Both postprandial glycaemia(Reference Howlett and Ashwell1, Reference Cavalot, Petrelli and Traversa2) and postprandial lipaemia(Reference Lopez-Miranda, Perez-Martinez and Marin3) are markers of increased vascular risk. After meal ingestion, the components of some foods could negatively influence a number of metabolic or cellular pathways which, repetitively and in the long term, could promote the development of atherosclerosis. This relationship has generally been ascribed to the effect of diet composition on metabolic factors epidemiologically linked to CVD. However, the direct effect that the diet could exert on cells involved in the atherogenic process has received less attention.
Atherosclerosis is a chronic inflammatory disease in which adhesion molecules of peripheral blood mononuclear cells (PBMC) play a key role in different stages of its development(Reference Ross4). L-selectin (CD62L) mediates leucocyte adhesion and rolling on the endothelial surface(Reference Rainer5), molecules of the β-integrin family, CD11a, CD49d and CD11b, are responsible for tight leucocyte attachments to endothelial receptors and, in a third stage, intercellular adhesion molecule-1 (ICAM-1) (CD54) interacts with its ligands of the extracellular matrix promoting PBMC transmigration into the subendothelial space(Reference Carlos and Harlan6).
The aim of the present study was to evaluate the acute effects of meals of different consumption on PBMC activation assessed by changes in the expression of key adhesion molecules implicated in leucocyte trafficking into the vessel wall.
Methods and volunteers
The present study was a randomised, cross-over study, performed in twenty normolipaemic subjects (seven men; mean age 32 years; age range 26–40 years; BMI 24·3 (sd 2·5) kg/m2). Participants were healthy volunteers recruited from the staff of the Hospital Carlos III. They were summoned at 07.30 hours after a 12 h fast, for three consecutive Mondays. Upon arrival, an intravenous cannula was placed for venous blood sampling. After the first blood extraction (time 0), and according to a list of randomisation, participants ingested one of three mixed, isoenergetic meals: rich in carbohydrates (CHO), rich in monounsaturated fat; rich in saturated fat. The meal rich in CHO contained 50 g white wheat bread, 400 ml skimmed milk, 40 g white sugar, 40 g strawberry jam and 60 g chocolate powder, had an energy content of 3231 kJ, a glycaemic index of 56, a glycaemic load of 84·5, 84 % energy as CHO, 11 % energy as protein, 2·2 % energy as saturated fat, 1·2 % energy as monounsaturated fat and 0·3 % energy as polyunsaturated fat. The fatty acid composition of the high-CHO meal was: 14 : 0, 0 g; 16 : 0, 1 g; 18 : 0, 1·2 g; 16 : 1, 0 g; 18 : 1, 1·2 g; 18 : 2, 0·3 g; 18 : 3, 0 g; EPA and DHA, 0 g. The meal rich in monounsaturated fat contained 50 g white wheat bread, 36 g olive oil, 400 ml skimmed milk and 30 g hazelnuts, had an energy content of 3327 kJ, 25 % energy as CHO, 11 % energy as protein, 6·7 % energy as saturated fat, 40·6 % energy as monounsaturated fat and 6·2 % energy as polyunsaturated fat. The fatty acid composition of the high-monounsaturated fat meal was: 14 : 0, 0 g; 16 : 0, 5·1 g; 18 : 0, 1·4 g; 16 : 1, 0·4 g; 18 : 1, 40 g; 18 : 2, 5·9 g; 18 : 3, 0·3 g; EPA and DHA, 0 g. The meal rich in saturated fat contained 50 g white wheat bread, 40 g butter, 400 ml whole milk and 30 g cheese, had an energy content of 3273 kJ, 24 % energy as CHO, 12 % energy as protein, 32·8 % energy as saturated fat, 16·2 % energy as monounsaturated fat and 1·6 % energy as polyunsaturated fat. The fatty acid composition of the high-saturated fat meal was: 14 : 0, 6·1 g; 16 : 0, 16·1 g; 18 : 0, 6·5 g; 16 : 1, 1·7 g; 18 : 1, 14 g; 18 : 2, 1 g; 18 : 3, 0·6 g; EPA and DHA, 0 g. After meal ingestion the volunteers were only allowed to consume mineral water. Blood samples were obtained at 2, 4, 6, 8 and 10 h after the first blood extraction.
