic compounds in humans from real-life doses of natural olive oils
Although non-absorbable phenolic compounds could show local antioxidant activities in the gastrointestinal tract(Reference Ursini, Zamburlini and Cazzolato24). One of the prerequisites for assessing the physiological significance of olive oil phenolic compounds is the ability to determine their bioavailability in human beings. Tyr and OHTyr, the main phenolic compounds in olive oil as free or conjugated forms(Reference Gimeno, Castellote and Lamuela-Raventós10, Reference Owen, Mier and Giacosa12), are absorbed by humans in a dose-dependent manner with the phenolic content of the olive oil administered(Reference Covas, de la Torre and Farré-Albaladejo25). Despite a short half-life in the plasma (around 2·5 h)(Reference Miró-Casas, Covas and Farré26), both Tyr and OHTyr increase in plasma and urine in a dose-dependent manner after sustained olive oil consumption(Reference Marrugat, Covas and Fitó27, Reference Weinbrenner, Fitó and de la Torre28). This implies an accumulation in the body even from moderate doses of olive oil (25 ml/d), which are lower than the traditional daily dietary intake in Mediterranean countries(Reference Helsing29). The bioavailability of OHTyr, however, has been shown to change largely depending on the matrix in which the phenolic compounds are administered, the most effective matrix being the olive oil(Reference Visioli, Galli and Grande30). Around 98 % of Tyr and OHTyr are present in the plasma and urine in conjugated forms, glucuronoconjugates or sulphates. This fact indicates the existence of an extensive first-pass intestinal/hepatic metabolism of the ingested primary forms(Reference Miró-Casas, Covas and Farré26, Reference Kotronoulas, Pizarro and Serra31). Data from experimental models also point out to a dose-dependent metabolic disposition of OHTyr, the main metabolites being the glucuronates or sulphates at low and high doses of OHTyr, respectively(Reference Rubió, Valls and Macià32).
Human studies on the postprandial effect of olive oil on oxidative stress and oxidative damage
After meals, particularly rich in fats, a postprandial hyperlipaemia and hyperglycaemia occurs, which is linked to oxidative stress. Although fasting hyperlipidaemia is considered an important risk for CVD, postprandial serum lipid levels have been found to correlate more closely to CVD than fasting lipids(Reference Roche and Gibney33). Activation of PPARα suppresses postprandial lipidaemia through fatty acid oxidation in enterocytes(Reference Kimura, Takahashi and Murota34). A functional olive oil enriched with its own phenolic compounds has shown to enhance the gene expression of PPARα in mononuclear cells of pre-hypertensive and stage I hypertensive patients(Reference Farràs, Valls and Fernández-Castillejo35).
Several data on the effect of olive oil rich in phenolic compounds, such as VOO, on the postprandial oxidative stress have been reported. They are, however, difficult to compare because some studies do not mention whether or not postprandial lipaemia and/or hyperglycaemia occurs, which could lead to oxidative stress, after olive oil ingestion, while in other studies neither hyperlipaemia nor hyperglycaemia occurs at postprandial state after olive oil ingestion(Reference Covas, Ruiz-Gutiérrez and de la Torre9). Ingestion of a 25 ml olive oil dose did not promote postprandial oxidative stress independent of the phenolic content of the olive oil(Reference Weinbrenner, Fitó and Farré-Albaladejo36), whereas single doses of 40 ml(Reference Covas, de la Torre and Farré-Albaladejo25)and 50 ml did(Reference Fitó, Gimeno and Covas37). With olive oil doses at which oxidative stress occurs, data from randomised, cross-over, controlled human studies showed (1) an increase in the serum antioxidant capacity after VOO ingestion, but not after ordinary olive oil, in comparison with maize oil, suggesting a role for the phenolic compounds of the VOO(Reference Bogani, Galli and Villa38); and (2) the phenolic content of an olive oil modulates the degree of lipid and LDL oxidation, the lipid oxidative damage being lower after high- than after low-phenolic content olive oil(Reference Covas, de la Torre and Farré-Albaladejo25, Reference Ruano, López-Miranda and Fuentes39). In comparison with sunflower oil, meals, submitted to a deep frying, with olive oils rich in phenolic compounds, both natural and added, have shown to reduce the postprandial oxidative stress in obese people(Reference Pérez-Herrera, Rangel-Zuñiga and Delgado-Lista40). Recently, it has been described that food fried in extra-VOO improves postprandial insulin response, an oxidative stress-associated phenomenae, in obese, insulin-resistant women(Reference Farnetti, Malandrino and Luciani41).
