Benefits of maternal nutrition during pregnancy are well documented. A healthy and balanced nutrition among pregnant mothers leads to healthy birth outcomes and, consequently, better offspring health in later life(Reference Chia, Chen and Lai1–Reference Abu-Saad and Fraser7). In low-and-middle-income countries, inadequate intake of energy, protein, vitamins and minerals to meet maternal and fetal needs is rampant and dietary intake of vegetables, meat, dairy products and fruit is often insufficient to meet these essential requirements(3–Reference Salam, Das and Bhutta5). India is no exception(Reference Sharma, Kishore and Roy8–Reference Green, Milner and Joy10), where an unacceptably high undernutrition among pregnant women is modelled to be a risk factor for poor birth outcomes(11–Reference Ramakrishnan, Lowe and Vir13). The Global Burden of Disease estimates highlight an unacceptably high burden of various indicators of undernutrition among women in India. For example: in 2016, Fe-deficiency anaemia accounted for 11 % of all disability among women in India(14). A high burden of Fe-deficiency anaemia among pregnant mothers has severe consequences on the health of their children(Reference Cappellini, Musallam and Taher15,Reference Rai, Fawzi and Barik16) , and nutrition supplements could be helpful in treating Fe-deficiency anaemia.
To mitigate the burden of undernutrition among pregnant women, providing nutrition supplements to pregnant mothers is recommended to be a scalable intervention and has positive effects on a range of health outcomes(Reference Dean, Lassi and Imam4,Reference Bhutta, Das and Rizvi6,Reference Barker, Dombrowski and Colbourn17,Reference da Silva Lopes, Ota and Shakya18) . In line with the evidence, the Indian government established Anganwadi centres (AWC) (meaning ‘courtyard shelter’) in 1975 as part of the Integrated Child Development Services programme to combat hunger and malnutrition among women and children(Reference Singh, Dubey and Singh19,Reference Kumar and Rai20) . India currently has over 1·3 million operational AWC managed by Anganwadi workers, and among their various responsibilities, Anganwadi workers encourage pregnant mothers to eat locally available nutritious foods; they provide education and counselling on exclusive breast-feeding practices to mothers and advise them on supplementary food required for the healthy development of their children(Reference Kumar and Rai20,21) . The need of the Integrated Child Development Services programme was confirmed in the 2013 National Food Security Act (NFSA) where distribution of supplementary nutrition to pregnant and lactating mothers was mandated as part of the right to food, and it was instructed that Anganwadi workers will continue providing daily ‘meal, free of charge, during pregnancy and six months after the child birth, through the local Anganwadi, so as to meet the nutritional standards’ – 2510 kJ of energy content/energy and 18–20 g of protein(22). Empirical study on the effect of NFSA on reduction of undernutrition in India is non-existent, but some preliminary studies indicate that NFSA is unlikely to greatly affect food consumption, and even if it does, it could only make a small dent to reduce undernutrition(Reference Kjelsrud and Somanathan23). Pregnant women registered with AWC are supposed to receive hot cooked meals and micronutrient-fortified and energy-dense food as take-home ration across the country. NFSA instructs all states and union territories of India to develop a menu of commonly accepted nutritious food which will provide the required nutrition. However, there is significant variation in the take-home ration provided across states, reflecting how take-home ration is frequently not aligned with Integrated Child Development Services guidelines for micronutrient composition, and a systematic lack of accountability at AWC remains a key barrier to programmatic effectiveness(24).
To our knowledge, no up-to-date studies exist on whether nutritional supplementation provided to pregnant mothers has helped to improve the health of children born to them. Against this knowledge gap, using two successive rounds of the nationally representative cross-sectional National Family Health Survey data (collected during 2005–2006 and 2015–2016) from India, we investigated if nutrition supplement given to pregnant mothers through AWC was associated with select child health indicators – extremely low birth weight (ELBW), very low birth weight (VLBW), low birth weight (LBW) and neonatal mortality (death during day 0–27) stratified by death during day 0–1, day 2–6 and day 7–27. This study’s findings aim to be helpful for guiding national policies on the role of nutrition intervention during pregnancy in improving child health.