Cholesterol and TAG concentrations were determined from plasma using enzymic colorimetric methods. HDL-cholesterol was measured after precipitation of apoB lipoproteins with phosphotungstate–MgCl2. LDL were isolated after sequential ultracentrifugation, oxidised with Cu2SO4 and conjugated dienes were measured as previously described(Reference Esterbauer, Striegl and Puhl7).
Leucocyte activation markers were measured from heparinised blood within 1 h of venepuncture. A quantity of 50 μl of each sample was incubated with the following monoclonal antibody mixtures: CD11a (clone G-25·2, IgG2a)-fluorescein isothiocyanate (FITC); CD49d (clone L25, IgG2b)-phycoerythrin (PE); CD62L (clone SK11, IgG2a)-FITC; CD54 (clone LB-2, IgG2b)-PE; CD11b (clone ICRF44, IgG1)-FITC; CD162 (clone KPL-1, IgG1)-PE. CD14 (clone MΦP9, IgG2b)-peridinin chlorophyll protein (PerCP) was added to all assays. Samples were also incubated with isotypic control monoclonal antibodies to detect non-specific staining. Monoclonal antibodies were provided by BD Biosciences (Erembodegem, Belgium) except CD11b-FITC, provided by AbD Serotec Inc. (Oxford, Oxon, UK). A quantity of 1 ml FACS Lysing solution (BD Biosciences) was added for 15 min at room temperature. After centrifugation and washing with PBS, cells were re-suspended with 300 μl of 1 % paraformaldehyde and acquired in a FACScan flow cytometer (BD Biosciences). A quantity of 20 μl of each monoclonal antibody, except for CD11b (5 μl), was added to the samples.
Lymphocytes were gated according to their light-scattering characteristics and monocytes according to their high CD14 expression. At least 1000 CD14++ cells and 5000 lymphocytes were analysed with CellQuest Pro software (BD Biosciences, San Jose, CA, USA). Fluorescence intensity of each cellular population was expressed as the mean fluorescence intensity (MFI) in arbitrary units. All samples were processed blinded to the type of diet consumed.
Results are presented as mean values with their standard errors. For each adhesion molecule, repeated-measures ANOVA was used for testing the effect of time, diet and time–diet interactions, after adjustment for sex, age, BMI and their MFI baseline values. When statistical significance was found, Bonferroni's test was used for post hoc comparisons. The Greenhouse–Geisser statistic was used when the sphericity assumption was not satisfied. SPSS (version 15.0; SPSS, Inc., Chicago, IL, USA) was used for statistical analysis.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Clinical Trials Ethics Committee of the Hospital Carlos III (Madrid, Spain). Written informed consent was obtained from all subjects.
Results
All diets raised plasma TAG. The increase was significantly higher with both of the fat-enriched meals. There were neither significant time changes nor differences among the diets on time to LDL oxidation ex vivo.
In lymphocytes, the expression of CD162, CD11a and CD54 increased after the ingestion of the CHO-enriched meal in comparison with both of the fat-enriched meals (Table 1). The CD11a increase was due to a raise in the percentage of lymphocytes expressing high levels of CD11a (CD11a-bright lymphocytes), returning to baseline values at t = 8 h (Fig. 1). There were no changes in the expression of CD62L, CD11b or CD49d with any of the diets. Lymphocytes did not change their size at the different time points after meal consumption.
Table 1 Time-dependent expression of different adhesion molecules in lymphocytes, after the consumption of a carbohydrate (CHO)-, monounsaturated fat- or saturated fat-enriched meal*
(Mean values with their standard errors)

a,b Mean values within a column (diet comparison) with unlike superscript letters were significantly different after correcting for the basal value (P < 0·05).
* Results are expressed as mean fluorescence intensity in arbitrary units.
