Rice is a daily dietary staple food for more than half of the world's population, and the major single food source of carbohydrate and energy in China and many other Asian countries( Reference Kennedy, Burlingame and Nguyen 1 ). In South India, for example, nearly half of daily energy intake come from refined grains, and white polished rice constitutes >75 % of refined grain intake( Reference Kumar, Mohanraj and Sudha 2 ). In China, brown rice is rarely consumed( Reference Zhang, Malik and Pan 3 ). As a result, in Asian populations, white rice makes large contributions to dietary glycaemic load, an index reflecting the acute blood glucose-raising potential of foods or diets( Reference Mohan, Radhika and Vijayalakshmi 4 ). Higher levels of postprandial glycaemic exposure have been implicated in the development of chronic metabolic diseases, particularly type 2 diabetes mellitus and CVD( Reference Blaak, Antoine and Benton 5 ). A recent systematic review and meta-analysis has shown a clear relationship between white rice intake and the risk of type 2 diabetes mellitus, with higher levels of rice intake being more strongly associated with the risk in Asian than in Western populations( Reference Hu, Pan and Malik 6 , Reference Neal 7 ).
There are many varieties of rice grain in the world, which vary considerably in the postprandial blood glucose (PPG) response they produce( Reference Foster-Powell, Holt and Brand-Miller 8 ). The results of glycaemic index (GI) studies around the world( Reference Brand Miller, Pang and Bramall 9 ) report values ranging from 64 to 93. Moreover, the post-harvest treatment of rice and the method of consumer preparation can also play a significant role in this variation. Starch comprises two glucose polymers: amylose and amylopectin. Amylose is a linear and relatively short polymer of glucose units linked by α(1 → 4) bonds. Amylopectin is a branched and longer polymer where glucose units are arranged linearly through α(1 → 4), with branches emerging via α(1 → 6) bonds occurring every twenty-four to thirty glucose units( Reference Sajilata, Singhal and Kulkarni 10 ). It is well known that starches with a higher amount of amylose are more resistant to digestion( Reference Hu, Zhao and Duan 11 ).
In addition to the variation in amylose content, cooking (and cooling) processes can influence starch digestibility via the degree of gelatinisation and retrogradation of rice starch. Gelatinisation is the collapse (disruption) of molecular order (breaking of H bonds) within the starch granule, manifested in irreversible changes in properties such as granular swelling, native crystallite melting, loss of birefringence and starch solubilisation during hydrothermal treatment( Reference Atwell, Hood and Lineback 12 ). This leads to the dissociation of crystalline regions in starch with associated hydration and swelling of starch granules, leading to higher starch availability to human digestive enzymes( Reference Tester and Sommerville 13 ). Retrogradation is the recrystallisation of amorphous phases created by gelatinisation( Reference Faraj, Vasanthan and Hoover 14 ) and, in the case of amylose, results in the formation of type 3 resistant starch (RS3)( Reference Mitra, Bhattacharya and Roy 15 ). RS3 is resistant to digestion, because it is heat stable and melts above 120°C( Reference Sievert and Pomeranz 16 ). In contrast, retrograded amylopectin is thought to melt upon reheating (cooking) due to the low melting point (46–65°C) of these crystallites, and therefore it is digestible upon cooking.
Post-harvest processing includes milling, parboiling and quick-cooking. The rice milling process starts with the husking stage to remove the husk from paddy rice, followed by the whitening–polishing stage to transform brown rice into polished white rice, and finally the grading and blending stage to obtain head rice with predefined amounts of broken rice. However, while this may affect the overall nutritional value, the effects on digestibility and PPG are less clear( Reference Dipti, Bergman and Indrasari 17 ). Other post-harvest treatments such as parboiling can also play a role in digestibility. Parboiling is a hydrothermal treatment that includes soaking in water, heating, drying and milling of paddy rice. During the parboiling process, the crystalline structure of the starch present in rice is transformed into an amorphous form. Pressure parboiling is accomplished by soaking paddy rice in warm water (65–68°C) for 4–5 h followed by steaming under pressure and drying( Reference Ranawana, Henry and Lightowler 18 ). Other post-harvest processes are used to produce quick-cooking rice. The latter is a precooked rice where the starch has been partially gelatinised by soaking in water and heating( Reference Owens 19 ). For consumer consumption, additional processes include cooking, storage and reheating. There are different ways of rice cooking depending on the ratios between rice and water, equipment (pressure cooking and steaming), and consumer preference (sticky rice, aromatic basmati, etc.). Cooking of polished white rice strongly affects gelatinisation. Retrogradation is affected by cooling and storage conditions (see also Fig. 3).
Given that reductions in PPG responses are generally seen as a beneficial dietary change( Reference Blaak, Antoine and Benton 5 ), it is useful to objectively establish the variation in the range of PPG responses to rice and the primary intrinsic and processing factors known to affect such responses. Therefore, we performed a systematic search of the literature characterising the range of PPG and PPI responses to different rice types, and considered this alongside available data on rice grain and processing characteristics. The main emphasis is on in vivo studies conducted in human subjects, supplemented in places by the in vitro literature related to specific mechanisms that may be relevant (e.g. influence of microstructure on rice).