Methods
Data set
Two successive rounds of India’s Demographic and Health Survey (collected during 2005–2006 and 2015–2016), commonly known as the National Family Health Survey (NFHS), were used(25,26) . NFHS2005–2006 (NFHS-3) and NFHS2015–2016 (NFHS-4) are cross-sectional surveys and widely used for programme and policymaking in India(Reference Dandona, Pandey and Dandona27). NFHS-3 covered twenty-nine states/union territories, whereas NFHS-4 included thirty-six states/union territories. For NFHS-3, 2001 Census of India and, for NFHS-4, 2011 Census of India sampling frame were used to draw the sample for both rural and urban areas using two-stage stratified random sampling. Villages in rural areas and census enumeration blocks in urban areas served as the primary sampling units or clusters. By virtue of their sampling design, estimates from both rounds of surveys are comparable(Reference Corsi, Neuman and Finlay28). Details of sampling procedures are furnished elsewhere(25,26) . Six administrative regions (Andaman and Nicobar Islands, Chandigarh, Dadra and Nagar Haveli, Daman and Diu, Lakshadweep and Puducherry) were not part of NFHS-3(25), leaving twenty-nine common states/union territories covered in both waves of the NFHS, representing over 99·6 % of India’s population. A household response rate of over 95 % was registered in both NFHS rounds.
To analyse birth weight, 148 019 children were eligible for this study – 14 856 children from NFHS-3 and 133 163 children from NFHS-4. The denominator for analysing neonatal mortality was 205 593, consisting of 33 263 samples from NFHS-3 and 172 330 samples from NFHS-4. NFHS records information on birth weight for children born in the 5 years preceding the survey date, and age at death was recorded for children ever born. However, this study includes the record of birth weight or child mortality only for index children, for two reasons – it would minimise recall errors, and information on primary variable and covariates used in this study was collected only for the index birth.
Outcome events
Two sets of outcome events were investigated. For the first set of events, three child health indicators related to LBW were considered. Following the guideline developed by the WHO, children’s LBW (in kg) was categorised into three groups: LBW with weight of <2·5 kg, VLBW with weight of <1·5 kg and ELBW with weight of <1·0 kg(29). In both rounds of NFHS, women were asked if the children born to them were weighed at birth, and if the response was affirmative, the weight at birth was recorded in kg. Enumerators were advised to record birth weight from the health card when available; otherwise reporting of birth weight was based on mother’s recall which could have been affected by recall errors, and digit preference which would cause heaping(Reference Swaminathan, Kim and Subramanian30).
As a second set of outcome events, neonatal mortality (death during day 0–27) stratified by death during day 0–1, day 2–6 and day 7–27 was analysed. The NFHS asked women about the birth and death history of children ever born to them. In case of death of a child, the age at which the child died was reported. Age at death was recorded in days if the child died within the first month of life, in months if the child died between one month and the second birthday or otherwise in years. In this study, death of children during the first 28 d of life (0–27 d) was defined as neonatal mortality. Neonatal mortality was further investigated by age at death: day 0–1, day 2–6 and day 7–27. This categorisation of age of death is critical as neonatal death varies greatly with days(Reference Sankar, Neogi and Sharma12).
Primary variable and covariates
As a primary variable of interest, this study assessed if women received nutritional supplementation during their last pregnancy. In NFHS, for their last birth in the 5 years preceding the survey date, eligible mothers were asked: ‘Did you receive any supplementary nutrition from the Anganwadi Centre during this pregnancy?’ If the response was affirmative, a follow-up question was posed: ‘During this pregnancy, were you always able to get the supplementary nutrition from the Anganwadi Centre?’ Responses were coded into ‘yes, always’ and ‘no’. Using this information, the primary variable of interest was computed into three groups about receipt of supplementary nutrition – never received, received but not always and always received. As nutritional supplementation during pregnancy is crucial, one may expect that women who always received supplements will have higher odds of experiencing favourable health outcomes of children born to them, compared with women who only sometimes received (but not always) supplements.
