Phyto-oestrogens are naturally occurring polyphenolic plant compounds with hormone-like activity(Reference Adlercreutz, Bannwart and Wahala1, Reference Adlercreutz and Mazur2). Lignans, present in, for example, wholegrain products, berries, fruits, vegetables, flaxseed and sesame seed, are the most abundant phyto-oestrogens in Western diets(Reference Adlercreutz3). After consumption, lignans are converted by the gut microflora into the so-called enterolignans, enterolactone and its immediate precursor enterodiol(Reference Adlercreutz and Mazur2, Reference Borriello, Setchell and Axelson4, Reference Setchell, Lawson and Borriello5). Until now, six plant lignans have been identified as precursors of enterolactone, including secoisolariciresinol (SEC), matairesinol (MAT), lariciresinol (LAR), pinoresinol (PIN), medioresinol (MED) and, to a minimal extent, syringaresinol (SYR)(Reference Adlercreutz3). Some studies have indicated that dietary phyto-oestrogens protect against CVD(Reference Vanharanta, Voutilainen and Lakka6, Reference Reynolds, Chin and Lees7), breast cancer(Reference Yamamoto, Sobue and Kobayashi8–Reference Pietinen, Stumpf and Mannisto10), prostate cancer(Reference Hedelin, Klint and Chang11–Reference Severson, Nomura and Grove13) and menopause-related symptoms(Reference Nagata, Takatsuka and Kawakami14–Reference Murkies, Lombard and Strauss16), but not all(Reference Hedelin, Lof and Olsson17, Reference Stattin, Adlercreutz and Tenkanen18). A possible reason for this inconsistency is the measurement error of phyto-oestrogen intake based on FFQ(Reference Keinan-Boker, van Der Schouw and Grobbee19–Reference dos Santos Silva, Mangtani and McCormack24). For instance, the long-term intake estimation of lignans based on FFQ might be subject to with-person random error due to the day-to-day fluctuation in dietary intake. In addition, systematic between-person errors might also have been produced due to the omission of lignan-containing food items in a standardised FFQ or the lack of a complete lignan composition database. Due to these measurement errors, the estimated level of lignan intake among study subjects might be inaccurate and therefore dilute the true association with disease(Reference Willett and Willett25). Four validation studies of dietary phyto-oestrogens have compared FFQ with relevant biomarkers(Reference Hedelin, Klint and Chang11, Reference Kilkkinen, Valsta and Virtamo26–Reference Bhakta, dos Santos Silva and Higgins28). One Finnish study and one Swedish study of men indicated significant correlations (both r 0·19) when comparing the FFQ-based estimate with the serum concentration of enterolactone, the biomarker of lignan intake(Reference Hedelin, Klint and Chang11, Reference Kilkkinen, Valsta and Virtamo26). However, two English studies did not observe a correlation between dietary intake of lignan and serum concentration of enterolactone(Reference Bhakta, Higgins and Sevak27, Reference Bhakta, dos Santos Silva and Higgins28). The purpose of the present study was to assess the validity of two versions of FFQ, the FFQ-87 with a shorter and the FFQ-97 with a longer list of food items containing lignans, in the measurement of dietary intake of lignans, as compared to serum concentrations of enterolactone, among Swedish women.
Method
Study subjects and study design
The present validation study included women randomly drawn from the Swedish Mammography Cohort. The Swedish Mammography Cohort was established between 1987 and 1990. All 90 303 women born between 1914 and 1948 and residing in the Västmanland and Uppsala counties in central Sweden were invited by mail to participate in a population-based mammography-screening programme. Enclosed with this invitation was the FFQ-87 that elicited information on diet, weight, height and education. The participation rate was 74 %. In 1997, a more comprehensive dietary questionnaire, the FFQ-97, was sent to the 56 030 Swedish Mammography Cohort members who were residing in the study area, and 70 % of them returned the completed questionnaire. During 2003–4, 140 women aged 55–75 years were randomly selected from the cohort for the present validation study. None of them had used antibiotics in the past year. This exclusion was necessary as antibiotics are known to influence phyto-oestrogens' metabolism(Reference Adlercreutz, Fotsis and Bannwart29). All 140 participants answered the FFQ-87 and the FFQ-97 in 2003–2004, and fasting blood samples were collected within 3 months of completing these questionnaires. The information on history of gastrointestinal disease was obtained from the National Patient Register, while the diabetes data was collected from the combination of the National Patient Register, the National Diabetes Register and self-reported questionnaires. The study was approved by the Regional Ethics Committee at the Karolinska Institutet in Stockholm, Sweden.
