Folate is a water-soluble B vitamin that is vital for DNA synthesis, repair and methylation, and for protein production in processes such as erythropoiesis and growth development(1,Reference Lamers2) . Given overwhelming evidence that folate deficiency in early pregnancy is associated with an increased risk of neural tube defects, considerable effort has been made to prevent folate deficiency in early pregnancy, typically by promoting folic acid supplementation and/or fortification(3–5). The WHO clearly recommends a daily folic acid intake of 400 μg for women from preconception until 12 weeks’ gestation(5), while recommendation after early pregnancy has not been uniformly formulated. Women who do not increase their folate intake during later pregnancy or lactation may be predisposed to folate deficiency, because their folate demand might be increased as pregnancy progresses.
Plasma folate, a transport form of folate to the tissue and to the fetus(Reference Tamura and Picciano6), plays crucial roles in maintaining maternal and offspring health. Accumulating evidence shows that a low plasma or serum folate concentration in mid- to late pregnancy is likely associated with increased risks of pre-eclampsia(Reference Wen, Chen and Rodger7), preterm birth(Reference Siega-Riz, Savitz and Zeisel8), low birth weight(Reference Zhu, Wei and Cao9) and poor language development in young children(Reference Huang, Ye and Li10). Animal models also showed that folate deficiency during lactation could alter cerebellar synapsin expression in offspring(Reference Pourié, Martin and Bossenmeyer-Pourié11). Therefore, it is of significance to explore the changing trend of maternal folate status from pregnancy to lactation, for directing folate nutritional recommendations beyond early pregnancy. However, up-to-date data on trends and determinates of folate status in women at later pregnancy and lactation are sparse. To the best of our knowledge, only three earlier studies from Western countries reported folate status throughout pregnancy and in postpartum period(Reference Willoughby and Jewell12–Reference Milman, Byg and Hvas14). Data from Western countries might not be applied to Chinese population, since folic acid fortification in foods is not mandatory in China, and the recommendation of folic acid supplements for Chinese women in early pregnancy is lower than some Western countries.
Therefore, we conducted a cross-sectional study in Chinese pregnant and lactating women to explore the trends in plasma folate status during mid-pregnancy, late pregnancy and lactation and to evaluate the potential influencing factors associated with folate status.
Materials and methods
Subjects and study design
The present study included 1254 women at 15–19 weeks’ gestation (mid-pregnancy), 37–41 weeks’ gestation (late pregnancy) or 35–49 d postpartum (lactation) drawn approximately equally from the cities of Yueyang, Weihai and Baotou between May and July 2014. Yueyang (latitude: 29°37′ N), located in Hunan Province, was selected to represent the southern region of China, Weihai (latitude: 37°25′ N) in Shandong Province represented the central region and Baotou (latitude: 40°15′ N) in the Inner Mongolia Autonomous Region represented the northern region. Details of the study including the study design and subject characteristics have been described elsewhere(Reference Li, Li and Trasande15–Reference Zhou, Li and Trasande17). Briefly, pregnant or lactating women who were attending routine prenatal or postpartum clinics at local hospitals were recruited by obstetricians if they were in good general health, aged 18–35 years, local permanent residents and had a singleton pregnancy or had just given birth to a healthy singleton. An additional inclusion criterion for lactating women was current exclusive breast-feeding or partial breast-feeding (mixed feeding of formula and breast milk). Of the 1254 women initially recruited, forty-three were excluded due to age >35 years old (n 23) or not being in the aforementioned mid- or late pregnancy or lactation periods (n 20), leaving 1211 women in the final analysis.
The study protocol was approved by the Institutional Review Board at Peking University Health Science Center (IRB00001052-14012; date of approval: 22 April 2014), and written informed consent was obtained from all participating women.