Human studies of the sustained effect of olive oil on oxidative stress and oxidative damage
Controversial results have been obtained in short sample size (n< 30), randomised, controlled human studies performed up to now on the effect of sustained doses of olive oil phenolic compounds on oxidative stress(Reference Fitó, de la Torre and Covas42). It must be pointed out that extensive differences existed among the studies in the experimental design, control of diet, sample population, age of participants, measurement or not of markers of the compliance of the intervention, as well as in the sensitivity and specificity of the oxidative stress biomarkers evaluated.
The results of the EUROLIVE (The effect of olive oil consumption on oxidative damage in European populations) study, however, have provided final evidence of the in vivo protective role of phenolic compounds from olive oil on lipid oxidative damage in humans, at real-life olive oil doses(Reference Covas, Nyyssönen and Poulsen43). The EUROLIVE study was a large, cross-over, multi-centre, clinical trial performed in 200 individuals from five European countries. Participants were randomly assigned to receive 25 ml/d of three similar olive oils, but with differences in their phenolic content (from 2·7 to 366 mg/kg of olive oil), in intervention periods of 3 weeks preceded by 2-week washout periods. All olive oils increased the HDL-cholesterol and the ratio between the reduced and oxidised forms of glutathione. In the EUROLIVE study, consumption of medium- and high-phenolic content olive oil decreased lipid oxidative damage biomarkers such as plasma oxidised LDL, un-induced conjugated dienes and hydroxy fatty acids, without changes in F2-isoprostanes. However, the most important results of the EUROLIVE study were that an increase in HDL-cholesterol and a decrease in the lipid oxidative damage were linear with the phenolic content of the olive oil consumed. The results of the EUROLIVE study have provided the first level evidence that olive oil is more than a MUFA fat. Recent data from a subsample (n 990) of the PREDIMED study have shown that a MedDiet, only when enriched in VOO with high phenolic content (316 mg/kg), decreases the LDL oxidation in a significant manner compared with control group (low-fat diet)(Reference Fitó, Estruch and Salas-Salvadó44), confirming previous data obtained(Reference Fitó, Guxens and Corella45).
HDL lipoprotein protects LDL from oxidation(Reference Fogelman46). Also, oxidation of HDL impairs HDL functionality rendering the lipoprotein less useful for the cholesterol efflux from macrophages(Reference Girona, LaVille and Solà21, Reference Fogelman46). From the results of the EUROLIVE study, a key question stems: Could phenolic compounds in VOO, besides to increase HDL quantity, also increase HDL functionality? Few data are available at present for answering this question. In a linear (non-randomised, not control group) study, extra-VOO consumption improves the capacity of HDL to mediate cholesterol efflux and increases ATP-binding membrane cassette system (ATP binding cassette transporter-A1 (ABCA1) and ATP binding cassette transporter-G1 (ABCG1)) expression, one of the main mechanisms for HDL-mediated cholesterol efflux from macrophages(Reference Helal, Berrougui and Loued47). We recently performed a randomised, controlled clinical trial in which pre/hypertensive patients were assigned 30 ml of two similar olive oils with high (961 mg/kg, a functional VOO enriched with its phenolic compounds) and standard (289 mg/kg) phenolic content. The results indicate a significant role of olive oil polyphenols in the up-regulation of genes involved in the cholesterol efflux from cells to HDL in vivo in humans(Reference Farràs, Valls and Fernández-Castillejo35). The fact that olive oil phenolic compounds increase the functionality of human HDL has been recently provided from a subsample of the EUROLIVE study. High-phenolic content olive oil increased the HDL-mediated cholesterol efflux from macrophages compared with low-phenolic content olive oil(Reference Hernáez, Fernández-Castillero and Farràs48). Thus, data are promising, and further randomised, controlled studies are required to establish the role of VOO and its phenolic compounds on HDL functionality.