Assessment of dietary phyto-oestrogen intake using FFQ
The FFQ-87 and the FFQ-97 included sixty-seven and ninety-three food items, respectively. In the FFQ-87, participants were asked to report their average frequency of consumption for each type of food or beverage using eight predefined frequency categories: ‘never/seldom’, ‘one to three times/month’, ‘one time/week’, ‘two to three times/week’, ‘four to six times/week’, ‘one time/d’, ‘two to three times/d’ or ‘four times/d’. In the FFQ-97, close-ended questions with similar response categories were set for most food items, but open-ended questions (open answers, not pre-specified categories) were designed for some commonly consumed foods including bread, milk, cheese, soft drinks, beer, coffee, tea and sugar. Energy content was obtained from the Swedish National Food Administration database(Reference Bergström, Kylberg and Hagman30). Total lignan intake was estimated using published content values of the six most prevalent dietary precursors of enterolactone: SEC, MAT, LAR, PIN, MED and SYR(Reference Adlercreutz and Mazur2, Reference Milder, Arts and van de Putte31–Reference Mazur, Fotsis and Wahala37). Of the sixty-seven and ninety-three food items listed in the FFQ-87 and the FFQ-97, forty-five (69·2 %) and sixty-five (69·9 %) items were assigned lignan values, respectively. The remaining had no values assigned because the lignan content was assumed to be negligible. Nutrient intake was computed by multiplying the frequency of food items by the nutrient content of the age-specific servings. The estimations of total intake of lignans were adjusted for total energy intake, using the residual method(Reference Willett and Stampfer38). As the activity of the gut microflora influences the metabolism of dietary lignans to enterolactone(Reference Borriello, Setchell and Axelson4, Reference Setchell, Lawson and Borriello5, Reference Setchell, Adlercreutz and Rowland39), we also used a formula based on experimental results to calculate the expected amount of mammalian lignans, which had been converted from dietary lignans(Reference Heinonen, Nurmi and Liukkonen40):
Analysis of serum enterolactone
Blood samples were processed and separated for sera that were stored at − 80°C until analysis. Samples were shipped frozen to the Folkhälsan Institute for Preventive Medicine, Nutrition and Cancer (Helsinki, Finland), where they were thawed and subjected to overnight enzymatic hydrolysis and diethyl ether extraction. Sample extracts were then diluted in assay buffer, with europium label, internal standards and subsequently analysed by time-resolved fluoroimmunoassay according to previously reported protocols for assessment of enterolactone(Reference Wang, Lapcik and Hampl41–Reference Stumpf, Uehara and Nurmi43). The intra- and inter-assay CV % of the time-resolved fluoroimmunoassay method was low (3·3–6·0 and 6·9–9·9 % for enterolactone, depending upon the serum concentrations)(Reference Wang, Lapcik and Hampl41–Reference Stumpf, Uehara and Nurmi43). Serum isoflavone genistein was analysed using the same method, but only forty of the total 140 women had a detectable serum concentration within the range 0–48 (median 3) nmol/l, indicating low consumption of the isoflavone genistein in the Swedish diet. Therefore, genistein was not included in the final analysis.
Statistical analysis
Lignan values were not normally distributed; therefore, the log-transformed values of lignan intake and serum enterolactone were used in the Pearson's correlation analyses. A total of five observations outside the 95 % CI of the corresponding values were excluded, leaving 135 participants for final analysis. The following variables were considered as potential confounders, as they might influence the metabolism of dietary lignans: age (categorised into three groups: 55–61, 62–69 and >69 years), BMI ( ≤ 24·9, 25–29·9 and ≥ 30 kg/m2), constipation (yes or no), gastrointestinal disease history (yes or no) and diabetes (yes or no). The analyses were implemented in both crude and multivariable models. The basic model included adjustment for age only, while the full multivariable model included all variables listed earlier. The partial Pearson's correlation was used to calculate the adjusted correlation coefficients in the full model. Test of linear trend across BMI categories was conducted by assigning the median of BMI in each BMI category and then treating these values as a continuous variable in the model. As previous studies have indicated BMI as an important determinant of the serum enterolactone concentration(Reference Kilkkinen, Stumpf and Pietinen44), additional analyses stratified by BMI subgroups were also conducted. ANOVA and t tests were used to compare the mean values of serum concentration of enterolactone in the subgroups when appropriate. All statistical analyses were performed using SAS 9.0 (Statistical Analysis System, version 9.0; SAS Institute).