Data and blood sample collection
Maternal socio-demographic information including age, ethnicity, educational status, height, weight before pregnancy, gestational age and parity was collected by trained obstetricians or nurses using a structured questionnaire. Information on delivery mode and feeding practice was recorded for lactating women. Pre-pregnancy BMI (kg/m2) was calculated as pre-pregnancy weight in kg divided by squared height in m.
Detailed procedures for blood sampling and processing have been reported previously(Reference Li, Li and Trasande15–Reference Zhou, Li and Trasande17). Briefly, a fasting venous blood sample (about 5 ml) was drawn during morning from each woman. The blood samples were collected into ethylene diamine tetraacetic acid-containing tubes and centrifuged to obtain plasma aliquots within 4 h of collection. Then, the plasma aliquots were stored at −20°C at the local hospitals for about 10 d. Then, the samples were sent on dry ice to the National Health Commission Key Laboratory of Reproductive Health at Peking University Health Science Center in China, where they were stored at −80°C until to be analysed.
Analysis of plasma folate
Plasma folate concentration was determined by microbiological assay in a ninety-six-cell microtiter plate using a chloramphenicol-resistant strain of Lactobacillus casei (NCIB 10463)(Reference Molloy and Scott18). Briefly, plasma was diluted 1:100 by adding 10 μl plasma to 990 μl 0·5 % Na l-ascorbate solution. Diluted plasma, folic acid casei medium and Lactobacillus casei were added to each well of the plate, which was sealed and incubated at 37°C in the dark for about 42 h. Turbidity was then measured by absorbance at 590 nm on a multifunctional microplate reader (Biotek). A cubic spline was applied to the calibrator curve which was produced with eleven concentration points from 0 to 1 nmol/l of folic acid. To ensure the accuracy of the assay, in-house quality controls at high, medium and low levels were used at each sample plate, and samples in the plate were retested if two or more of the quality control results were over the mean +/− 2 sd limit or if any of the quality control results were over the mean +/− 3 sd limit. In plates that passed quality control, samples were retested if the CV of all four replicates exceeded 15 % and the CV of the three remaining replicates still exceeded 10 % after removal of the largest outlier. The limit of detection of this assay was 2·0 nmol/l. The intra- and inter-assay CV were <10 %. All sample analyses were performed in a biosafety level II laboratory under yellow light by trained staff who were qualified to perform the folate assay, which was validated by the Centers for Disease Control and Prevention in the USA.
According to cut-off values defined by the WHO(1), folate deficiency was defined as concentrations <6·8 nmol/l, and marginal deficiency was defined as concentrations of 6·8–<13·5 nmol/l. Folate deficiency and marginal deficiency were regarded as suboptimal folate status in the present study.
Statistical analysis
Categorical variables are expressed as frequency and percentages, and normally distributed continuous variables are expressed as mean values and standard deviations. Plasma folate concentrations are expressed as medians and interquartile ranges (IQR) because of the skewed distribution according to the Kolmogorov–Smirnov D test (P values <0·001). Adjusted medians (IQR) were estimated using multivariable quantile regression models. The median differences across physiological period, geographic region and other polytomous variables were assessed using Kruskal–Wallis tests, followed by Bonferroni corrected Mann–Whitney U tests for multiple comparisons. The median differences according to parity and other dichotomous variables were assessed using Mann–Whitney U tests. The potential influencing factors of suboptimal folate status were explored using univariable and multivariable modified Poisson regression models with robust error variance.
Variables in the multivariable models included physiological period (mid-pregnancy, late pregnancy and lactation), geographic region (south, central and north), maternal age (≤25, >25–30 and >30 years), pre-pregnancy BMI (underweight <18·5, normal weight 18·5–<25 and overweight/obese ≥ 25 kg/m2), parity (nulliparous and multiparous), educational level (middle school or less, high school and college or higher) and ethnicity (Han and other). Delivery mode (vaginal delivery and Caesarean delivery) and feeding practice (exclusive breast-feeding and partial breast-feeding) were additionally included in the models for lactating women.