Select covariates representing socio-economic characteristics are current age group (in completed years) of mother (15–19, 20–29, 30–39 and ≥40), mother’s age at marriage (<17, 18–20, 21–25 and ≥26), education of mother (no or incomplete primary, primary or incomplete secondary, and secondary or higher), sex of child (male and female), birth order (1, 2, 3, 4 and ≥5), place of residence (urban and rural), social group (others, Scheduled Castes, Scheduled Tribes and Other Backward Classes), religion (Hinduism, Islam, Christianity and others), economic group (poorest, poorer, middle, richer and richest) and state of residence (non-high-focus states and high-focus states). In addition, covariates on maternal healthcare service utilisation included number of antenatal care visits (≥4 and <4), institutional delivery (yes and no) and maternal nutrition status represented by BMI of the mother (underweight, optimum, and overweight and obesity). Variable on sources of birth weight data (from written card and from mother’s recall) was used to analyse LBW, and waves of NFHS (2005–2006 and 2015–2016) were used to capture variation of outcome events by time of survey.
Primary education refers to studying ≤8th grade, and secondary education refers to study of 9th–10th grade. For social group, as per the Constitution of India(31), Scheduled Castes, Scheduled Tribes and Other Backward Classes are historically socially and economically disadvantaged populations; the ‘Others’ category represents the population that has historically been relatively more privileged. NFHS provides the variable of wealth index in its data set. The wealth index is calculated using household assets and durables. The method of constructing the wealth index representing economic groups is presented elsewhere(Reference Rutstein and Johnson32). Due to both high fertility and high mortality indicators, nine states are regarded as high-focus states in need of special attention; these are Bihar, Chhattisgarh, Jharkhand, Madhya Pradesh, Odisha, Rajasthan, Uttarakhand, Uttar Pradesh and Assam(Reference Kumar, Singh and Rai33). According to the WHO, a BMI of <18·5 kg/m2 is considered a measure of underweight, 18·5–22·99 kg/m2 as optimum weight and ≥23 kg/m2 is labelled as overweight, including obesity, for the Asian population(34).
Statistical approach
This study used a combination of descriptive and multivariate analyses. To run the analysis, data from both NFHS were pooled to increase the power of the study, totalling 148 019 children to study LBW, and 205 593 children for studying neonatal mortality. Bivariate analysis was run to understand the proportional difference of outcome events – ELBW, VLBW, LBW and neonatal mortality (death at age 0–27 d) – stratified by age at death (day 0–1, day 2–6 and day 7–27). Multivariable logistic regression analyses were conducted for all outcome variables coded in binary terms (0 and 1). For each outcome, four regression models were run. Model I included the primary variable of interest – receipt of nutritional supplementation during pregnancy and waves of the NFHS survey; model II included all variables from model I and variables representing socio-economic characteristics; model III included all variables of model II and variables representing maternal healthcare service utilisation (ANC and delivery care); model IV included all variables from model III and maternal BMI. This four-step modelling helped separate the possible role of other variables while understanding the association between receiving nutritional supplementation during pregnancy and select outcome events. For all the outcomes on birth weight, one additional variable representing sources of birth weight data was adjusted as birth weight reporting could differ between health card and maternal recall(Reference Balarajan, Subramanian and Fawzi35). Adjusting NFHS year for all the regression models helped in accounting for secular time trend. Before executing the multivariate analysis, the variables included in the model were tested for multicollinearity by estimating the variance inflation factor and all variance inflation factors were <5·0, indicating a low probability of multicollinearity(Reference Chatterjee and Hadi36).
Recording of birth weight through mother’s recall is likely to have digit preference, often in multiples of 500 g(Reference Balarajan, Subramanian and Fawzi35) which leads to heaping(Reference Channon, Padmadas and McDonald37). To check the sensitivity of this, an alternate analysis with alternate definition of ELBW of ≤1·0 kg, VLBW ≤ 1·5 kg and LBW of ≤2·5 kg was run (data not shown separately), hoping the magnitude of negative association of nutritional supplementation received during pregnancy would be smaller than the standard definition of respective categories. Appropriate sample weighting available with the NFHS data set was used, and ‘svy’ suite available to adjust sample weighting with the statistical software Stata, version 14,(38) was used. P value (two-tailed) of <0·05 obtained from logistic regression models was included in the Results and Discussion.
Ethics statement
Prior to conducting the NFHS, ethical approval was obtained by the implementing institute, the International Institute for Population Sciences, from an independent ethics review committee constituted by the Ministry of Health and Family Welfare, Government of India. NFHS data sets are available in the public domain with all participant identifiers removed. Thus, no separate ethical approval was required for this study.