Results
Dietary intake of lignans and serum enterolactone
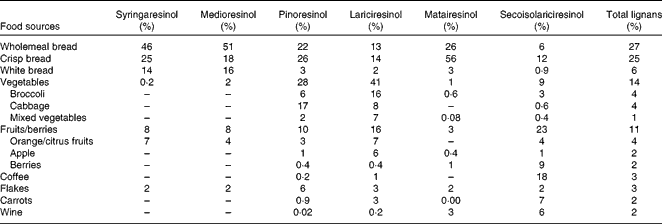
Among the 135 study participants aged 55–75 years, with a mean BMI of 26·7 kg/m2, the average energy-adjusted dietary intake of total lignans was 1616 (sd 424) μg/d according to the FFQ-87 and 1516 (sd 409) μg/d for the FFQ-97 (Table 1). The estimates of dietary lignan intake were different for the two FFQ (t= 3·2, P= 0·002). The average intake of lignans using the FFQ-97 in obese subjects (BMI ≥ 30 kg/m2) was 1634 (sd 589) μg/d and 1464 (sd 368) μg/d in normal-weight (BMI < 24·9 kg/m2) subjects (t= − 1·42, P= 0·16). The mean concentration of serum enterolactone was 23·2 (sd 15·4) nmol/l (Table 2). No statistically significant difference of serum enterolactone was observed among subgroups of BMI, age groups, constipation, diabetes and gastrointestinal disease history. Approximately 60 % of the daily intake of total lignans was derived from bread, while fruit and vegetable intake accounted for 25 % (Table 3). Lignan LAR (32·4 %) and PIN (33·3 %) were the main contributors of total lignan intake (data not shown).
Table 1 Dietary lignan intake among 135 Swedish women (Mean values and standard deviations; medians and ranges)

* FFQ-87: FFQ, 1987 version (forty-five food items containing lignans); FFQ-97: FFQ, 1997 version (sixty-five food items containing lignans).
† Nutrient intakes are energy-adjusted via the residual method.
‡ Five outliers (95 % CI) were excluded.
§ Two subjects had missing BMI values.
∥ Expected amount of dietary lignans was converted to enterolactone in the intestine using conversion factors: matairesinol = 0·62, secoisolariciresinol = 0·72, lariciresinol = 1·01, pinoresinol = 0·55, syringaresinol = 0·44, pinoresinol = 0·55, syringaresinol = 0·04 and medioresinol = 0·8.
Table 2 Serum concentration of enterolactone by characteristics of study subjects (Mean values and standard deviations; medians and ranges)
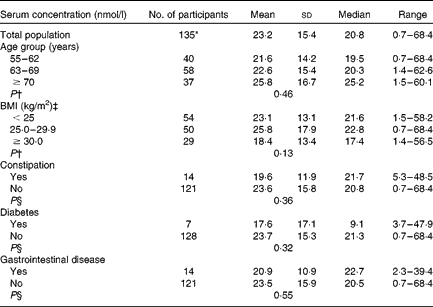
* Five outliers (95 % CI) were excluded.
† ANOVA was used to compare the mean values.
‡ Two subjects had missing BMI values.
§ The t test was used to compare the mean values.
Table 3 Food sources for major contribution of dietary intake of lignans among 135 Swedish women
Correlation between dietary lignans and serum enterolactone
Pearson's correlation coefficients between lignan intake and serum concentration of enterolactone are presented in Table 4. Dietary lignans assessed by the FFQ-97 were statistically significantly correlated with serum enterolactone in the crude and multivariable-adjusted models (r 0·16, P= 0·06; r 0·22, P= 0·01, respectively), as well as when converted lignan values were used (r 0·17, P= 0·04; r 0·24, P= 0·006, respectively) (Table 4). However, no such correlation was found for the FFQ-87 with lignan intake (r 0·09, P= 0·30), nor with converted lignans (r 0·12, P= 0·19). The correlation between the two FFQ was statistically significant (r 0·59, P< 0·0001).
Table 4 Pearson's correlation between energy-adjust dietary intake of lignans based on the FFQ and serum concentration of enterolactone*
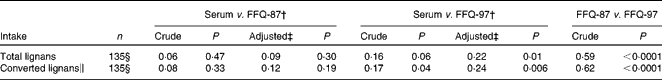
* All dietary intakes of lignans and serum enterolactone were log-transformed.