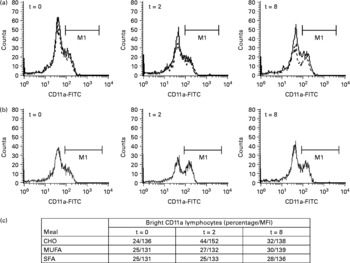
Fig. 1 Changes in the percentage of lymphocytes expressing high levels of CD11a (CD11a-bright lymphocytes; M1) after consumption of meals enriched in carbohydrate (CHO), saturated fat (SFA) and monounsaturated fat (MFA). (a) Histograms represent changes following monounsaturated fat (—) and saturated fat (- - -) diets. (b) Histograms show changes after the CHO diet. Three time points (t = 0, 2 and 8 h) are depicted. FITC, fluorescein isothiocyanate. (c) Percentage and mean fluorescence intensity (MFI) of CD11a-bright lymphocytes.
In monocytes, we only observed a differential expression of CD49d after the ingestion of the CHO diet compared with the enriched-fat diets (time × diet interaction, P = 0·035) (results not shown).
Discussion
The present report is the first to compare the effect of mixed meals of different composition on the expression of key adhesion molecules implicated in the atherogenic process. The present results demonstrate that an acute consumption of a CHO-enriched meal increases the expression of CD162, CD49d, CD11a and CD54 on PBMC. Globally, these time-dependent changes were of greater magnitude than the ones observed after fat-enriched meals, either monounsaturated or saturated, and were more evident in lymphocytes than in monocytes. A similar, although more marked, activation pattern of expression of the adhesion molecules has been described in subjects with inflammatory diseases(Reference Bhatnagar, Wig and Majumdar8), coronary artery disease(Reference Kawamura, Miura and Murayama9, Reference Meisel, Shapiro and Radnay10) and in individuals with cardiovascular risk factors(Reference Weber, Erl and Weber11, Reference van Oostrom, Van Wijk and Sijmonsma12). Moreover, this phenotypic pattern has been associated with an increased adhesiveness of PMBC to the endothelium(Reference van Gils, Zwaginga and Hordijk13). Although the observed changes are small in magnitude, a repetition of this pattern whenever a meal is consumed could, in the long term, have adverse consequences. In fact, it has been shown that chronic consumption of high-CHO diets with a high dietary glycaemic load and glycaemic index are associated with an increased cardiovascular risk(Reference Beulens, de Bruijne and Stolk14, Reference Liu, Willett and Stampfer15).
Previous studies have demonstrated that either chronic hyperglycaemia or ex vivo cellular exposure to glucose induces changes in the expression of adhesion molecules(Reference Kado, Wakatsuki and Yamamoto16) and stimulates leucocyte adhesion to the endothelium(Reference Kim, Berliner and Natarajan17) by a NF-κB-mediated mechanism(Reference Morigi, Angioletti and Imberti18). Also, acute CHO consumption induces monocyte activation, as assessed by an increased expression of TNFα, IL-β(Reference Motton, Keim and Tenorio19), CD11a, CD11b and CD54(Reference Sampson, Davies and Brown20), and endothelial dysfunction(Reference Kawano, Motoyama and Hirashima21, Reference Title, Cummings and Giddens22).
The effect of both fat-enriched meals on adhesion molecule expression was small. Most studies evaluating the effect of high-fat diets on PBMC have administered a fat load instead of a mixed fat-enriched meal. It has previously been demonstrated that the addition of either proteins(Reference Westphal, Taneva and Kastner23) or glucose(Reference van Oostrom, van Dijk and Verseyden24, Reference Knuth, Remias and Horowitz25) to a high-fat meal can modify its effect.
We conclude that the acute consumption of a mixed meal enriched in CHO is associated with up-regulation of adhesion molecules on circulating lymphocytes. This effect was higher than the one observed after monounsaturated and saturated fat consumption. These potentially harmful findings could contribute to the increased cardiovascular risk that has been attributed to high-CHO diets.
Acknowledgements
The present study has been funded by a research grant from Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III (no. 03/1211) and from Fundación para el fomento y Desarrollo de la Investigación Clínica.
J. M. conceived of and designed the study, interpreted the data and drafted the article; M. G.-M. and C. L. conceived of and designed the study, interpreted the data and revised the article; E. T. was in charge of all the laboratory work, interpreted the data and revised the article.
There are no conflicts of interest.