Results
Table 1 represents prevalence of ELBW, VLBW and LBW, and prevalence of day of neonatal mortality (day 0–1, day 2–6 and day 7–27) and neonatal mortality with 95 % CI by select background characteristics. Overall, the prevalence of ELBW, VLBW and LBW was 0·13 % (95 % CI 0·11, 0·16), 1·29 % (95 % CI 1·21, 1·37) and 17·7 % (95 % CI 17·4, 18·0), respectively. Women who never received nutritional supplementation experienced a higher prevalence of ELBW, VLBW and LBW of their babies. On the other hand, prevalence of neonatal mortality during day 0–1, day 2–6, day 7–27 and neonatal mortality was 1·12 (95 % CI 1·06, 1·18), 0·53 (95 % CI 0·49, 0·57), 0·35 (95 % CI 0·31, 0·38) and 1·99 (95 % CI 1·91, 2·07), respectively. Women who never received nutrition supplements during their pregnancy had higher prevalence of any type of neonatal mortality, compared with women who received nutritional supplementation.
Table 1 Prevalence of extremely low birth weight, very low birth weight and low birth weight, and prevalence of timing of neonatal mortality (day 0–1, day 2–6 and day 7–27) and neonatal mortality (day 0–27) by select background characteristics*
(Numbers and percentages, 95 % confidence intervals)
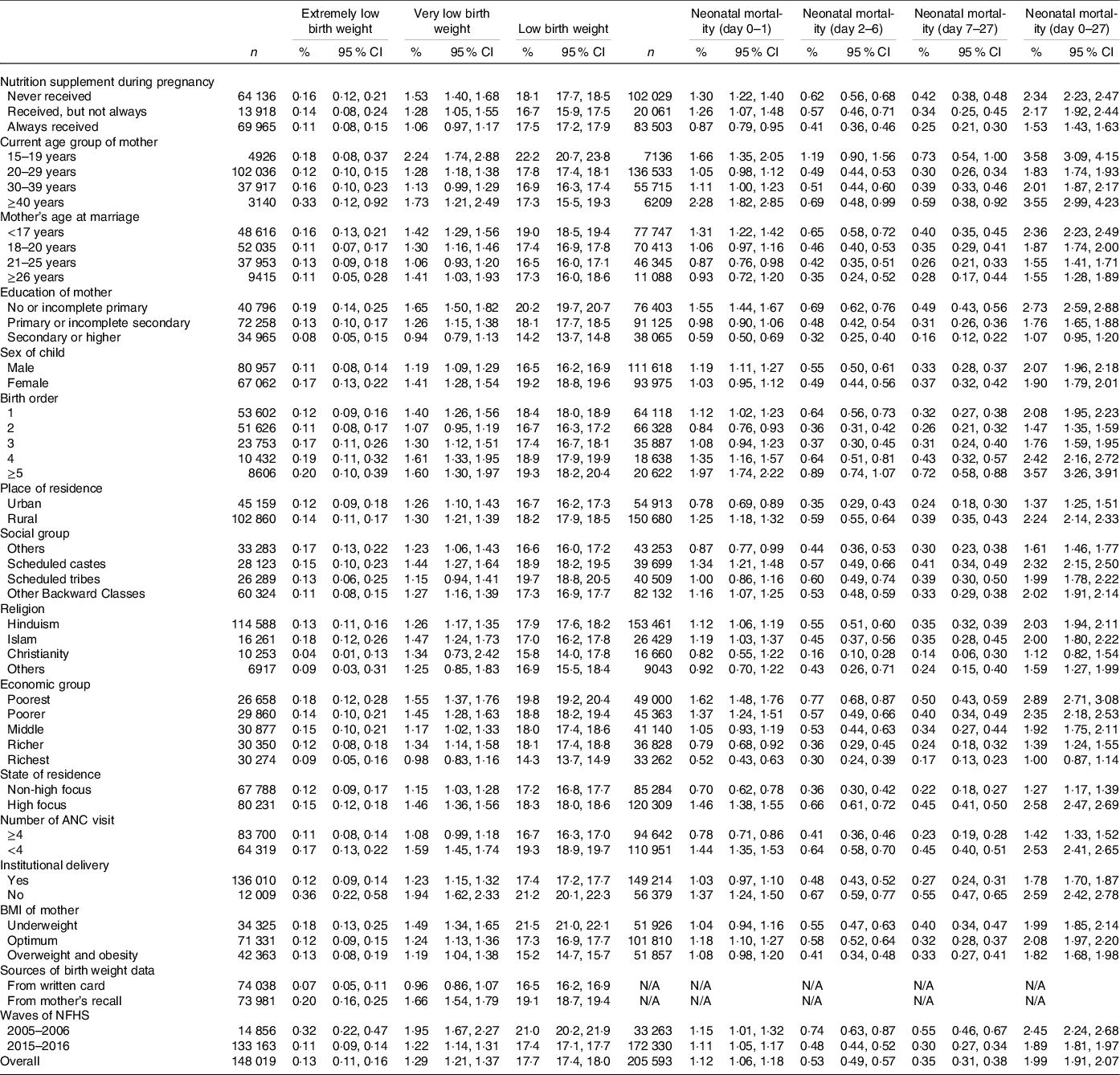
ANC, antenatal care; N/A, not applicable; NFHS, National Family Health Survey.
