Conjugated linoleic acids (CLA) include positional and geometric isomers of octadecadienoic fatty acids with a conjugated double bond(Reference Bauman, Perfield and Harvatine1). CLA naturally originates mainly from bacterial isomerisation and biohydrogenation of PUFA in the rumen and from the desaturation of vaccenic acid (trans-11-18 : 1) in the adipose tissue and mammary gland(Reference Griinari, Bauman, Yurawecz, Mossoba and Kramer2). Although there are many isomers, cis-9, trans-11-CLA and trans-10, cis-12-CLA have received the most attention due to their known biological effects(Reference Hayashi, de Medeiros and Carvalho3) and ability to chemically synthesise. Studies have revealed that the trans-10, cis-12-CLA isomer is responsible for milk fat depression in lactating cows(Reference Baumgard, Corl and Dwyer4), ewes(Reference Oliveira, Gama and Fernandes5,Reference Sandri, Camêra and Sandri6) , goats(Reference Baldin, Gama and Dresch7,Reference Fernandes, Gama and Ribeiro8) , mice(Reference Loor, Lin and Herbein9) and pigs(Reference Poulos, Azain and Hausman10,Reference Lee, Joo and Lee11) , while cis-9, trans-11-CLA has been proposed to have healthful properties for humans to combat cancer(Reference Ip, Chin and Scimeca12,Reference Belury13) , inflammation(Reference Cook, Miller and Park14) and atherogenesis(Reference Lee, Kritchevsky and Parizaa15).
In lactating sows, milk is the major source of nutrients for suckling piglets and their maximal growth performance and survival largely depend on enough milk being produced by sows(Reference Wu, Bazer and Wallace16). Moreover, it has been documented that sows utilise energy from their body stores for milk production(Reference Noblet and Etienne17), resulting in a loss of body weight (BW) during lactation(Reference Quesnel, Etienne and Père18). Additionally, several studies have reported that BW loss during lactation reduces reproductive performance in the subsequent pregnancy(Reference Clowes, Aherne and Foxcroft19,Reference Thaker and Bilkei20) . Thus, it is important to minimise BW loss in sows during lactation as well as to maintain both maximal growth of piglets and subsequent reproductive performance(Reference Lee, Joo and Lee11).
Feeding ruminants rumen-protected trans-10, cis-12-CLA supplements presents an opportunity to manipulate milk fat synthesis, since these supplements may improve the energy balance of lactating animals by reducing the amount of energy required for milk synthesis(Reference Griinari, Bauman, Sejrsen, Hvelplund and Nielsen21). Spared energy can be partitioned towards the synthesis of other milk components(Reference Medeiros, Oliveira and Aroeira22), or, alternatively, depending on the lactation period, it could also be used to replenish body fat reserves(Reference Harvatine, Perfield and Bauman23), which may improve reproductive performance. Research at the cellular level has demonstrated a coordinated down-regulation in the transcript abundance of genes involved in milk fat synthesis in the mammary gland of lactating cows(Reference Baumgard, Matitasshvili and Corl24) and bovine mammary primary cells and cell lines(Reference Kadegowda, Bionaz and Piperova25) and also in ewes(Reference Ticiani, Urio and Ferreira26) and goats(Reference Shi, Zhang and Li27), caused by supplementation with trans-10, cis-12-CLA.
However, the molecular mechanisms behind the inhibitory effect of trans-10, cis-12-CLA on mammary lipid synthesis in sows remain unclear. Therefore, we evaluated the effect of CLA on milk composition and also mammary and adipose lipogenic gene expression, mainly focusing on genes involved in the metabolism of fatty acids in the mammary gland and adipose tissue of lactating sows.
Materials and methods
Animals, design and treatments
All procedures were approved by the Santa Catarina State University Ethical Committee, protocol no. 3162250216, and were performed at a commercial farm in Concórdia City, SC (27º 14′ 03″ S and 52º 01′ 40″ W). Twenty multiparous sows from a commercial genotype (Aurora Genetics) in their 1st–5th parities and weighing (BW) 200 (se 10) kg were randomly assigned to one of the following treatments: (1) control, without CLA added to the diet; or (2) 1 % CLA (containing 4·1 % palmitic acid, 3·6 % stearic acid, 27·4 % oleic acid, 1·2 % linoleic acid, 29·8 % cis-9, trans-11-CLA, 29·9 % trans-10, cis-12-CLA and 3·0 % other fatty acids) mixed into the diet. The amount of CLA mixed into the feed was based on the procedure outlined in Lee et al. (Reference Lee, Joo and Lee11).
Management, feeding, experimental period, sampling and analysis
The experimental period was 18 d and CLA feeding started on day 7 of lactation and was maintained till day 25 of lactation. The sows were moved into farrowing rooms after 108 d of gestation and were housed individually in pens (2·2 × 1·6 m) with slatted floors and controlled temperature and relative humidity. Experimental diets were formulated according to the company to which the farm was integrated to meet the recommended nutrients required by the animals and contained ground maize (68·8 %), soyabean meal (23·2 %), a commercial vitamin/mineral mix (5 %) and soyabean oil (3 %). The soyabean oil was replaced by CLA in the experimental treatment (1 % of the total amount). The nutritional composition of diets contained, on average, 18 % crude protein, 6·5 % diethyl ether extract, 0·65 % Ca, 0·5 % total P, 2·7 % fibre and 4·6 % mineral matter. Sows were fed twice per d and received 7·4 (se 0·1) kg/d (as fed) of the ration. Water access was ad libitum.
