Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by Crossref.
Rodríguez-Bermúdez, Ruth
Herrero-Latorre, Carlos
López-Alonso, Marta
Losada, David E.
Iglesias, Roberto
and
Miranda, Marta
2018.
Organic cattle products: Authenticating production origin by analysis of serum mineral content.
Food Chemistry,
Vol. 264,
Issue. ,
p.
210.
van der Reijden, Olivia L.
Galetti, Valeria
Hulmann, Marie
Krzystek, Adam
Haldimann, Max
Schlegel, Patrick
Manzocchi, Elisa
Berard, Joel
Kreuzer, Michael
Zimmermann, Michael B
and
Herter-Aeberli, Isabelle
2018.
The main determinants of iodine in cows’ milk in Switzerland are farm type, season and teat dipping.
British Journal of Nutrition,
Vol. 119,
Issue. 5,
p.
559.
Rodríguez-Bermúdez, R.
López-Alonso, M.
Miranda, M.
Fouz, R.
Orjales, I.
and
Herrero-Latorre, C.
2018.
Chemometric authentication of the organic status of milk on the basis of trace element content.
Food Chemistry,
Vol. 240,
Issue. ,
p.
686.
Orjales, I.
Herrero-Latorre, C.
Miranda, M.
Rey-Crespo, F.
Rodríguez-Bermúdez, R.
and
López-Alonso, M.
2018.
Evaluation of trace element status of organic dairy cattle.
Animal,
Vol. 12,
Issue. 6,
p.
1296.
van der Reijden, Olivia L.
Galetti, Valeria
Herter-Aeberli, Isabelle
Zimmermann, Michael B.
Zeder, Christophe
Krzystek, Adam
Haldimann, Max
Barmaz, Andrea
Kreuzer, Michael
Berard, Joel
and
Schlegel, Patrick
2019.
Effects of feed iodine concentrations and milk processing on iodine concentrations of cows’ milk and dairy products, and potential impact on iodine intake in Swiss adults.
British Journal of Nutrition,
Vol. 122,
Issue. 2,
p.
172.
Mullan, Karen
Hamill, Lesley
Doolan, Katy
Young, Ian
Smyth, Peter
Flynn, Albert
Walton, Janette
Meharg, Andrew A.
Carey, Manus
McKernan, Claire
Bell, Marcia
Black, Neil
Graham, Una
McCance, David
McHugh, Cathy
McMullan, Paul
McQuaid, Siobhan
O’Loughlin, Aonghus
Tuthill, Antoinette
Bath, Sarah C.
Rayman, Margaret
and
Woodside, Jayne V.
2020.
Iodine status of teenage girls on the island of Ireland.
European Journal of Nutrition,
Vol. 59,
Issue. 5,
p.
1859.
Woodside, Jayne V.
and
Mullan, Karen R.
2021.
Iodine status in UK–An accidental public health triumph gone sour.
Clinical Endocrinology,
Vol. 94,
Issue. 4,
p.
692.
Witard, Oliver C.
Bath, Sarah C.
Dineva, Mariana
Sellem, Laury
Mulet-Cabero, Ana-Isabel
Dongen, Laura H. van
Zheng, Ju-Sheng
Valenzuela, Carina
and
Smeuninx, Benoit
2022.
Dairy as a Source of Iodine and Protein in the UK: Implications for Human Health Across the Life Course, and Future Policy and Research.
Frontiers in Nutrition,
Vol. 9,
Issue. ,
Niero, G.
Visentin, G.
Censi, S.
Righi, F.
Manuelian, C.L.
Formigoni, A.
Mian, C.
Bérard, J.
Cassandro, M.
Penasa, M.
Moore, S.
Costa, A.
and
De Marchi, M.
2023.
Invited review: Iodine level in dairy products—A feed-to-fork overview.
Journal of Dairy Science,
Vol. 106,
Issue. 4,
p.
2213.
Tattersall, Joanne K.
Peiris, Manishka S.