Concerning DNA oxidative damage, the urinary excretion of 8-oxo-deoxyguanosine is advocated as a biomarker of the whole-body DNA oxidation(Reference Poulsen49). Protective effects of olive oil phenolic compounds on in vivo DNA oxidation, measured as 8-oxo-deoxyguanosine in mononuclear cells and in urine, were found in healthy male subjects in a short-term study in which participants were submitted to a very low antioxidant diet(Reference Weinbrenner, Fitó and de la Torre28). A protective effect on DNA oxidation, measured by the comet assay in peripheral blood lymphocytes, was observed in postmenopausal women(Reference Salvini, Sera and Caruso50). Results of the EUROLIVE study, however, have shown that consumption of 25 ml of olive oil per day during 3 weeks reduced DNA oxidation in 182 healthy males, as measured by the 24 h urinary excretion of 8-oxo-deoxyguanosine, irrespective of the phenolic content in olive oil (Reference Machowetz, Poulsen and Gruendel51). Differences in the type of population involved (with or without oxidative stress) could explain the differences among the results. In this sense, one conclusion of the Consensus Report made by the Expert Panel in the International Conference of Olive Oil and Health held in Jaen, Spain, October 2004(8, Reference Pérez-Jimenez, Alvarez de Cienfuegos and Badimon52), was that the protective effects on oxidation markers in human trials were better displayed in oxidative stress conditions.
Oxidative stress is linked to other pathological conditions present in chronic degenerative diseases such as inflammation, endothelial dysfunction, hypertension, etc. Here, we revise the available information related to concerning the role of VOO on these issues.
Inflammation
Oxidative stress is linked to other pathological conditions present in chronic degenerative diseases such as inflammation, endothelial dysfunction, hypertension, etc. Here, we revise the available information related to concerning the role of VOO on these issues.
Although the protective mechanism of oleic acid-rich diets on inflammation has been attributed to a decrease in the LDL linoleic acid content, oleic acid is not the single responsible factor for the anti-inflammatory properties of olive oil. The minor components of olive oil have been shown to have anti-inflammatory, antihypertensive and anti-endothelial activation properties in experimental studies(Reference Perona, Cabello-Moruno and Ruiz-Gutıerrez53). Several studies have examined the anti-inflammatory and vasculoprotective effect of olive oil phenolic compounds in humans (Table 1). In these studies, VOO with high phenolic content has been shown to be effective in reducing the eicosanoid inflammatory mediators derived from arachidonic acid, such as thromboxane B2and 6-keto-PG F1α(Reference Bogani, Galli and Villa38, Reference Visioli, Caruso and Grande54, Reference Oubiña, Sanchez-Muniz and Rodenas55), as well as other inflammatory markers, such as high-sensitivity C-reactive protein or IL-6(Reference Fitó, Cladellas and de la Torre56, Reference Moreno-Luna, Muñoz-Hernandez and Miranda57). Concerning the effect on cell adhesion molecules, a decrease in serum intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 levels at postprandial state after VOO ingestion when compared with refined olive oil ingestion has been reported(Reference Pacheco, Bermúdez and Lopez58). Also, the postprandial inflammatory response after ingestion of heated oils in obese persons was reduced by the presence of oils with phenolic compounds or non-natural antioxidants(Reference Pérez-Herrera, Delgado-Lista and Torres-Sanchez59). In this study(Reference Pérez-Herrera, Delgado-Lista and Torres-Sanchez59), although it is not possible to differentiate the effect of MUFA to that of olive oil phenolics, VOO or a mix of sunflower and rapeseed oil artificially enriched with olive oil phenolic compounds and other antioxidants, mitigated postprandial inflammation (reduced NF-κB activation, increased IκB-α and decreased lipopolysaccharide plasma concentration), compared with sunflower oil. In sustained consumption studies, no differences in ICAM-1 or vascular cell adhesion molecule-1 levels were reported after sustained virgin or refined olive oil consumption in CHD patients(Reference Fitó, Cladellas and de la Torre56). However, a decrease in ICAM-1 level after VOO ingestion, but not after VOO plus epigallocatechin-3-galate ingestion, in early atherosclerosis patients has been reported(Reference Widmer, Freund and Flammer60). The effect of olive oil polyphenols modulating, towards a protective mode, the expression of pro-inflammatory genes is described in the Nutrigenomic Section.
Table 1 Randomised, controlled studies on the effect of virgin olive oil (VOO) on inflammatory markers

SFO, sunflower oil; TXB2, thromboxane B2; PRP, platelet-rich plasma; LTB4, leukotriene B4, ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; hs-CRP; high-sensitivity C-reactive protein; BP, blood pressure; ADMA, asymmetric dimethylarginine; MIF, macrophage migration inhibitory factor; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide.