† FFQ-87: FFQ, 1987 version (forty-five food items containing lignans); FFQ-97: FFQ, 1997 version (sixty-five food items containing lignans).
‡ Adjusted for age, BMI ( < 25, 25–29·9, ≥ 30) kg/m2, constipation (yes/no), gastrointestinal disease history (yes/no) and diabetes (yes/no).
§ Five outliers (95 % CI) were excluded.
∥ Expected amount of dietary lignans was converted to enterolactone in the intestine using conversion factors: matairesinol = 0·62, secoisolariciresinol = 0·72, lariciresinol = 1·01, pinoresinol = 0·55, syringaresinol = 0·44, pinoresinol = 0·55, syringaresinol = 0·04 and medioresinol = 0·8.
Pearson's correlations between intake of total lignans and of converted lignans and serum enterolactone in BMI subgroups are shown in Table 5. The highest correlation was observed in obese women (BMI ≥ 30 kg/m2). A borderline positive trend between increasing BMI and increasing correlation coefficients for lignans was indicated, but due to the limited sample size, chance errors cannot be ruled out.
Table 5 Pearson correlations between energy-adjusted dietary intake of lignans based on the FFQ and serum concentration of enterolactone stratified by BMI*

* All dietary intakes of lignans and serum enterolactone were log-transformed.
† FFQ-87: FFQ, 1987 version (forty-five food items containing lignans); FFQ-97: FFQ, 1997 version (sixty-five food items containing lignans).
‡ Expected amount of dietary lignans could be converted to enterolactone in the intestine.
§ Adjusted for age, constipation (yes/no), gastrointestinal disease history (yes/no) and diabetes (yes/no), when applicable.
∥ Two subjects had missing BMI values.
¶ Test for trend across BMI categories was conducted by assigning the median of BMI in each BMI category and then treating these values as a continuous variable.
Discussion
The present study shows that the validity of FFQ-based estimates of dietary lignan intake depends on the number of food items containing lignans in the FFQ, and potentially the BMI of the study subjects.
Limitations of the present study include the small sample size, although it was not smaller than that of other phyto-oestrogen validation studies(Reference Hedelin, Klint and Chang11, Reference Bhakta, dos Santos Silva and Higgins28, Reference Heald, Bolton-Smith and Ritchie45). A limitation of the serum enterolactone assessment was that only a single fasting blood sample was used. The high intra-individual variation with poor precision when using a single measurement might lead to misclassification of the enterolactone exposure(Reference Sonestedt and Wirfalt46). However, the concentration of serum enterolactone in the present study was in line with the concentrations observed in other Swedish studies(Reference Sonestedt, Ericson and Gullberg47, Reference Hulten, Winkvist and Lenner48). Among strengths were the adjustments for confounders, including diabetes and gastrointestinal diseases, that might interact with phyto-oestrogens' metabolism through enzymatic modification and gut microflora(Reference Barnes, Sfakianos and Coward49–Reference Takaishi, Matsuki and Nakazawa51). Furthermore, none of the participants had used antibiotics during the previous 12 months, which might otherwise be a matter of concern(Reference Adlercreutz, Fotsis and Bannwart29). The use of antibiotics is known to reduce the amount of gut bacteria and subsequently serum concentrations of enterolactone(Reference Kilkkinen, Pietinen and Klaukka52). Additionally, the use of fasting blood samples was an advantage, as non-fasting samples might have lower reliability regarding enterolactone measurements(Reference Sonestedt and Wirfalt46, Reference Sonestedt, Ericson and Gullberg47, Reference Hausner, Johnsen and Hallund53). Another strength was the inclusion of four newly identified enterolactone precursors (LAR, PIN, SYR and MED) in the calculation of total dietary lignan intake, while most of the previous studies included only SEC and MAT for such calculations(Reference Kilkkinen, Valsta and Virtamo26–Reference Bhakta, dos Santos Silva and Higgins28).