All statistical analyses were performed using SPSS software version 24.0 (SPSS Inc.). All tests were two-tailed, and P values <0·05 were considered to indicate statistical significance.
Results
The participant characteristics are shown in Table 1. The study included 1211 women with a mean age of 27·8 (sd 3·0) years, and mean pre-pregnancy BMI of 20·9 (sd 2·9) kg/m2. In total, 33·6 % (n 407) of the women were in mid-pregnancy (mean gestational age, 17·4 (sd 1·5) weeks), 32·8 % (n 397) in late pregnancy (mean gestational age, 38·5 (sd 1·5) weeks) and 33·6 % (n 407) in lactation (mean postpartum days, 42·2 (sd 3·5) d); 33·0 % (n 399) of the women lived in the central region, 33·2 % (n 402) in the southern region and 33·8 % (n 410) in the northern region; 83·5 % (n 1011) of the women were primiparous. Among the 407 lactating women, 59·7 % (n 243) had a vaginal delivery, and 59·0 % (n 240) breastfed exclusively. Detailed information on participant characteristics has been reported previously(Reference Li, Li and Trasande15-Reference Zhou, Li and Trasande17).
Table 1. Characteristics of pregnant and lactating women
(Frequencies and percentages)
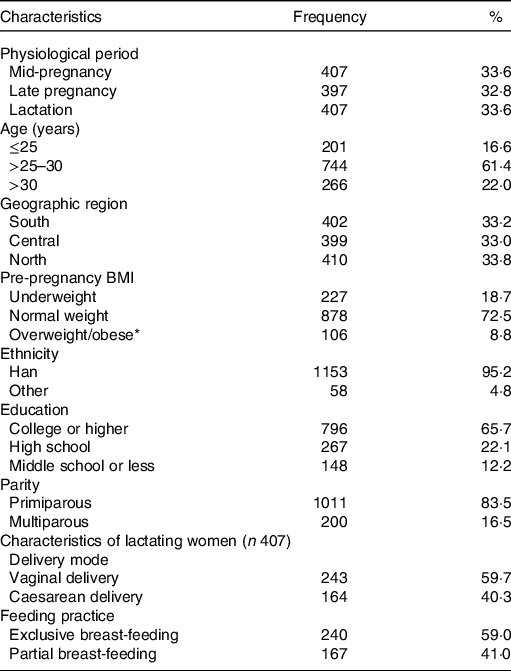
* Of 106 women classified as overweight/obese, eleven were obese.
The crude median of plasma folate concentration for total pregnant and lactating women was 19·0 (IQR 11·9, 29·8) nmol/l, and the adjusted estimate was 19·5 (IQR 13·6, 27·6) nmol/l. The folate concentrations in pregnant and lactating women significantly differed by physiological period, geographic region and socio-demographic characteristics (Table 2). The adjusted folate concentrations decreased from mid-pregnancy (28·8 (IQR 19·9, 38·2) nmol/l) to late pregnancy (18·5 (IQR 13·2, 26·4) nmol/l) and lactation (17·0 (IQR 12·3, 22·5) nmol/l) (P for trend <0·001), which were consistently observed in subgroups stratified by geographic region (Fig. 1). The folate concentrations were the highest in southern (21·5 (IQR 15·3, 29·8) nmol/l) region, followed by central (19·2 (IQR 13·7, 29·0) nmol/l), and the lowest in northern region (11·2 (IQR 7·1, 19·2) nmol/l). Additionally, lower folate concentrations were observed among women who were younger in age, had higher pre-pregnancy BMI and lower educational status, and were multiparous (Table 2). Most of the differences in folate concentrations by maternal characteristics persisted across periods of mid-pregnancy, late pregnancy and lactation (online Supplementary Table S1). In lactating women, maternal folate concentrations were also lower in those who had a Caesarean delivery and those who breastfed exclusively.