The number of piglets in each litter was adjusted (twelve piglets per sow) by cross-fostering piglets within 24 h of birth. Litters’ weights were recorded immediately prior to initiation of CLA treatment (7th d of the lactation) and termination of treatment (25th d of lactation), and piglets were weaned at 28 d of age. In the first 5 d after birth, the litters were subjected to normal management procedures, including cutting of milk teeth, tail docking, ear notching, Fe shots and castration of males. At 2 weeks old, the litters began to receive supplemental dry feed for adaptation to a solid diet. The litters from both treatments received the same ration, and the intake was approximately 0·80 kg (as fed) during this period.
Milk samples were collected on days 7 and 25 of lactation. Approximately 50 ml of milk was obtained by manual stripping after intravenous injection of 0·5 ml of oxytocin (Ocitovet©; Ceva Santé Animal). The samples were stored at 4°C with a preservative (Bronopol tablet; D & F Control Systems Inc.). Milk fat, protein, lactose and total solids were determined by infrared analysis (method 972.160(28)) and fatty acid profile on day 25 as below.
Weaning-to-oestrus interval was determined by monitoring oestrus by daily exposure to a boar on days 3–7 after weaning.
Milk fatty acid profile analysis
Milk fatty acid profile was determined on milk collected on the last day of treatment (day 25 of lactation). Milk fat cake was obtained by centrifuging refrigerated milk at 3000 rpm for 15 min at 4°C. Approximately 50 mg was then methylated following the methodology of O’Fallon et al. (Reference O’Fallon, Busboom and Nelson29). The resulting fatty acid methyl esters (FAME) were determined using a GC (model Focus GC; Thermo Scientific) equipped with a flame ionisation detector and a fused silica capillary column SP-2560 (100 m × 25 mm × 0·2 µm of film thickness; Supelco). H2 was used as a carrier gas (1 ml/min), and N2 was used as an auxiliary gas. Detector and injector temperatures were both set at 250°C with an injection split ratio of 15:1. Initial oven temperature was set to 70°C for 4 min, was increased by 13°C/min to 175°C, at which point it was maintained for 27 min, and then increased 4°C/min to 215°C, where it was maintained for 31 min(Reference Kraemer, Fellner and Dugan30). The FAME were identified by comparing with three FAME references (Supelco FAME mix no. C4-C24, trans-9, cis-11-CLA no. 16413 and trans-10, cis-12-CLA no. 04397; Sigma Aldrich). The cis/trans-18 : 1 isomers were identified according to their order of elution reported under the same chromatographic conditions(Reference Kraemer, Fellner and Dugan30).
Mammary and adipose tissue biopsies
Mammary biopsies were taken on day 25 of lactation, after 18 d of treatment. A tranquiliser was administered (2 ml/sow intramuscular and 6 ml/sow intravenous of Destress injectable; Des-Far Laboratories LDTA) to immobilise the animals, and lidocaine hydrochloride subdermal (2 ml/sow) was then administered above the incision site. A coaxial needle with a trocar was introduced to the first or second thoracic mammary glands. The biopsy was collected using a Bard Max-Core Disposable Core Biopsy Instrument (Bard Biopsy Systems). Briefly, a 16-gauge biopsy needle was partially inserted through the coaxial needle and two tissue samples (approximately 35 mg tissue/biopsy) were collected. These were inspected to verify tissue homogeneity, rinsed with saline solution, placed in cryotubes containing 1 ml of Dulbecco’s PBS (Gibco Laboratories) and were immediately stored in liquid N2 until RNA extraction. The biopsy procedure resulted in minimal bleeding and no intra-mammary infections were observed.
The adipose tissue biopsy was taken from the tail head region immediately cranial and lateral to the last lumbar vertebra (dorsal subcutaneous depot). Prior to the biopsy, site asepsis was performed and lidocaine hydrochloride subdermal was administered in a circular pattern surrounding the incision site (2 ml/sow). A small incision was made in the skin, and adipose tissue was dissected. Two samples of adipose tissue (approximately 100 mg) from the same site were obtained, rinsed with sterile saline solution, placed in cryotubes with PBS and frozen in liquid N2 until RNA extraction. The incision was closed with number 1 Nylon using a blanket stitch. After biopsies of adipose and mammary tissues, an anti-inflammatory was administered (flunixin meglumine; 1·1 mg/kg of BW).
RNA extraction, synthesis of complementary DNA and quantitative real-time RT PCR
Total RNA extraction, synthesis of complementary DNA and quantitative real-time PCR (RT-qPCR) were all carried out according to the methodology of Sandri et al. (Reference Sandri, Camêra and Sandri6). Briefly, total RNA was extracted from both mammary and adipose tissue samples using the RNeasy Lipid Tissue Mini Kit (Qiagen Sciences) with on-column DNAse treatment (On-Column DNase I Digestion Set; Sigma-Aldrich). The RNA concentration was measured using a spectrophotometer (NanoDrop ND-2000; NanoDrop Technologies), and using the same spectrophotometer, the quality was evaluated by the A260/280 ratio, which was approximately 2·03 (se 0·01). Total RNA was transcribed to form complementary DNA using the GoScript™ Reverse Transcription Mix (Promega Corporation) with random primers. PCR amplification was performed in triplicates in a 48-well reaction plate (MicroAmp™; Applied Biosystems) with 15 μl volume reaction with 30 ng of complementary DNA and 7·5 μl GoTaq qPCR Master Mix (Promega) in a StepOne Real-Time machine (Applied Biosystems) under the following conditions: 95°C for 2 min, forty cycles of 95°C for 15 s, 62°C for 1 min and 95°C for 15 s. The data were analysed using StepOne software version 2.1 (Applied Biosystems). All primers used were previously validated for the formation of a single product by melting curve and amplification efficiency. Each sample was run using seven-point standard curves with a ‘pool’ of complementary DNA from the mammary or adipose tissues with serial dilutions (100, 50, 25, 12·5, 6·25, 3·125 and 1·5625 %). Subsequently, a regression equation was generated by plotting the cycle threshold values from RT-qPCR against the log of each value from the standard curve. The slope of the equation was used to determine efficiency of the reaction.