* All n are unweighted.
Prior to running multivariable logistic regression analysis, variance inflation factor was estimated to check the presence of multicollinearity and all estimates were <5·0 indicating low possibility of multicollinearity (data not shown separately). Whether nutritional supplementation to mothers was associated with ELBW, VLBW and LBW was tested in a logistic regression model, presented with OR in Table 2, whereas full modelling results are presented as supplementary material online (online Supplementary Table S1). ELBW was not associated (>0·05) with mothers receiving nutritional supplementation, irrespective of covariates added in the model (model 1 through model IV). Receipt of supplementary nutrition (whether not always/sometimes or always) appears to have helped reduce VLBW and LBW (model II through IV). For neonatal mortality (stratified by day of death), role of nutrition supplement is presented in Table 3, with elaboration of the full models in the online supplementary material (online Supplementary Table S2). As shown in model IV, compared with women who never received nutritional supplementation during their pregnancy, women who always received nutrition supplements were less likely to experience neonatal mortality (OR: 0·67, 95 % CI 0·61, 0·73, P < 0·001). Women always receiving supplementation were also less likely to experience death of the infant in various day increments: day 0–1 (OR: 0·66, 95 % CI 0·58, 0·74, P < 0·001), day 2–6 (OR: 0·69, 95 % CI 0·58, 0·82, P < 0·001) and day 7–27 (OR: 0·68, 95 % CI 0·53, 0·87, P = 0·002), and the unadjusted (model I) association also showed similar results. However, women who only sometimes received nutrition supplements had no association (P ≥ 0·05) with prevention of neonatal mortality on day 2–6 and day 7–27, whereas a protective association was observed for death on day 0–1 and neonatal mortality for women who sometimes received nutrition supplements during pregnancy.
Table 2 Association between receipt of nutrition supplement during pregnancy and extremely low birth weight, very low birth weight and low birth weight (Odds ratio and 95 % confidence intervals)
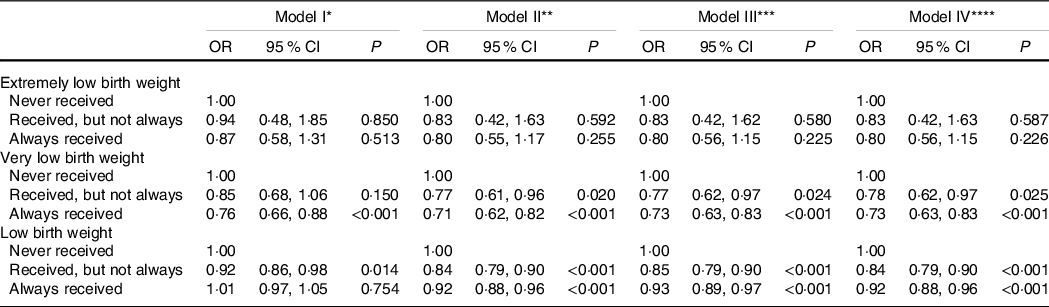
P, level of significance; ANC, antenatal care; NFHS, National Family Health Survey.
* Model is adjusted for receipt of nutrition supplement, sources of birth weight data, and waves of NFHS.
† Model is adjusted for receipt of nutrition supplement, current age group of mother, mother’s age at marriage, education of mother, sex of child, birth order, place of residence, social group, religion, economic group, state of residence, sources of birth weight data and waves of NFHS.