Few studies(Reference Hedelin, Klint and Chang11, Reference Kilkkinen, Valsta and Virtamo26–Reference Bhakta, dos Santos Silva and Higgins28) have investigated the correlation between lignan intake estimates and serum enterolactone as a biomarker (Table 6). In a study of 140 women in England, the correlation between dietary intake measured by FFQ and serum enterolactone was low (r 0·10, P= 0·20)(Reference Bhakta, Higgins and Sevak27). However, a Finnish study showed better validity of 24 h recalls (r 0·19, P< 0·0001) in a large study population (n 1784), but they assessed only the precursors SEC and MAT to estimate lignan intake(Reference Kilkkinen, Valsta and Virtamo26). The only previous study that estimated total lignan intake based on all six precursors of plant lignans was performed in Swedish men, and showed a correlation of 0·19 (P= 0·09)(Reference Hedelin, Klint and Chang11). The correlation observed in the present study of the FFQ-97 (r 0·22, P= 0·01) was similar.
Table 6 Summary of papers investigating correlation between dietary intake of lignans and serum concentration of enterolactone

The reasons for the low correlation between lignan intake and serum enterolactone might be diverse. FFQ might overestimate the consumption of a number of food groups, particularly lignan intake from fruits and vegetables(Reference Willett and Stampfer38), and the FFQ were not designed specifically for lignan intake; thus, it was difficult to obtain full coverage of related food sources. On the other hand, concentration of enterolactone might vary substantially over the course of a single day, or different seasons. Furthermore, potentially large inter-individual variation in the metabolism of plant lignans into mammalian enterolactone cannot be ignored(Reference Rowland, Wiseman and Sanders54). Upon ingestion, plant lignans are transformed into their immediate precursor enterodiol and then to enterolactone by certain gut microflora(Reference Borriello, Setchell and Axelson4, Reference Setchell, Lawson and Borriello5, Reference Setchell, Adlercreutz and Rowland39). Therefore, lack of certain gut microflora can result in different enterodiol:enterolactone ratios. Moreover, persons regularly consuming certain lignan-rich foods, such as fruits, vegetables and fibre-rich foods have more efficient transformation of plant lignans into mammalian enterolactone(Reference Nurmi, Mursu and Penalvo55, Reference Hutchins, Lampe and Martini56). Smoking and high BMI might decrease the serum concentration of enterolactone, while constipation might enhance the production of enterolactone due to the decrease of intestinal motility(Reference Kilkkinen, Stumpf and Pietinen44, Reference Johnsen, Hausner and Olsen57).
To improve the assessment of phyto-oestrogens using FFQ, some modifications might be warranted. For example, some improvements might be needed to develop a lignan-specific questionnaire, in which important exposures such as age, education, height and weight (to calculate BMI) will also be included. Furthermore, several criteria should be considered when adopting a biomarker to calibrate dietary assessment, e.g. by obtaining serum samples at several time points. Although hampered by low statistical power, it was interesting that obese participants seemed to show a higher correlation between estimate of plant lignan intake and serum concentration of enterolactone. Speculatively, obese people might be more prone to recall healthy dietary habits, such as intake of fruits and vegetables, compared to non-obese people. The difference in assessment of dietary lignan intake using the FFQ-97 and the FFQ-87 might be due to the fact that the FFQ-97 (sixty-five food items containing lignans) evaluated more lignan-containing food items than the FFQ-87 (forty-five food items containing lignans), and the fact that some questions in the FFQ-97 were more precise than those in the FFQ-87. Participants were asked to report food intake frequency within eight categories ranging from ‘never/seldom’ to ‘four times/d’ in the FFQ-87, whereas they were asked about the precise frequency (open answers, not pre-specified categories) for commonly consumed food items, e.g. tea, in the FFQ-97. In fact, there were differences between the FFQ-87 and the FFQ-97 in terms of assessment of tea.
In summary, the present study indicates that the correlation between plant lignan intake based on assessment with the FFQ and serum concentration of enterolactone is limited, which can partly depend on varying metabolism of lignans. Therefore, interpretation of FFQ-based results regarding lignan exposure should be very cautious, and direct measurement of serum enterolactone should be recommended.
Acknowledgements
The present work was supported by research grants from the Swedish Cancer Foundation, the Swedish Research Council/Committee for Infrastructure and the Faculty Funds for Partial Financing of New Doctoral Students from Karolinska Institutet (12059012/KID-medel 2010). None of authors has conflicts of interest to disclose. The authors' contributions to the present study were as follows: A. W. designed the study; A. W. carried out the data collection; J. L. P. and H. A. performed the laboratory analysis; N. H., Y. L. and Y. L. performed the statistical analysis; and Y. L., Y. L., J. L., J. L. P., H. A. and A. W. wrote the manuscript.