Primer design
Gene sequences for primer designs were obtained from the gene bank of the National Center for Biotechnology Information (NCBI, USA). All primers were designed by the Prime-BLAST tool of NCBI, synthesised at Invitrogen™ and were tested for their efficiency before use. Expression of the following genes was measured: acetyl-CoA carboxylase-α (ACACAα), fatty acid synthase (FASN), stearoyl-CoA desaturase 1 (SCD1), lipoprotein lipase (LPL), fatty acid binding protein 3 (FABP3), acyl glycerol phosphate acyltransferase 6 (AGPAT6), diacylglycerol acyltransferase 1 (DGAT1), casein-α S1, casein-β, casein-κ and α-lactalbumin. The primer sequences of the evaluated genes are listed in Table 1, and the description of the function of them is presented in online Supplementary Table S1.
Table 1. Swine primers used in the quantitative real-time RT PCR (RT-qPCR) analysis

RPS18, ribosomal protein S18; ACTB, β-actin; ACACAα, acetyl-CoA carboxylase-α; FASN, fatty acid synthase; SCD1, stearoyl-CoA desaturase 1; LPL, lipoprotein lipase; FABP3, fatty acid binding protein 3; AGPAT6, acyl glycerol phosphate acyltransferase 6; DGAT1, diacylglycerol acyltransferase 1; CSN1S1, casein-α S1; CSN2, casein-β; CSN3, casein-κ; LALBA, α-lactalbumin.
* Primers are reported as 5′ to 3′ sequence.
Statistical analysis
Data were analysed using the MIXED procedure in SAS(31). Milk yield and concentration and yield of milk components were analysed using the MIXED procedure with day ‘zero’ as a covariate, treatment as a fixed effect and the individual animals as a random effect. The data for all genes of interest were normalised by the geometric mean of the reference genes ribosomal protein S18 and β-actin(Reference Vandesompele, De Preter and Pattyn32), and the model for gene expression included the fixed effect of treatment and animal as a random effect.
Data points with Studentised residuals outside of ±2·5 were considered outliers and were excluded from analysis. When necessary, data were log2-transformed and back-transformed data are reported. Least-squares means (LSMEANS) were used to compare treatments, and significance was declared at P < 0·05 and a trend at P < 0·10.
Results
Milk composition, litter performance and weaning-to-oestrus interval
Milk composition, piglet weaning weight and weaning-to-oestrus interval are presented in Table 2. Milk fat and protein concentration were decreased 20 % (P = 0·004) and 11 % (P < 0·0001) by CLA, compared with the control. Milk casein content decreased 17 % (P < 0·0001) and total solids decreased 9·6 % (P = 0·0004) in the CLA treatment. There was also a trend for increased lactose content with CLA treatment (P = 0·10).
Table 2. Effect of trans-10, cis-12 conjugated linoleic acid (CLA) on milk composition, piglet weaning weight, and weaning-to-oestrus interval (WEI) of lactating sows
(Mean values with their pooled standard errors)
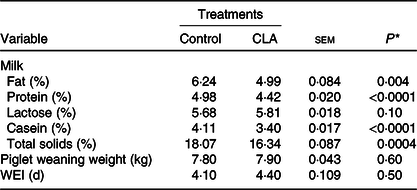
* Overall effect of treatment.
There was no effect of CLA on weaning-to-oestrus interval or piglet weight. The litters from both treatments received the same ration at 2 weeks of age, and intake was approximately 0·80 kg (as fed basis) during this period.
Milk fatty acid profile analysis
Milk fatty acid compositions are shown in Table 3. Dietary CLA increased the concentration of 14 : 0 (P = 0·003), 18 : 0 (P < 0·0001), trans-18 : 1 (P = 0·0004), C20 : 0 (P < 0·0001), C20 : 4n-6 (P = 0·03) and cis-9, trans-11-CLA (P = 0·0004) and increased the proportion of SFA by 16 % (P < 0·0001). In contrast, CLA reduced the concentration of 14 : 1 (P = 0·0002), 16 : 1 (P = 0·0001), 18 : 1n-9 (P = 0·03), 18 : 2n-6 (P = 0·03) and 18 : 3n-3 (P = 0·03) and decreased the proportion of MUFA by 17·6 % (P < 0·0001). The CLA treatment did not alter total PUFA concentration.
Table 3. Effect of trans-10, cis-12 conjugated linoleic acid (CLA) on fatty acid (FA) composition (% total FA)
(Mean values with their pooled standard errors)

* Overall effect of treatment.
† Trans-10, cis-12-CLA was not detected in the control treatment.