‡ Model is adjusted for receipt of nutrition supplement, current age group of mother, mother’s age at marriage, education of mother, sex of child, birth order, place of residence, social group, religion, economic group, state of residence, number of ANC visit, institutional delivery, sources of birth weight data and waves of NFHS.
§ Model is adjusted for receipt of nutrition supplement, current age group of mother, mother’s age at marriage, education of mother, sex of child, birth order, place of residence, social group, religion, economic group, state of residence, number of ANC visit, institutional delivery, BMI of mother, sources of birth weight data and waves of NFHS.
Table 3 Association between receipt of nutrition supplements during pregnancy and timing of neonatal mortality (day 0–1, day 2–6 and day 7–27) and neonatal mortality (day 0–27)(Odds ratio and 95 % confidence intervals)
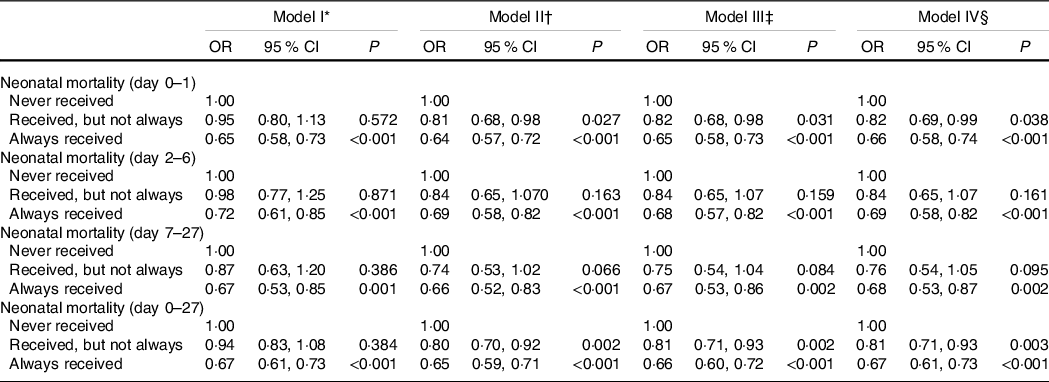
P, level of significance; ANC, antenatal care; NFHS, National Family Health Survey.
* Model is adjusted for receipt of nutrition supplement, and waves of NFHS.
† Model is adjusted for receipt of nutrition supplement, current age group of mother, mother’s age at marriage, education of mother, sex of child, birth order, place of residence, social group, religion, economic group, state of residence and waves of NFHS.
‡ Model is adjusted for receipt of nutrition supplement, current age group of mother, mother’s age at marriage, education of mother, sex of child, birth order, place of residence, social group, religion, economic group, state of residence, number of ANC visit, institutional delivery and waves of NFHS.
§ Model is adjusted for receipt of nutrition supplement, current age group of mother, mother’s age at marriage, education of mother, sex of child, birth order, place of residence, social group, religion, economic group, state of residence, number of ANC visit, institutional delivery, BMI of mother and waves of NFHS.
Discussion
Nutritional supplementation to women during their pregnancy is crucial for health of their children. In India, Anganwadi Workers of the AWC under the Integrated Child Development Services programme are given responsibility for distributing nutritional supplementation, primarily in the form of take-home ration, to pregnant mothers. In absence of a nationwide evaluative study, using nationally representative NFHS data, this study assesses whether nutritional supplementation given to women during their last pregnancy was associated with select child health indicators – ELBW, VLBW and LBW, and neonatal mortality as well as day of neonatal mortality (day 0–1, day 2–6 and day 7–27).
Multivariate analysis revealed that receipt of nutrition supplements during pregnancy is not associated (P ≥ 0·05) with ELBW. The primary causes of ELBW are pre-term birth and intra-uterine growth restriction, and prevention of pre-term birth and intra-uterine growth restriction is multi-factorial where biological pathways and preventive measures for these two conditions are different(Reference Cutland, Lackritz and Mallett-Moore39). But whether the role of nutrition supplements during pregnancy outweighs the biological pathways and preventive measures requires further investigation. On the other hand, multivariable analysis revealed that providing nutritional supplementation (sometimes/not always and always) during pregnancy might be helpful in reducing VLBW and LBW. This finding is consistent with interventions confirming the role of supplementary nutrition in mitigating LBW(Reference da Silva Lopes, Ota and Shakya18,Reference Ramakrishnan40) , but evidence on the role of supplementary nutrition in VLBW in India is absent, and this study offers some insight into the issue.