Arai, Maika
McCully, Katherine
Pearce, Neeve
Rayman, Margaret P.
Stergiadis, Sokratis
and
Bath, Sarah C.
2024.
Variation in milk‑iodine concentration around the world: a systematic review and meta-analysis of the difference between season and dairy-production system.
Food Chemistry,
Vol. 459,
Issue. ,
p.
140388.



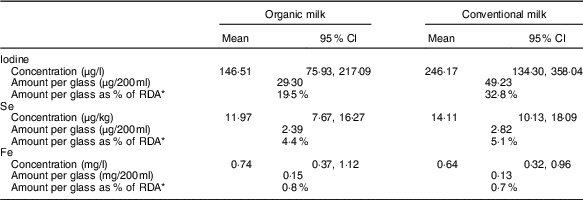

We have been asked to comment on differences in trace-element concentrations between organic and conventional milk found in the recent meta-analysis by Średnicka-Tober et al.( Reference Średnicka-Tober, Barański and Seal 1 ): Higher PUFA and n-3 PUFA, conjugated linoleic acid, α-tocopherol and iron, but lower iodine and selenium concentrations in organic milk: a systematic literature review and meta- and redundancy analyses. Such a comment is important because in fact the most significant difference revealed between organic and conventional milk in terms of contribution to nutrient requirements was that of iodine. In many countries, and particularly in the UK where iodised salt is rarely used( Reference Bath, Button and Rayman 2 ), milk is the single biggest contributor to iodine intake( Reference Bates, Lennox and Prentice 3 ). In contrast, milk is a relatively inconsequential source of fatty acids, particularly of those desirable long-chain n-3 PUFA. This calls into question the emphasis placed on the n-3 PUFA both in the paper and in the press release.
We will concentrate our comment on the differences in iodine, Se and Fe concentrations. We will use the standard meta-analysis data presented by the authors as these are weighted according to the size of the studies (unweighted meta-analyses are generally not considered appropriate) and were the only analyses to find significant differences in mineral concentrations between organic and conventional milk samples. For the same reason, we will use the weighted mean percentage differences derived from the standard meta-analyses.
Deficit in iodine
The standard meta-analysis included six( Reference Jahreis, Leiterer and Fechner 4 – Reference Hanus, Vorlicek and Sojkova 9 ) of the seven studies( Reference Bath, Button and Rayman 10 ) that had iodine data and indicated that conventional milk had a higher iodine concentration than organic milk (Table 1). The mean percentage difference was reported as −73·85 (95 % CI −115·19, −32·5) %, P<0·001. In terms of the iodine content of a glass of milk, this means that organic milk would provide 13·3 % less of the adult RDA (150 μg) than conventional milk (19·5 v. 32·8 %; Table 1).
Table 1 Mean trace-element concentration in organic and conventional milk (concentration figures taken from the online Supplementary Table S10b of the meta-analysis( Reference Średnicka-Tober, Barański and Seal 1 )) (Mean values and 95 % confidence intervals)
* RDA as set by the Institute of Medicine for females, aged 19–50 years( 33 , 37 ).
These findings are very relevant to population health, particularly in the UK, for two reasons. First, iodine deficiency is prevalent in certain UK population groups, notably in pregnant women and women of childbearing age( Reference Bath and Rayman 11 ). This has implications for fetal brain development, as iodine, a crucial component of the thyroid hormones, is essential during pregnancy and early life to ensure appropriate neurological development; for instance, we have shown that iodine deficiency in UK pregnant women is associated with lower intelligence quotient and reading ability in their offspring( Reference Bath, Steer and Golding 12 ). Second, milk and dairy products are the primary source of iodine in the UK diet, contributing 33 % to adult intake and 51 % to child (4–10 years) intake, according to National Diet and Nutrition Survey (NDNS) data( Reference Bates, Lennox and Prentice 3 ). We, and others, have found a positive relationship between milk intake and iodine status in pregnant women( Reference Bath, Walter and Taylor 13 ), school-aged children( Reference Bath, Combet and Scully 14 , Reference Vanderpump, Lazarus and Smyth 15 ) and women of childbearing age( Reference Bath, Sleeth and McKenna 16 ) in the UK.