‡ ∑ SFA, ∑ MUFA, ∑ PUFA: sum of SFA, MUFA and PUFA, respectively.
As expected, the trans-10, cis-12-CLA isomer was not detectable in milk fat of the control; however, it was increased to 0·58 % of fatty acids in the CLA treatment.
Expression of lipogenic genes in mammary gland and adipose tissue
In the mammary gland, CLA reduced mammary expression of ACACAα by 37 % (P = 0·003), FASN by 64 % (P = 0·002) and SCD1 by 52 % (P = 0·003) (Fig. 1(A)). In genes involved in the uptake and transport of fatty acids, CLA reduced mammary LPL expression by 26 % (P = 0·03, Fig. 1(B)), whereas CLA tended to reduce the expression of FABP3 (P = 0·09, Fig. 1(B)). Finally, mammary expression of the TAG synthesis genes AGPAT6 was reduced by 15 % (P = 0·02), and DGAT1 was reduced 27 % (P = 0·02) (Fig. 1(C)).
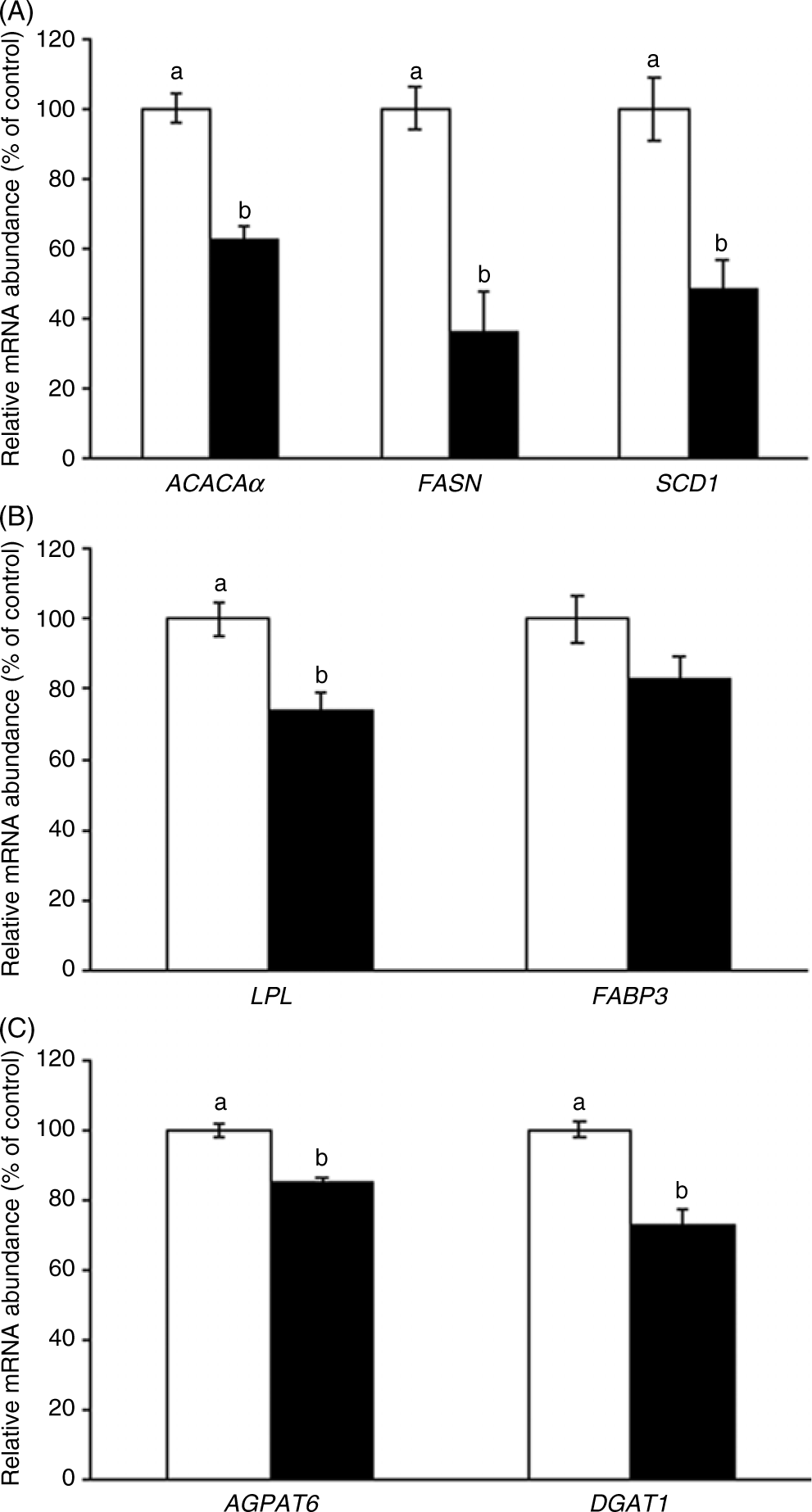
Fig. 1. Acetyl-CoA carboxylase-α (ACACAα), fatty acid synthase (FASN), stearoyl-CoA desaturase 1 (SCD1) (A), lipoprotein lipase (LPL), fatty acid binding protein 3 (FABP3) (B), acyl glycerol phosphate acyltransferase 6 (AGPAT6) and diacylglycerol acyltransferase 1 (DGAT1) (C) gene expression in the mammary gland of sows supplemented with trans-10, cis-12 conjugated linoleic acid ( ), compared with control (
), compared with control ( ). Values are means with their standard errors. a,bMean values with unlike letters were significantly different (P < 0·05).