Haemostasis, endothelial function and blood pressure
The assessment of different aspects of endothelial dysfunction in cardiovascular medicine has been the focus of intense research and includes vasomotor, haemostatic, antioxidant and inflammatory activities(Reference Shantsila, Wrigley and Blann61). The effect of VOO reducing oxidative stress and oxidative damage, as well as the inflammatory markers, has been described above. With respect to haemostatic factors, a huge body of work exists concerning the benefits of olive oil consumption reducing thrombogenesis through a decrease of coagulation factors and platelet aggregation(Reference Delgado-Lista, García-Rios and Pérez-Martínez62). The role of specific olive oil components on these effects is, however, still controversial(Reference Delgado-Lista, García-Rios and Pérez-Martínez62). In randomised, controlled human studies, the intake of phenol-rich VOO, compared with a low-phenol one, improved the postprandial prothrombotic profile in healthy subjects(Reference Pacheco, Lopez and Bermudez63) and hypercholesterolaemic patients(Reference Ruano, Lopez-Miranda and de la Torre64). The role of phenolic compound-rich oils compared with a low-phenolic content oils on thromboxane B2, an inflammatory mediator and potent platelet aggregation inductor, has been discussed above (Table 1). Several studies have reported the beneficial effects of VOO on the endothelial function. An improved post-ischaemic hyperaemia via reduced oxidative stress and increased NO metabolites was reported after the intake of phenol-rich VOO in comparison with a low-phenol olive oil in hypercholesterolaemic patients(Reference Ruano, López-Miranda and Fuentes39). The effect of VOO phenols on the postprandial endothelial function seems to be mediated by the NOS3 Glu298Asp polymorphism in patients with the metabolic syndrome(Reference Jiménez-Morales, Ruano and Delgado-Lista65). Beneficial effects improving the endothelial function have been observed after a 4-month diet with polyphenol-rich olive oil in patients with early atherosclerosis(Reference Widmer, Freund and Flammer60).
Consumption of olive oil is known to reduce blood pressure(Reference López-Miranda, Pérez-Jiménez and Ros7). However, phenolic compounds in VOO also play a beneficial role. A 2-month diet with olive oil rich in polyphenols decreased systolic (SBP) and diastolic blood pressure and improved endothelial function in young women with mild hypertension compared with the same diet with low-polyphenol content olive oil(Reference Moreno-Luna, Muñoz-Hernandez and Miranda57). In this study(Reference Moreno-Luna, Muñoz-Hernandez and Miranda57), changes in blood pressure and endothelial function were concomitant with markers related with vasodilatation, such as an increase in NO and a decrease in serum asymmetric dimethylarginine (ADMA), as well as a reduction in oxidised LDL and high-sensitivity C-reactive protein. VOO, but not high-oleic sunflower oil, reduced SBP in hypertensive women, indicating a role for the minor olive oil components on blood pressure levels(Reference Ruiz-Gutierrez, Muriana and Guerrero66). A decrease in SBP after VOO consumption, in comparison with refined olive oil, in hypertensive stable patients with CHD has been reported(Reference Fitó, Cladellas and de la Torre67), together with a decrease in lipid oxidation. In a parallel study comparing the effect of VOO on blood pressure in diabetic patients and healthy individuals, a reduction in SBP was observed in both conditions(Reference Perona, Montero and Sánchez-Domínguez68).
Mechanisms by which virgin olive oil and its phenolic compounds can exert their benefits in vivo in humans
There is a huge body of experimental studies concerning the mechanisms by which VOO and its phenolic compounds could exert their beneficial effects. From these, only two have been reported to occur in vivo in humans: (1) the increase in the antioxidant content of the LDL; (2) a nutrigenomic effect.