In the case of neonatal mortality, women who always received nutrition supplements during their last pregnancy experienced higher odds of survival of their neonates of all ages. However, women who sometimes received nutrition supplement had no association (P ≥ 0·05) with prevention of mortality on day 2–6 and day 7–27. These estimates indicate the benefits of systematic and intense nutrition supplement intervention in reducing neonatal mortality(Reference Costello and Osrin41) and unorganised and untimely intervention of nutritional supplementation may not be useful(42) in preventing mortality on day 2–6 and day 7–27. In India, about 57 % of all neonatal deaths occur in the first 3 d after birth and two-thirds of these deaths occur on the first day, that is, within 24 h of birth(Reference Sankar, Neogi and Sharma12). Prevention of neonatal mortality would require quality prenatal care including nutrition-sensitive interventions, coupled with prevention strategy of death due to prematurity, malformations and sepsis(Reference Sankar, Neogi and Sharma12). While nutrition supplements such as Fe-and-folic-acid, Ca and other supplements to pregnant mothers are crucial for mitigating neonatal death, the prevention of overall death also had to do more with quality of emergency obstetric care of pregnant women in health facilities as well as special care to the new-born unit and postnatal care(Reference Sankar, Neogi and Sharma12,Reference Sankar, Natarajan and Das43) . Well-organised home-based postnatal care could be useful for preventing neonatal death on day 2–6 and day 7–27.
This study acknowledges certain limitations in light of the findings. First, data on all possible determinants of birth weight and neonatal mortality were not available in the NFHS, thus not included in regression modelling. Second, the most information was self-reported or reported by mothers on behalf of their children, thus reporting might be affected by recall errors and social desirability bias. Third, information on birth weight was based on data from the health card and mother’s recall, which reduced the sample size and may underestimate the association. However, the prevalence of LBW in this study being comparable to the general population offers confidence about generalisability of the study findings. Fourth, supported by the sensitivity analysis (data not shown separately), the multivariable models adjusted for recording of birth weight data indicated that mothers’ recall had higher likelihood to record ELBW, VLBW and LBW (online supplementary Table S1). Fifth, while the association between nutritional supplementation to pregnant mothers and outcome events should be interpreted cautiously as detailed information on nutrition supplements (e.g. consumption pattern, nutritional content, intra-household distribution, preparation methods, etc. among other dimensions of nutrition) were not available with NFHS data. Sixth and finally, the response against receipt of nutritional supplementation from AWC is heavily contingent upon the pregnant mother and her family member’s knowledge, awareness and nutrition supplement-seeking behaviours. Thus, reporting about receipt of supplementation could be variable, and a careful interpretation of study findings is needed. Despite these limitations, with a coverage of 99·6 % of India’s children aged 6–59 months and high external validity (no sample selection bias was recorded), findings of this study successfully demonstrated the importance of nutritional supplementation for pregnant mothers in reducing the burden of various stages of LBW and neonatal mortality in India.
Reduction of LBW at the rate of 2 % per annum has been a prime target of the Prime Minister’s Overarching Scheme for Holistic Nutrition or POSHAN Abhiyaan (44). Additionally, the 2017 National Health Policy emphasised the prevention of neonatal mortality by ensuring required nutrition to mothers and children(45). Health has been a state subject in India, dictated by the constitution of India. Thus, central government must encourage every state and union territory to follow NFSA guidelines and strengthen the performance of AWC while exercising appropriate monitoring and evaluation. Pregnant mothers enrolled with AWC should be encouraged to receive and consume nutritional supplementation as directed. Failure to improve child health through the nutritional supplementation programme designed for pregnant mothers would not only compromise the targets set under POSHAN Abhiyaan and the National Health Policy, but also erode the foundation of health for coming generations.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
R. K. R. conceived and designed the study. R. K. R. performed the analysis and prepared the first draft. S. S. K., D. J. P., S. K. and H. R. critically reviewed results and commented on subsequent versions of the manuscript. All authors approved content presented in the study.
The authors report no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114521000982