). Values are means with their standard errors. a,bMean values with unlike letters were significantly different (P < 0·05).
In adipose tissue, CLA treatment had no effect on the gene expression of all lipogenic genes evaluated (ACACAα, P = 0·92; FASN, P = 0·67 and SCD1, P = 0·80, Fig. 2(A); LPL, P = 0·73 and FABP3, P = 0·88, Fig. 2(B); and AGPAT6, P = 0·69 and DGAT1, P = 0·40, Fig. 2(C)).
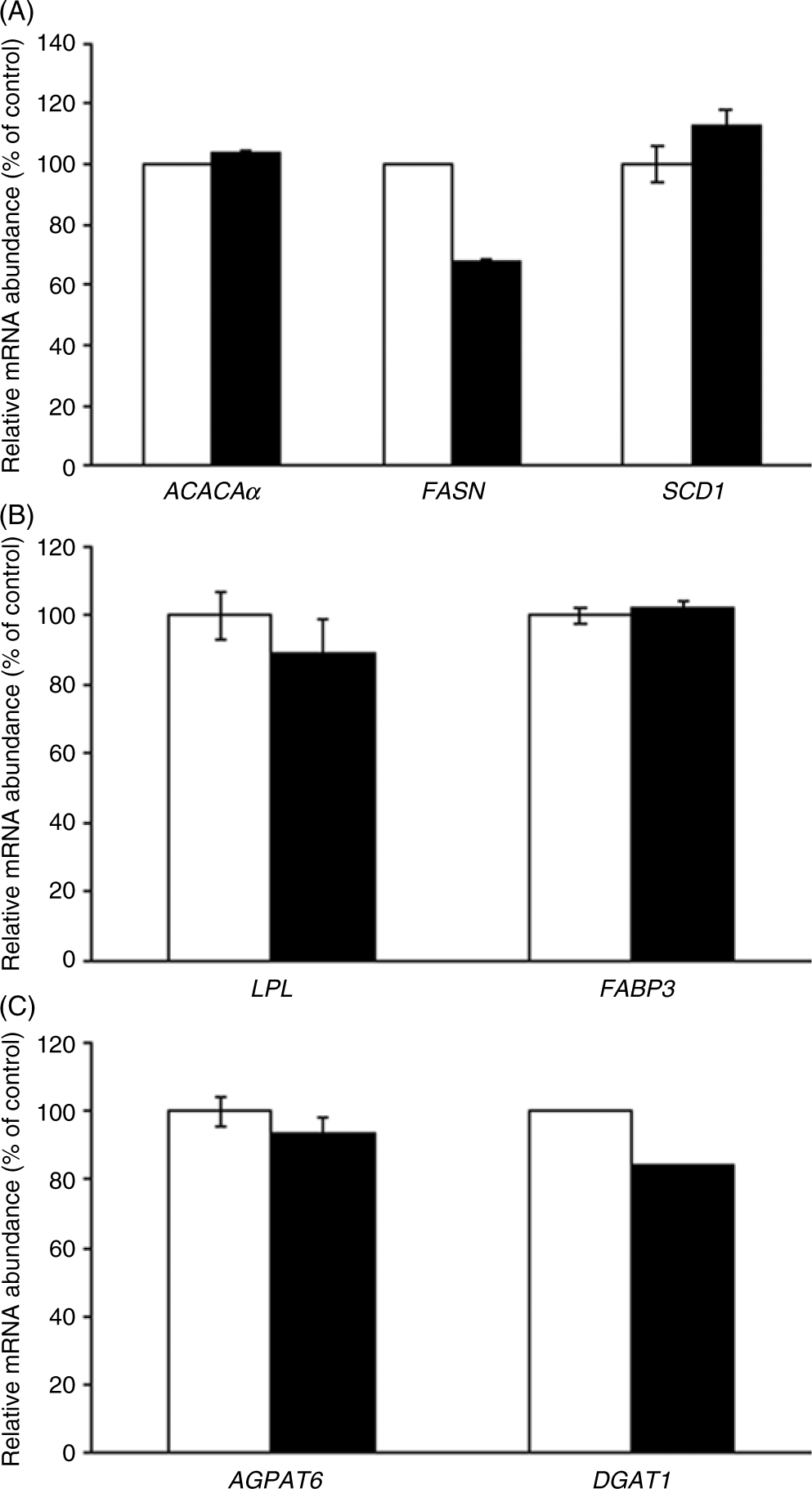
Fig. 2. Acetyl-CoA carboxylase-α (ACACAα), fatty acid synthase (FASN), stearoyl-CoA desaturase 1 (SCD1) (A), lipoprotein lipase (LPL), fatty acid binding protein 3 (FABP3) (B), acyl glycerol phosphate acyltransferase 6 (AGPAT6) and diacylglycerol acyltransferase 1 (DGAT1) (C) gene expression in the adipose tissue of sows supplemented with trans-10, cis-12 conjugated linoleic acid ( ), compared with control (
), compared with control ( ). Values are means with their standard errors.
). Values are means with their standard errors.
Expression of genes coding for milk protein in mammary gland
Since milk protein was modified by CLA treatment (Table 2), the expression of individual milk protein genes was examined (Fig. 3). Among the caseins, CLA treatment reduced β-casein gene expression by 68 %, compared with the control (P = 0·0004) and reduced whey protein α-lactalbumin expression by 62 % (P = 0·005).