Thus, the finding of lower iodine content in organic milk is an important message for consumers to hear; for this reason, we were surprised that it was not given more prominence in the article (e.g. by presenting the iodine data in the abstract) and in the press release. The figures show that organic milk is still a reasonable source of iodine – a glass would provide a fifth of the adult recommendation (compared with a third in conventional milk; Table 1). However, the lower iodine message must not be lost when promoting organic milk, and consumers need to be directed to alternative dietary sources( Reference Bath and Rayman 17 ) to ensure that they have adequate iodine intake overall.
Factors that may result in lower iodine concentration in organic milk
The authors of the meta-analysis proposed several possible reasons for the lower iodine concentration of organic milk, including reduced use of mineral supplements and iodophor disinfectants in organic farming. The issue of iodine supplementation of cattle feed was touched upon in the discussion of the meta-analysis. The authors highlighted the recent recommendation by the European Food Safety Authority (EFSA) to reduce the maximum permitted level of iodine in cattle feed from 5 to 2 mg/kg( 18 ). However, it is important to note that EFSA received comments from various member states including Belgium, Finland and the UK, raising concern that a reduction in the maximum permitted level for iodine could potentially exacerbate iodine deficiency in the population( 18 , 19 ). As a result, the European Commission asked EFSA to review the opinions and the evidence; the legal maximum iodine in cattle feed remains at 5 mg/kg but 2 mg/kg is recommended where possible( 20 ).
In fact, iodine supplementation of cattle feed tells only a part of the story in terms of iodine concentration in milk – so-called ‘iodine antagonists’ or goitrogens also play a significant role( Reference Troan, Dahl and Meltzer 21 , Reference Flachowsky, Franke and Meyer 22 ). The authors did not discuss the goitrogenic potential of forage as an explanation for the lower iodine in organic milk. For example, clover is used more extensively in organic farming as a natural fixer of N in place of the prohibited synthetic fertilisers( 23 ). Certain strains of white clover contain cyanogenic glucosides (linamarin and lotaustralin) that are degraded to thiocyanate and act as competitive inhibitors of iodine transport into cows’ milk by the sodium–iodide symporter in the mammary gland( Reference Flachowsky, Franke and Meyer 22 ).
A German study has found that the presence of goitrogens from rapeseed cake in cattle feed lowered the iodine concentration of milk by 50–78 %( Reference Flachowsky, Franke and Meyer 22 , Reference Franke, Meyer and Wagner 24 ). Goitrogens in the feed at various levels of iodine supplementation (up to 5 mg/kg) were shown to reduce the carry-over of iodine from feed to milk( Reference Franke, Meyer and Wagner 24 ). Previous research has focused on glucosinolates from rapeseed cake( Reference Franke, Meyer and Wagner 24 , Reference Hejtmankova, Kuklik and Trnkova 25 ) or crambe cake (Crambe abyssinica)( Reference Flachowsky, Franke and Meyer 22 ) but a potentially similar effect from white clover needs to be quantified.
The meta-analysis did not make it clear whether season was accounted for in the analysis. Of the six studies, some were conducted in a single season (either summer( Reference Gabryszuk, Sloniewski and Sakowski 8 ) or winter( Reference Rey Crespo, Miranda and Lopez-Alonso 7 )), some studies sampled milk in both summer and winter( Reference Dahl, Opsahl and Meltzer 5 , Reference Köhler, Fechner and Leiterer 6 , Reference Hanus, Vorlicek and Sojkova 9 ) and others did not state the season( Reference Jahreis, Leiterer and Fechner 4 ). As the differences between organic and winter milk may not be consistent throughout the year, season of sampling may explain some of the heterogeneity in the data.