Table 2. Adjusted plasma folate concentrations (nmol/l) by maternal characteristics*
(Medians and interquartile ranges (IQR))
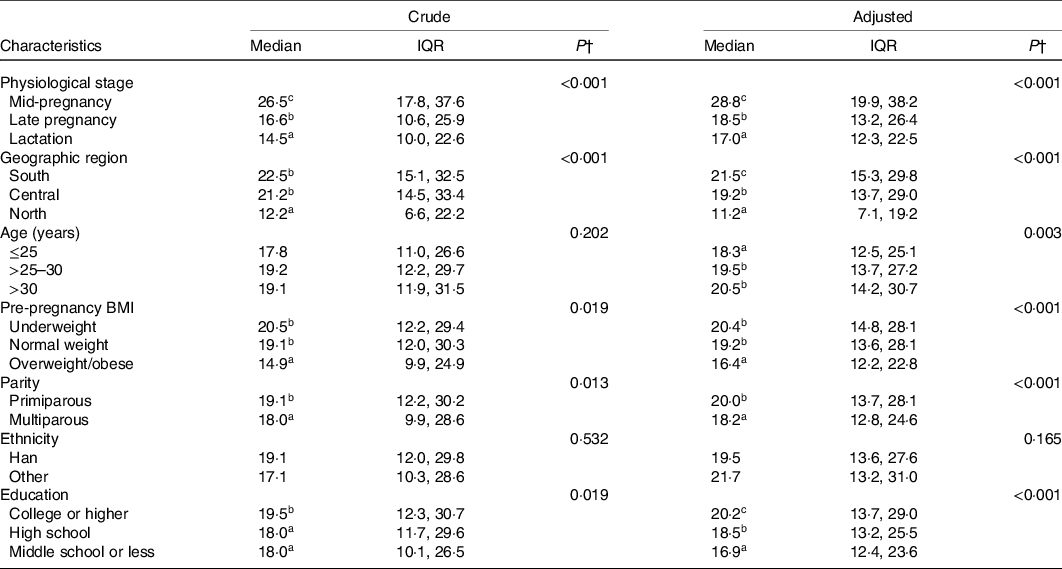
* Adjusted medians were estimated from multivariable quantile regression models adjusting for physiological period, geographic region, maternal age, pre-pregnancy BMI, parity, educational level and ethnicity; delivery mode and feeding practice were additionally included in the models for lactating women.
† The adjusted median folate concentrations were significantly different according to maternal characteristics (P < 0·001); values with varied superscript letters (a, b, c) denote significantly different concentrations: a < b < c; Bonferroni corrected Mann–Whitney U tests were used for multiple comparisons.
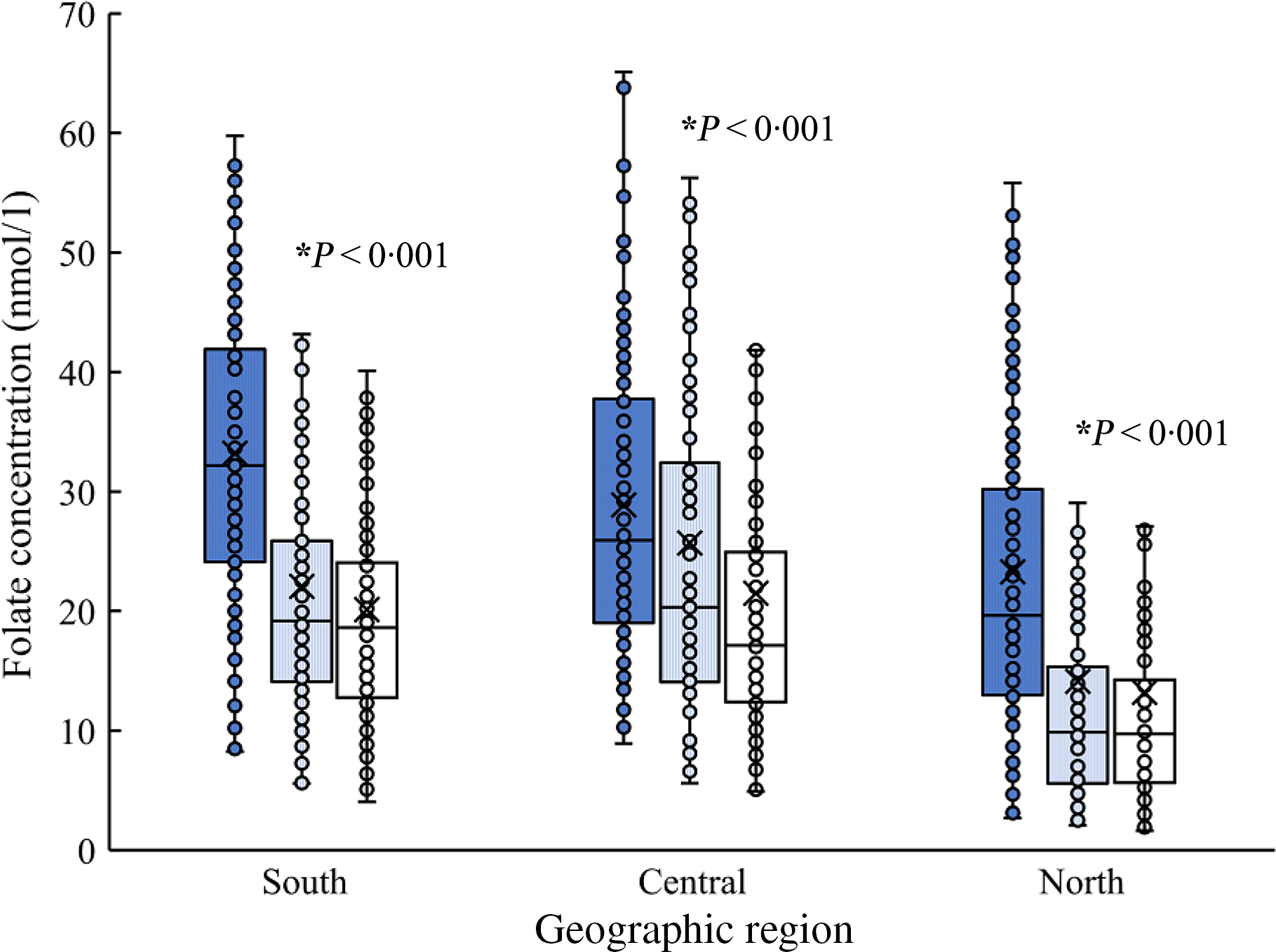
Fig. 1. Maternal plasma folate concentrations (nmol/l) by physiological period, according to geographic region. ![]() , Mid-pregnancy;
, Mid-pregnancy; ![]() , late pregnancy;
, late pregnancy; ![]() , lactation.
, lactation.
Among the 1211 women, 124 (10·2 %) were folate deficient and 256 (21·4%) were marginally deficient, leading to 380 (31·6%) women having a suboptimal folate status. Compared with women at mid-pregnancy, those at late pregnancy (adjusted relative risk (RR) 3·01, 95 % CI 2·31, 3·94) or lactation (adjusted RR 3·50, 95 % CI 2·69, 4·55) had a higher risk of suboptimal folate status (Table 3). The risks were higher among women residing in northern region than those in southern (adjusted RR 2·91, 95 % CI 2·35, 3·60), higher among multiparous women than primiparous (adjusted RR 1·25, 95 % CI 1·02, 1·53) and higher among women with middle school or lower education than those with college or higher (adjusted RR 1·35, 95 % CI 1·08, 1·69). As compared with women aged >25–30 years, those aged >30 years were less likely to have a suboptimal folate status (adjusted RR 0·74, 95 % CI 0·61, 0·90). Some of the associations persisted in subgroups stratified by physiological period (online Supplementary Table S2).