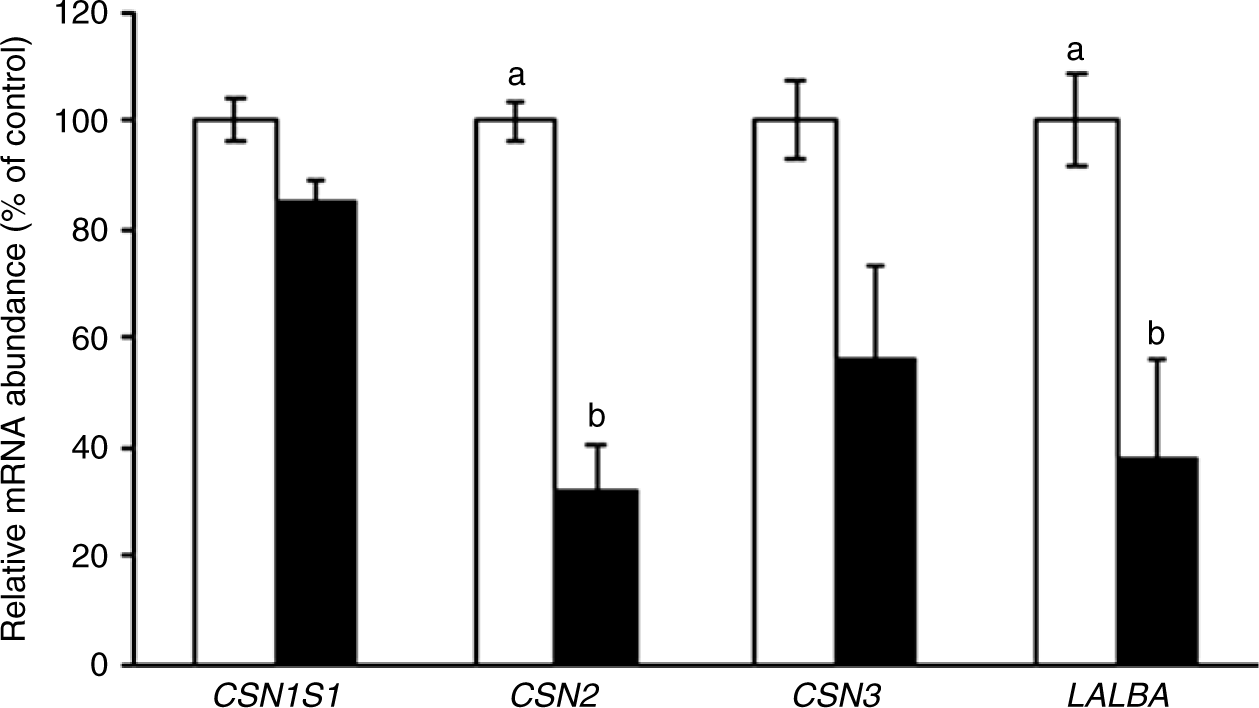
Fig. 3. Casein-α S1 (CSN1S1), casein-β (CSN2), casein-κ (CSN3) and α-lactalbumin (LALBA) gene expression in the mammary gland of sows supplemented with trans-10, cis-12 conjugated linoleic acid ( ), compared with control (
), compared with control ( ). Values are means with their standard errors. a,bMean values with unlike letters were significantly different (P < 0·05).
). Values are means with their standard errors. a,bMean values with unlike letters were significantly different (P < 0·05).
Discussion
Supplementing lactating sows with CLA reduced fat content in milk similar to previous studies. In the present study, the reduction in milk fat concentration was approximately 20 %, which is in accordance with the results of other studies in sows (approximately 14–36 %(Reference Cordero, Isabel and Morales33,Reference Harrell, Phillips and Boyd34) ). Variation in responses may be due to the level and time of supplementation, the major isomer used, and the genotype and physiological state of the individual animals(Reference Lee, Joo and Lee11). A negative effect of CLA on milk protein content was observed in the present study. As milk protein concentration is positively associated with dietary energy content(Reference Bocquier and Caja35), the CLA-induced reduction in energy level may have reduced milk protein synthesis. In addition, trans-10, cis-12-CLA, but not the cis-9, trans-11 isomer, stimulates expression of the mammalian target of rapamycin in the mammary gland of the sows before initiating lactation, and it effects protein synthesis in this species(Reference Chung, Brown and Sandberg36,Reference Manjarín, Steibel and Kirkwood37) . As a mixture of these isomers was used, the milk protein reduction in the CLA-treated animals may be due to the isomer-specific action of cis-9, trans-11-CLA on mammalian target of rapamycin expression.
Unlike other studies, the weight of the piglets did not differ between the treatments. Corino et al. (Reference Corino, Pastorelli and Rosi38) and Cordero et al. (Reference Cordero, Isabel and Morales33) found increased weaning weight in piglets from sows receiving CLA, which may be a reflection of the increase in milk yield of the animals, which was not measured in our study. Moreover, the dry feed intake of the piglets was not different between treatments, and there was a trend for increased lactose content in CLA treatment (Table 2). As this is the drive for milk synthesis, we suggest that milk yield was increased by CLA treatment. Lastly, the weaning-to-oestrus interval of the sows was not affected by the treatments, which corroborates the work of Lee et al.(Reference Lee, Joo and Lee11).