Indeed, it has been suggested that the goitrogenic potential of fresh forage is lower than that of feed given in the winter( Reference Hejtmankova, Kuklik and Trnkova 25 ). In the UK, during winter, silage is used on both conventional and organic farms to feed cattle. Silage from organic farms is likely to contain a higher proportion of clover than that from conventional farms. However, the silage-making process may reduce the goitrogenic properties of white clover, as it has been shown to reduce cyanogenic glycosides in other goitrogenic species (Acacia sieberiana)( Reference Ngwa, Nsahlai and Iji 26 ). Therefore, if the goitrogenic effect of clover is reduced by converting it to silage, the difference in iodine concentration between organic and conventional milk may be smaller in winter than in summer. Furthermore, red clover is often used for silage making, being better suited to silage than grazing, as it has a high forage yield and lower persistence in grazed land than white clover( 27 ). Fewer strains of cultivated red than white clover contain cyanogenic glycosides( Reference Muzashvili, Moniuszko-Szajwaj and Pecio 28 ), and red clover contains less cyanide than white clover( Reference Yong-An, Zirkler and Ellis 29 ). Thus, red clover may have a lower goitrogenic potential and its use in silage may also result in a smaller difference in iodine concentration between organic and conventional milk in the winter. Further research in this area is required to quantify these potential effects.
Deficit in selenium in the context of the whole diet
Dietary intake of Se is relatively low in Europe( Reference Rayman 30 ). For instance, in the UK, 38 % of adults aged 19–64 years (26 % of men, 51 % of women) do not even meet the UK lower reference nutrient intake (LRNI) of 40 µg/d( Reference Bates, Lennox and Prentice 3 ), which is considered to be adequate for only 2·5 % of people. Therefore, it is important to consider whether a difference between organic and conventional milk may be important.
Only four( Reference Rey Crespo, Miranda and Lopez-Alonso 7 , Reference Gabryszuk, Sloniewski and Sakowski 8 , Reference Malbe, Otstavel and Kodis 31 , Reference Emanuelson and Fall 32 ) of the eight studies that gave data on Se were included in the standard meta-analyses; the mean Se concentrations in organic and conventional milk were 11·97 and 14·11 µg/kg, respectively (P=0·015). Thus, a glass of organic milk (200 ml) would supply 4·4 % of the daily Se requirement of a woman of childbearing age (RDA 55 µg( 33 )), whereas a glass of conventional milk would supply 5·1 % of the daily Se requirement (see Table 1). Clearly this difference is minimal in terms of the total Se dietary supply. In any case, the percentage contribution to daily Se intake supplied by milk (of all types) is only 2·4 % in the UK( Reference Bates, Lennox and Prentice 3 ) or no more than 6 % if data from the UK Total Diet Study are used( 34 ).
Higher iron intake from organic milk in the context of the whole diet
A number of population groups, most notably menstruating women, are at risk of Fe deficiency( Reference Lopez, Cacoub and Macdougall 35 , Reference Radlowski and Johnson 36 ). In the UK, for instance, Fe intake below the UK LRNI (8 mg/d) was found in 46 % of girls aged 11–18 years, in 29 % of women aged 25–49 years and in 23 % of women aged 19–64 years, with evidence of Fe deficiency in 4·9 % of girls and 4·7 % of women( Reference Bates, Lennox and Prentice 3 ). Therefore, if organic milk can supply more Fe, this may be important. as adequate Fe status is vital for many aspects of human health( Reference Lopez, Cacoub and Macdougall 35 – 37 ).