Table 3. Risk of suboptimal folate status by maternal characteristics*
(Numbers and percentages; relative risks (RR) and 95 % confidence intervals)
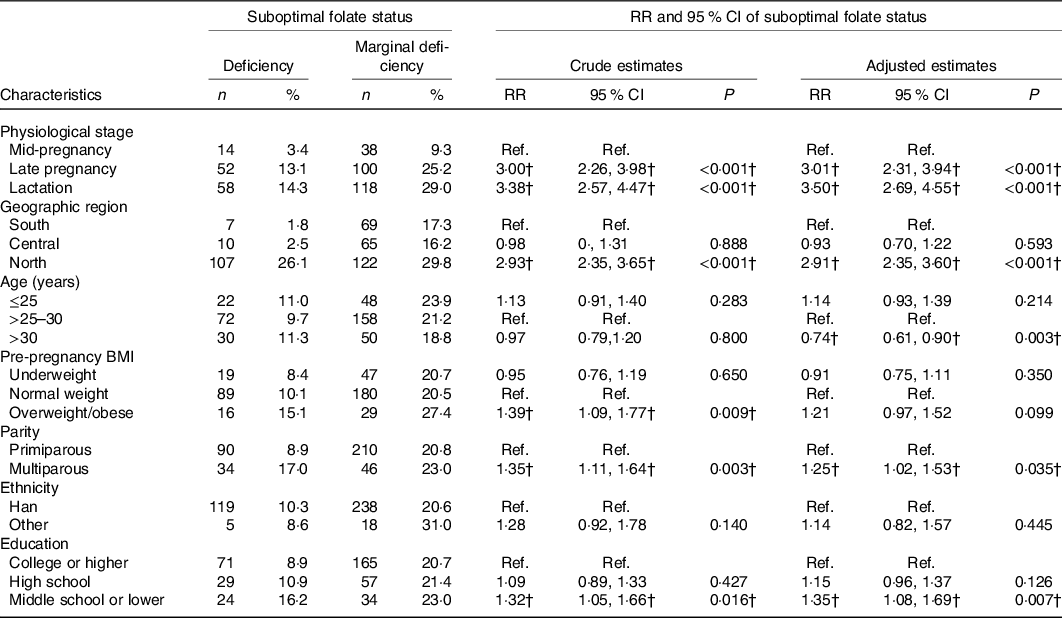
Ref, reference.
* Adjusted RR and 95 % CI were estimated using multivariable Poisson regression models adjusting for physiological period, geographic region, maternal age, pre-pregnancy BMI, parity, educational level, and ethnicity.
† Significantly adjusted RR and 95 % CI.
Discussion
In this cross-sectional study of healthy pregnant and lactating Chinese women, we found that maternal plasma folate concentrations decreased markedly from mid-pregnancy to late pregnancy and lactation. We also found that maternal folate status was associated with geographic region, maternal age, pre-pregnancy BMI, educational status and parity. Folate status of lactating women was additionally associated with delivery mode and feeding practice.
Data on folate status for Chinese women during mid- and late pregnancy are sparse and even lacking for lactating women. Our study showed 28·8 nmol/l of the median folate concentration for women during mid-pregnancy, comparable to the estimate (29·5 nmol/l) reported previously for women during early mid-pregnancy in a local setting, Shanxi Province of China where a prevalence of neural tube defects was as high as 31·5 cases per 10 000 births(Reference Zhang, Liu and Jin19), but lower than that for mid-pregnant women in the USA (45·5 nmol/l)(Reference Siega-Riz, Savitz and Zeisel8). Our study showed 18·6 nmol/l of the median folate concentration for women during late pregnancy, which has not been reported previously for Chinese women. This estimate was much lower than those for late pregnant Korean women (29·5 nmol/l)(Reference Kim, Hwang and Kim20) or for low-income late pregnant women in the USA (30·0 nmol/l)(Reference Cheng, Mistry and Wang21). Regarding lactating women, our study showed the median folate concentration of 17·0 nmol/l, which was even lower than that for Brazilian lactating women (22·2 nmol/l)(Reference Barnabe, Alessio and Bittar22). The low maternal folate levels indicate that Chinese reproductive women might have insufficient intake of folate-enriched foods or supplements, suggesting a need to explore an intervention to improve folate status for Chinese women during later pregnancy and lactation. Besides folic acid supplementation, the USA and Brazil have implemented food fortification policies. The appropriateness of these policies to the Chinese women is warranted to be evaluated.