Supplementation with CLA had a marked effect on milk fatty acid profile, with an increase in total SFA, a decrease in MUFA and no effect on total PUFA. These results are in agreement with those of previous studies(Reference Cordero, Isabel and Morales33,Reference Bee39) . The distinct shift towards higher SFA and lower MUFA synthesis indicate the potential down-regulation of SCD1 expression by dietary CLA(Reference Smith, Hively and Cortese40). This can be evidenced by the reduction in expression of lipid synthesis genes (Fig. 1(A)) and the effect observed on 16 : 1, a product of the 16 : 0 desaturation process through SCD1, and by the observed reduction in LPL expression (Fig. 1(B)), which hydrolyses and captures fatty acids from the diet. These observations may indicate a lower presence of these fatty acids in the milk and, consequently, a greater mobilisation of the reserve fatty acids, composed of SFA.
Another important aspect is that the concentration of milk fatty acids synthesised de novo in the mammary gland did not change or was increased with CLA treatment (e.g. 8 : 0, 10 : 0 and 12 : 0 were not modified, and 14 : 0 was increased by CLA), while the expression of the ACACAα and FASN genes, responsible for this synthesis pathway, was reduced by CLA. This discrepancy between the results may be explained by the presence of the enzyme thioesterase II in the mammary tissue of non-ruminants. Thioesterase II appears to function identically to thioesterase I in that it terminates the chain(Reference Smith41). However, thioesterase II specificity differs, producing 8 : 0 to 14 : 0 fatty acids, meaning that fatty acid synthesis in the mammary gland is terminated at the level of myristic acid (14 : 0) rather than palmitic acid (16 : 0), as in other tissues(Reference Chen, Pelletier and Hollywood42). Moreover, Duttaroy et al. (Reference Duttaroy, Crozet and Taylor43) investigated the effects of fatty acids on thioesterase activity in placental choriocarcinoma (BeWo) cells and found that its activity was increased by CLA, which could help explain the higher concentration of 14 : 0 in the CLA treatment. However, it is impossible to know how the de novo proportion of 16 : 0 was not impacted because it was not decreased even with higher termination at 14 : 0.
Regarding the CLA isomers, the presence of trans-10, cis-12 isomer was not detectable in the control and because of this, it could not be analysed statistically. However, the isomer was present in the CLA treatment. The concentration of the cis-9, trans-11 isomer increased by more than 4-fold with CLA treatment. Given that the dietary CLA supplement contained the two isomers in similar proportions, studies have shown that the trans-10, cis-12 isomer is linked to a less efficient mechanism of incorporation into tissues than the cis-9, trans-11 isomer(Reference Fiego, Macchioni and Santoro44,Reference Ostrowska, Cross and Muralitharam45) .
To our knowledge, this was the first study that evaluated effect of CLA on gene expression in mammary gland and adipose tissue of lactating sows; therefore, comparisons were made with ruminants or growing pigs. As in our study, previous works in cows, ewes and goats have established the anti-lipogenic effect of the trans-10, cis-12-CLA and have shown that its causes a reduction in the expression of genes involved in many lipid synthesis enzymes including ACACAα, FASN, SCD1, LPL, AGPAT and DGAT (Reference Baumgard, Matitasshvili and Corl24,Reference Shi, Zhang and Li27,Reference Hussein, Harvatine and Weerasinghe46) .
Our results revealed a more pronounced reduction in the expression of genes involved in de novo fatty acid synthesis (e.g. more than 60 % reduction in FASN), whereas the TAG synthesis genes and genes involved in the uptake and transport of preformed fatty acids were less affected. This was contrary to our hypothesis that genes related to preformed acids would be more affected by supplementation with CLA.
Spincer et al. (Reference Spincer, Rook and Towers47) used the arteriovenous difference technique to identify milk precursors in lactating sows between the 5th and 6th week of lactation. Nutrients that had a high percentage of extraction from mammary gland included glucose (31 %), essential amino acids (22–38 %) and the TAG fatty acids, oleate (23 %) and palmitate (19 %). The percentage of extraction of NEFA (−6 %) and β-hydroxybutyrate (11 %) suggested a minor role for these metabolites in milk synthesis. The authors proposed that the incorporation of preformed fatty acids appears to be less important quantitatively, suggesting that de novo synthesis of milk fat is greater in pigs and that it is formed from glucose as opposed to acetate and β-hydroxybutyrate as in ruminants(Reference Spincer, Rook and Towers47). Therefore, it is worth emphasising that synthesis from reserve fatty acids is more intense at the beginning of the lactation and as we evaluated the gene expression at the end of the lactation, consequently, in this period, de novo synthesis playing an important role in mammary lipogenesis and this can explain the greater response of the ACACAα and FASN genes to CLA. This may have played a role in the relatively lower magnitude of reduction in LPL expression, and in the observed tendency to reduce FABP3, compared with de novo synthesis genes.
The expression of the FABP3 gene was the only one unaffected by CLA in the mammary gland, and this corroborates with the results of Peterson et al. (Reference Peterson, Matitashvili and Bauman48) and Hussein et al. (Reference Hussein, Harvatine and Weerasinghe46). The aforementioned studies observed the same result in lactating cows and ewes receiving diets that induced milk fat depression (high concentrate/low forage) and that were supplemented with CLA, respectively.