In all, eight studies( Reference Rey Crespo, Miranda and Lopez-Alonso 7 – Reference Hanus, Vorlicek and Sojkova 9 , Reference Malbe, Otstavel and Kodis 31 , Reference Zagorska, Ciprovica and Karklina 38 – Reference Florence, da Silva and do Espirito Santo 41 ) (though two were identical( Reference Zagorska, Ciprovica and Karklina 38 , Reference Zagorska and Ciprovica 39 ) and unlikely to have been peer-reviewed) were included in the standard Fe meta-analysis; the mean Fe concentrations in organic and conventional milk were 0·74 and 0·64 mg/kg, respectively (P=0·034). Thus, a glass of organic milk (200 ml) would provide only 0·1 % more of the daily Fe requirement of a woman of childbearing age (18 mg( 37 )) than would a glass of conventional milk (see Table 1). As the authors themselves acknowledge, the finding of a marginally higher concentration of Fe in organic than in conventional milk is largely inconsequential, as milk is known to be a poor source of dietary Fe. Indeed, data from the UK NDNS show that milk supplies only 0·22 % of our daily Fe intake( Reference Bates, Lennox and Prentice 3 ).
Quality of the data
Although the authors carried out a GRADE assessment (Grading of Recommendation Assessment, Development and Evaluation) of the strength of evidence for standard meta-analysis, there was no attempt to assess the quality of the analytical data in included papers, despite the fact that this study rests on showing that small differences between concentrations of nutrients in organic and conventional samples are meaningful. These data need to be robust in a meta-analysis that relies on comparisons in analytical data to draw conclusions.
Of the twelve papers used in the standard meta-analysis for iodine, Se and Fe, two had identical data( Reference Zagorska, Ciprovica and Karklina 38 , Reference Zagorska and Ciprovica 39 ) – one was a conference paper and the other was a book chapter, which suggests that neither had been peer-reviewed (although surprisingly, non-peer-reviewed articles were included in the meta-analysis). Moreover, one paper that measured iodine along with other elements( Reference Gabryszuk, Sloniewski and Sakowski 8 ) used acid digestion, which is inappropriate for iodine. Only three of the twelve( Reference Dahl, Opsahl and Meltzer 5 – Reference Rey Crespo, Miranda and Lopez-Alonso 7 ) gave quality-control data to show that their analytical data were accurate. Another study( Reference Jahreis, Leiterer and Fechner 4 ) used a certified reference material but did not report whether the result obtained was within the certified range. Emanuelson & Fall( Reference Emanuelson and Fall 32 ), in another conference paper, mentioned that their analysis had been carried out by the Swedish National Veterinary Institute; certified reference materials were used, but the results were not reported (N. Fall, personal communication). However, it is not clear why that conference paper was used rather than the later (2011) Fall & Emanuelson( Reference Fall and Emanuelson 42 ) paper in the Journal of Dairy Research with the same data, which would have been peer reviewed. Hanus et al.( Reference Hanus, Vorlicek and Sojkova 9 ) mentioned that their analyses were conducted by an accredited Czech laboratory in Rapotin, although it was unclear whether those analyses included that of Fe; five of the papers( Reference Gabryszuk, Sloniewski and Sakowski 8 , Reference Malbe, Otstavel and Kodis 31 , Reference Zagorska, Ciprovica and Karklina 38 , Reference Zagorska and Ciprovica 39 , Reference Florence, da Silva and do Espirito Santo 41 ) made no mention of any quality-control methods being applied.
Conclusion
We are concerned that the quality of the analytical data on which this comment relies with respect to the trace elements has not been taken into account; only three of the twelve studies cited demonstrated satisfactory quality of their analytical data. If articles are included that have no evidence of having adequate quality-control procedures in place, it calls into question the validity of the meta-analysis.
Setting that aside for the moment, of the three trace minerals, the only information that was meaningfully different between organic and conventional milk in terms of the total diet was for iodine. Indeed, for iodine, the difference was significant, the effect size was large and of all the nutrients investigated it was one of only two rated as having high reliability, yet it was not the low concentration of iodine in organic milk that made it to the headlines.
As nutritional differences are one of the factors that may influence the purchase of organic milk, it is important that scientists ensure that consumers are given a balanced picture so that they can weigh up the potential benefits and disadvantages of its consumption.
Acknowledgements
S. C. B. has received lecture fees from the Dairy Council. M. P. R. was awarded a grant from Wassen International, which partly funded a PhD studentship for S. C. B. (2009–2012).