As expected, we found a progressively decreasing trend in plasma folate concentration from mid-pregnancy to late pregnancy and to lactation, consistent with the findings of a longitudinal Danish study that showed a significant decreasing trend in plasma folate levels through 18, 32 and 39 weeks of gestation, and 8 weeks postpartum(Reference Milman, Byg and Hvas14). The underlying mechanisms include a uterus enlargement, maternal blood volume expansion, fetal and placental growth, and an increasing folate requirement(Reference Hall, Pirani and Campbell23), as well as an increasing rate of folate catabolism that reaches a peak in late pregnancy(Reference Higgins, Quinlivan and McPartlin24). After parturition, maternal folate demand continues to increase, as folate transferring from mothers to infants through breast milk is increasing during the first 3 months of lactation(Reference Ek25). Moreover, an insufficient intake of dietary folate or supplements may also be a contributor to the low folate levels for Chinese women during later pregnancy and lactation. A study conducted in Sichuan Province in China between 2010 and 2011 showed that 62·3 % of women took folic acid supplements for only 2·5 months during pregnancy, and less than 0·2 % took the supplements postpartum(Reference Tang, Lee and Yau26). However, in the USA, 60 % of women took folic acid supplements in early pregnancy, 79 % in mid-pregnancy, 89 % in late pregnancy(Reference Branum, Bailey and Singer27) and 73 % in lactation(Reference Stultz, Stokes and Shaffer28).
Our findings that maternal folate concentrations varied markedly across geographic region, with the highest in the southern region followed by the central and the northern regions, are consistent with the findings of previous Chinese studies(Reference Bi, Duan and Wang29,Reference Zhao, Hao and Zhang30) . This regional variation is likely due to distinct differences in dietary styles or habits. Individuals living in the southern region are likely to eat more green vegetables or fruits rich in folate than people in the northern. A nationwide Chinese study in women of reproductive age showed that the mean dietary folate intake was significantly higher in southern region (213 μg/d) than in the northern (188 μg/d)(Reference Zhao, Hao and Zhang30). Furthermore, regional variations might also be related to the gene polymorphism, methylenetetrahydrofolate reductase 677C–>T, in folate metabolism, and methylenetetrahydrofolate reductase 677 TT genotype is linking with lower folate concentration(Reference Crider, Zhu and Hao31). Previous studies have reported that the prevalence of the methylenetetrahydrofolate reductase 677 TT genotype was higher in women of reproductive age living in northern (35 %) than in those in southern China (28 %)(Reference Crider, Zhu and Hao31,Reference Jiajin, Shuyan and Ying32) .