Among the FABP isoforms, Bionaz & Loor(Reference Bionaz and Loor49) confirmed the predominance of FABP3 in cow mammary gland, which has also been found in mice(Reference Rudolph, McManaman and Phang50) and other species(Reference Haunerland and Spener51). According to Bionaz & Loor(Reference Bionaz and Loor49), the expression of FABP3, FABP4 and FABP5, the most abundant among the FABP isoforms, was up-regulated by the onset of lactation and increased greatly (1- to 78-fold) relative to their content prepartum. Among them, FABP3 is the most involved in bovine mammary lipid synthesis. We suggest that, just as the FABP3 is highly expressed in lactating cows, this could also be the same case in sows, to the point that the amount and period of CLA supplementation in the present study were not sufficient to cause a difference in its expression. In contrast, the lack of reduction in FABP3 may be due to the fact that the animals were in a positive energy balance and therefore fatty acid mobilisation from the reserves was not important.
In the adipose tissue, increased lipid synthesis during milk fat depression may be an indirect response to the reduction in energy required for milk fat synthesis(Reference Sandri, Camêra and Sandri6). Harvatine et al. (Reference Harvatine, Perfield and Bauman23) observed that cows abomasally infused with trans-10, cis-12-CLA had increased gene expression of enzymes involved in lipid synthesis (FASN, SCD1 and FABP1) and of lipid synthesis regulatory transcription factors (PPARγ) in adipose tissue, whereas fat synthesis decreased in the mammary gland. However, it is worth noting that in addition to a reduction in milk fat, there was a reduction in voluntary intake, resulting in an excess of available energy that was directed to adipose tissue. In our study, the voluntary intake was not measured as ration refusals were not observed, so there may not have been an excess of energy capable of stimulating greater fat synthesis in the adipose tissue, resulting in no changes in the expression of lipogenic genes in this tissue.
Specifically in pigs, some aspects must be taken into account: (1) in these animals, adipose tissue is the main site of lipogenesis(Reference O’Hea and Leveille52), and Duran-Montgé et al. (Reference Duran-Montgé, Theil and Lauridsen53) observed that SFA were equivalent (or more potent) inhibitors of lipogenesis in the adipose tissue of pigs to unsaturated fatty acids, since the animals fed on the diet with highest SFA content tended to have decreased abundance of ACACAα, FASN and SCD1 mRNA, relative to other diets. Therefore, the CLA in the adipose tissue of the sows in our experiment would not have as potent an effect as in other species; (2) Zhou et al. (Reference Zhou, Li and Yin54) observed that trans-10, cis-12-CLA, but not cis-9, trans-11-CLA, reduced the mRNA expression of adipocyte determination and differentiation factor-1, PPARγ, adipocyte fatty acid binding protein and LPL genes in subcutaneous adipose tissue cultures with a 6 d treatment. Therefore, when a mixture of isomers is used (as in the present study), different effects should be considered.
As a reduction in protein content was observed with CLA treatment, consequently the expression of β-casein and α-lactalbumin genes was reduced. It is interesting to note that even with the reduction in α-lactalbumin, one of the main routes of lactose synthesis, there was an increasing trend in lactose concentration. Lactose synthesis is a complicated process that requires the coordination of many genes encoding for enzymes involved in glucose uptake(Reference Kuhn and White55,Reference Zhao56) , glucose–galactose interconversion(Reference Mohammad, Hadsell and Haymond57), uridine diphosphate galactose transportation(Reference Mohammad, Hadsell and Haymond57,Reference Kuhn and White58) and synthesis of lactose(Reference Rudolph, McManaman and Phang50,Reference Neville59) . The synthesis itself occurs through lactose synthase composed of β1,4-galactosyltransferase 1 and α-lactalbumin, and according Zhang et al. (Reference Zhang, Zhang and Guan60), the increased lactose synthesis related to the coordinated up-regulation of genes or enzymes involved in the lactose synthesis pathway, glucose transportation and lactose synthetase (β1,4-galactosyltransferase 1 and α-lactalbumin) might be the critical step in the lactose synthesis pathway of sows during lactation. The reason why the lactose concentration trended to increase even with reduced expression of α-lactalbumin is unclear but may involve other mechanisms of synthesis or a differentiated effect of CLA.
For being the first study of our group with lactating sows, and due to management and routine of the farm, some additional analyses could have been done, which eventually limited the interpretation of our results, such as milk collection, blood collection, biopsy at the beginning of the experimental period. Furthermore, for being also a first study on the trans-10, cis-12 CLA action on gene expression in the mammary gland in this animal species some points need to be better elucidated (e.g. mechanisms of action of the lipogenic genes and the lactation metabolism in sows) in order to extrapolate and to validate these results in future studies, including in humans.
Conclusion
CLA reduces the fat and protein content of milk in sows, without affecting the performance of the litter. The expression of genes involved in the lipogenic pathways evaluated was reduced by CLA in the mammary gland, and a greater expression intensity was observed for de novo synthesis genes. The reduction of genes coding for milk protein is a breakthrough in the present study.
Acknowledgements
The authors acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes) for the scholarship to E. C. S.. The authors also would like to thank Mr. Rafael Dallagnol and his family from Dallagnol Farm and Priscila C. Carraro for assistance.
The authors gratefully acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (grant no. 403731/2016-0 to D. E. O.) for the financial support.
The authors’ contributions were as follows: E. C. S. and D. E. O. designed the research and E. C. S., D. E. O. and K. J. H. wrote the paper; E. C. S. conducted the animal experiment and data analysis with guidance from D. E. O. All authors read and approved the final manuscript.
The authors declare that they have no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114519003325