Increase in the antioxidant content of the LDL
Among the conclusions of the Consensus Report made by the Expert Panel in the International Conference of Olive Oil and Health held in Jaen, Spain, October 2004(Reference Covas, Ruiz-Gutiérrez and de la Torre9, Reference Pérez-Jimenez, Alvarez de Cienfuegos and Badimon52), one of them was that, as a general rule, the best results obtained on lipid oxidation, after VOO consumption, were displayed in those markers directly associated with LDL oxidation. This could be explained on one hand by the fact that ingestion of any type of olive oil increases the plasma oleic acid content of the LDL lipoprotein. As has been referred to before, oleate-rich LDL are less susceptible against oxidation than linoleate-rich particles(Reference Reaven, Parthasarathy and Grasse15). Further results of the EUROLIVE study(Reference Cicero, Nascetti and López-Sabater69) have shown that, after olive oil ingestion, oleic acid concentration in LDL increased and those of linoleic and arachidonic acid decreased. An inverse relationship between the oleic:linoleic acid ratio and biomarkers of oxidative stress was observed(Reference Cicero, Nascetti and López-Sabater69). On the other hand, the total polyphenol content bound to human LDL increases in a dose-dependent manner with the phenolic content of the olive oil administered(Reference Covas, de la Torre and Farré-Albaladejo25). OHTyr and Tyr metabolites, glucuronides and sulphates have been reported to bind human LDL after VOO ingestion, but were not detected after refined olive oil ingestion(Reference De la Torre-Carbot, Chávez-Servín and Jaúregui70). Experimental data have shown that the susceptibility of LDL to oxidation depends not only on its fatty content but also on the LDL antioxidant content bound to the LDL(Reference Fuller and Jialal71). In our previous work(Reference De la Torre-Carbot, Chávez-Servín and Jaúregui70), we confirmed that this occurs in vivo in humans after VOO ingestion, given that an inverse relationship was obtained between plasma levels of oxidised LDL and the content of phenols bound to LDL. Phenolic compounds that can bind LDL are likely to perform their peroxyl scavenging activity in the arterial intima, where full LDL oxidation occurs(Reference Witzum18).
Nutrigenomic effect of virgin olive oil and its phenolic compounds
Nutrigenomics embrace all ‘omics’ fields, such as genomics, transcriptomics, proteomics and metabonomics with the aim of understanding and characterising how nutrients and/or food act at molecular level. We here focus on the transcriptomic data available from randomised, controlled human studies, given that, at present, they are the only ‘omic’ data available. Particularly, we refer to studies in which different types of olive oils, but with differences in their phenolic content, have been tested with the same dietary pattern as background. Several studies have examined the transcriptomic profile of VOO in comparison with low-phenolic content olive oil at postprandial state. Using microarray techniques, it has been reported that a breakfast based on VOO, high in polyphenols (398 parts per million (ppm)), was able to postprandially repress the expression of proinflammatory genes when compared with a common olive oil-based breakfast (low in polyphenols, 70 ppm) in the metabolic syndrome individuals(Reference Camargo, Ruano and Fernandez J72). Microarray results showed nineteen up-regulated and seventy-nine down-regulated genes, linked to obesity, dyslipaemia and type 2 diabetes mellitus, after the intake of VOO(Reference Camargo, Ruano and Fernandez J72). Also and as it has been referred before(Reference Farràs, Valls and Fernández-Castillejo35), a VOO enriched with its own phenolic compounds enhanced the postprandial expression of cholesterol efflux-related genes in vivo in humans compared with a standard VOO. In our pre-hypertensive and stage I hypertensive patients, we observed an increase in ABCA1, scavenger receptor class B type 1 (SR-B1), PPAR and binding protein (PPARBP, PPARα, PPARγ and PPARδ) and cluster of differentiation 36 (CD36) gene expression in leucocytes at postprandial state after high polyphenol VOO ingestion when compared with standard VOO ingestion(Reference Farràs, Valls and Fernández-Castillejo35).