We also found that maternal folate status during pregnancy and lactation was associated with a series of maternal socio-demographic characteristics. The maternal folate concentrations were significantly lower in women who were overweight/obese in pre-pregnancy compared with those of normal weight. Potential underlying mechanisms may include increased utilisation, dilution, redistribution and tissue sequestration of folate in individuals with a higher BMI(Reference Hebert, Hurley and Hsieh33,Reference Kant34) . These findings highlight the importance of careful monitoring of folate status particularly for overweight or obese women during pregnancy and lactation. Adequate folate concentrations, besides the general benefits to maternal and child health, might also mitigate the adverse effects of maternal obesity on offspring metabolic outcomes(Reference Wang, Hu and Mistry35). We also found that low folate levels were associated with lower educational level, multiparity and younger age. Consistent results were also observed when we dichotomised the folate concentration. The risks of suboptimal folate status increased by 35 % for women with middle school or lower education v. those with college or higher, and by 25 % for multiparous women v. nulliparous, and decreased by 26 % for women older than 30 years v. those aged 25–30 years. These associations were likely due to different consumption profiles of folate-enriched foods or folic acid supplements. Previous studies have shown that pregnant women who complied with recommendations related to folic acid supplementation were more likely to be well educated, to be nulliparous and to be older(Reference McNulty, Pentieva and Marshall36,Reference Nilsen, Vollset and Gjessing37) . In lactating women, we found that those with exclusive breast-feeding had a lower plasma folate level than those with partial breast-feeding, presumably because the former need to secrete more folate into the breast milk to meet their infants’ demands. Additionally, we found for the first time that folate level was lower in lactating women who had a Caesarean delivery than in those who had a vaginal delivery, which is warranted to be further studied.
Our study has several strengths. We simultaneously assessed plasma folate status during mid-pregnancy, late pregnancy and lactation in women drawn equally from the northern, central and southern regions of China, each with distinct dietary habits. We used internationally standardised assay to assess the folate concentrations, making direct international comparison possible. Furthermore, examining the potential factors associated with folate status helped identifying the higher-risk populations who might need to be targeted for potential supplementation programmes. Our study has limitations. Our participants were all healthy women in urban areas, probably restricting the generalisation of the findings to other populations. Secondly, our study was a cross-sectional rather than a longitudinal study, restricting the investigation of the individual changes in folate status over time from pregnancy to lactation. Thirdly, the plasma folate was assayed 3 years later after the samples were collected and stored at −80°C, likely leading to slightly lower estimates of median folate level. Finally, data concerning folate intake, folic acid supplements or erythrocyte folate levels were not collected or assayed, restricting a further examination concerning long-term intake or folate stores.
Conclusions
In conclusion, our cross-sectional study of pregnant and lactating women from representative regions of China found that folate nutritional concentration decreased markedly as pregnancy progressed and was lower in northern than that in southern regions. Moreover, women most likely to be predisposed to folate depletion were those with younger age, higher pre-pregnancy BMI, lower education or multiple parity. Lactating women who had a Caesarean delivery or exclusively breastfed their infants were also more likely to have a low folate concentration. Our findings suggested that Chinese reproductive women, upon comprehensive assessments, need to consume diverse diets rich in natural folate or to take folic acid supplements from preconception through lactation, to maintain an adequate folate status(Reference McNulty, McNulty and Marshall38).
Acknowledgements
We thank all physicians, nurses and other staff members from Weihai Maternal and Child Health Hospital, Yueyang Maternal and Child Health Hospital, The First Affiliated Hospital of Baotou Medical School and The Third Hospital of Baogang Group for their outstanding assistance with the field work. We thank Ya-li Zhang, Lin-lin Wang and Zhao-xia Xiong from Institute of Reproductive and Child Health of Peking University for their contribution to data collection. We are also indebted to all women who participated in the present study for their cooperation.
This research was funded by the National Key Research & Development Program of China (grant number 2016YFC1000401, 2016YFC1000406-1); the National Natural Science Foundation of China (grant number 81801542) and the Wyeth Nutrition Science Center (project number 14.10.CN.INF). The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
J.-M. L. conceived, designed and supervised the study, critically revised the manuscript and takes responsibility for the integrity of the data and has full access to all of the data in the study. Y.-B. Z. designed the study, collected the data, did the analysis, interpreted the data and drafted the manuscript. K.-Y. S. analysed the samples, interpreted the data and drafted the manuscript. H.-T. L. designed the study, collected the data and critically reviewed and revised the manuscript. X.-C. L. and Y. M. analysed the samples, and reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
The authors declare no conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520004821