We also have data concerning the transcriptomic effect of VOO in sustained consumption studies. In the frame of the PREDIMED study, two studies have reported the differences when an enrichment of the MedDiet with VOO occurs. In the first study(Reference Llorente-Cortes, Estruch and Mena73), a 3-month intervention with VOO-enriched MedDiet prevented the increase in cyclooxigenase-2 (COX2) and LDL receptor-related protein (LRP1) genes, as well as reduced the expression of monocyte chemoattractant protein 1 (MCP1) gene, compared with a MedDiet enriched with nuts or with a low-fat diet. In the second, study(Reference Castañer, Corella and Covas74), we compared 3-month changes in the whole genome of peripheral blood mononuclear cells by means of whole transcriptome microarray analyses. Results of functional annotation analyses showed that from eighteen cardiovascular canonical pathways, nine were modulated by a MedDiet enriched with VOO and four when the MedDiet was enriched with nuts(Reference Castañer, Corella and Covas74). Also within the frame of a MedDiet, we have reported an in vivo nutrigenomic effect of VOO polyphenols in humans(Reference Konstantinidou, Covas and Muñoz-Aguayo75). In this parallel, controlled, 3-month intervention trial, ninety healthy volunteers were randomised to three intervention dietary patterns: (1) MedDiet supplemented with polyphenol-rich VOO (328 mg/kg); (2) MedDiet supplemented with washed VOO (low in polyphenols: 55 mg/kg); (3) control group with their habitual diet. Only after the supplementation of MedDiet with polyphenol-rich VOO, there was a significant decrease, v. the control group, in the expression of genes related with inflammation: interferon γ (IFNγ), Rho GTPase activating protein 15 (ARHGAP15) and IL-7 receptor (IL7R); and oxidative stress: adrenergic β-2 receptor (ADRB2). Changes in gene expression after the MedDiet supplemented with VOO were concomitant with decreases in lipid oxidative damage and systemic inflammation markers(Reference Konstantinidou, Covas and Muñoz-Aguayo75). In a substudy of the EUROLIVE study, we proposed for the first time an integrated scheme for the in vivo down-regulation of the CD40/CD40L system and its downstream products promoted by the consumption of VOO(Reference Castañer, Covas and Khymenets76). Our results showed a decrease in the expression of pro-atherogenic genes: CD40 antigen ligand (CD40L), IL-23A (IL23A), ADRB2, oxidised LDL (lectin-like) receptor 1 (OLR1), IL-8 receptor-a (IL8RA) and IL7R) after consumption of VOO high in polyphenols when compared with the refined olive oil, low in polyphenols. The decrease in these genes was concomitant with the decreasing trend of the interlinked genes such as vascular endothelial growth factor (VEGF), ICAM1 and MCP1. In this study(Reference Castañer, Covas and Khymenets76), the reduction in LDL oxidation and the increase in antioxidant polyphenols in LDL promoted by the regular dietary intake of polyphenol-rich VOO were associated with a down-regulation in the expression of genes related with the CD40/CD40L pathway. Fried sunflower oil, incorporated into muffins, but not VOO or oil to which natural antioxidants of the olives have been added, induced the postprandial increase in the mRNA levels of the endothelial reticulum stress-sensor serum X-box binding protein 1 (SXBP1), and the endothelial reticulum stress-related chaperones sbinding immunoglobulin protein (BiP) and calreticulin (CRT)(Reference Rangel-Zúñiga, Haro and Pérez-Martínez77). Due to all referred above, VOO and its phenolic compounds can modulate the expression of atherosclerosis-related genes towards a protective mode in vivo in humans.
Strengths and limitations
The design of clinical trials referred to in this review has several strengths. As has been referred to previously, all the included trials were of a randomised, controlled design. A large part of them were cross-over, thus permitted the same participants to be included in the different olive oil interventions, thus, minimising the interferences with other possible confounder variables. However, these studies also have limitations. One of them is the inability to determine whether the changes in cardiovascular risk factors could be attributed to a specific VOO component (e.g. phenolic compounds), an interaction among olive oil components (e.g. phenolic content plus MUFA), or among olive oil components and others from diet. It is difficult to separate the biological actions of nutrients from the global dietary pattern. In addition, the measurements of dietary intake relied on self-reporting and were, therefore, subjective. Also, the determination of the phenolic content of an olive oil has methodological difficulties, and a large variability exists between procedures. Another limitation is the short duration of the intervention periods, in the cross-over studies. It is unknown whether additional or different effects would have been observed over longer periods. A longer duration of the studies, however, could have impaired the compliance of the participants.
Conclusions
From all the data referred to in this review, the question: Why to choose VOO? can be answered. VOO has shown to promote additional benefits to those provided by other vegetable oils on the following: (1) increasing HDL-cholesterol; (2) reducing the oxidative damage to lipids; (3) decreasing inflammation; (4) improving endothelial function; (5) decreasing SBP. Mechanisms by which VOO can exert its beneficial effects are the increase in the antioxidant content of LDL and a nutrigenomic effect, modulating the expression of atherosclerosis-related genes towards a protective mode.
Acknowledgements
CIBEROBN is an initiative of the Instituto de Salud Carlos III, Madrid, Spain.
The authors' contributions are as follows: M.-I. C. prepared the first draft and the final version of the manuscript; M. F. and R. d. l. T. revised the first draft and made useful remarks, and revised the final version of the manuscript.
None of the authors has any conflict of interest to declare.