Methods
The literature database ‘Scopus’ was searched for the following combinations of keywords (without language or time restrictions): rice* AND glycaem* or glycem* or digestib* or glucose* or insulin* or hyperglycaem* or hyperglycem* or hypoglycaem* or hypoglycem* or normoglycaem* or normoglycem* AND combined with the title from 1980 through July 2014, resulting in ninety-four records. In addition, the PubMed and SciFinder databases were also searched using the same search terms, resulting in one additional article. A further three ‘missed’ articles were identified from the cited references in the articles identified in the formal searches, resulting in ninety-eight articles. From manual inspection of the ninety-eight abstracts, we identified twenty-eight original articles describing the results of thirty-two randomised clinical trials with rice as the test food and a measure of PPG (and in some cases also PPI) as an outcome measure (for a detailed flow chart, see Fig. 1).

Fig. 1 Flow chart of the systematic review article selection process. RCT, randomised controlled trial.
Results
Evidence base
Studies identified in the search and their key relevant results are presented in Table 1. In addition, specific comparisons of amylose content, parboiling and milling are presented in online Supplementary Tables S2, S3 and S4, respectively. The thirty-two randomised clinical trials on PPG responses to rice included different rice types (e.g. regional varieties) and different processes (milling, (par)boiling, ‘quick-cook’ and (pressure) cooking). Outcome measures for blood glucose included GI (twenty-seven studies) and/or the incremental area under the PPG response curve (iAUC, nineteen studies), or peak glucose values (eight studies). The iAUC is the actual blood glucose response to a given serving of rice, whereas the GI and the corresponding insulinaemic index (II) use a fixed available carbohydrate load (usually 50 g) and represent responses as a comparison with a reference (assigned a value of 100). Except where noted, the GI and II studies compared rice with glucose as the reference. A subset of studies reported the II (seven studies) or insulin AUC (eight studies). Furthermore, two studies took breath hydrogen into account as an indicator of carbohydrate malabsorption( Reference Li, Piao and Tian 20 , Reference Casiraghi, Brighenti and Pellegrini 21 ).
Table 1 Human in vivo studies on the postprandial glycaemic and insulinaemic effects of rice*
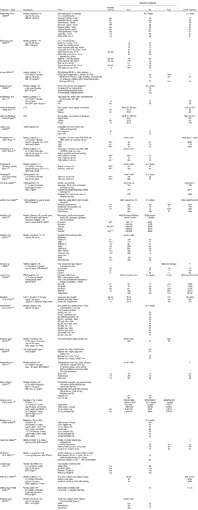
GI, glycaemic index; II, insulinaemic index; NR, not reported; RS, resistant starch; UBR, Uncle Ben's rice; HR, Hassawi rice; tAUC, total AUC; T2DM, type 2 diabetes mellitus; iAUC, incremental AUC; PB, parboiled; NP, not parboiled; Bg, Bathalagaoda; Bw, Bombuwala; NIDDM, non-insulin-dependent diabetes mellitus; IDDM, insulin-dependent diabetes mellitus.
* For the GI and II values, 50 g of available carbohydrates were used, with glucose as the reference (except where noted) being assigned the value of 100.
† The AUC was not calculated by the trapezoidal method but by the following formula: (time 1)/4+(time 2)/2+¾ time 3+time 4+time 5.
Characterisation of rice and processing
In most studies, rice was well characterised with respect to the percentage of amylose (nine studies), dietary fibre (four studies), RS (two studies) and available starch (sixteen studies). In some studies, gelatinisation or amylograph measurements of milled rice flour were taken into account( Reference Al-Mssallem, Hampton and Frost 22 – Reference Panlasigui and Thompson 26 ), while in others, in vitro glucose release assays were included( Reference Casiraghi, Brighenti and Pellegrini 21 , Reference Juliano, Perez and Komindr 24 , Reference Kim, Kim and Kong 27 ). A few studies reported grain size, rheology or retrogradation determined by differential scanning calorimetry (a thermo-analytical technique to identify phase transition)( Reference Larsen, Rasmussen and Rasmussen 28 ). The processes explored in the studies involved post-harvest treatments such as parboiling and milling (Fig. 2) .
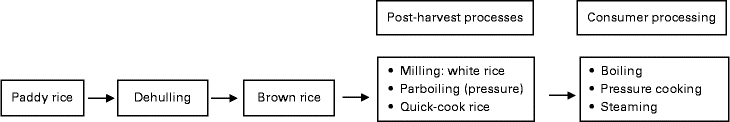
Fig. 2 Rice processing steps.
Variation observed in the glycaemic index and insulinaemic index and its causes
The observed GI values ranged from 48 to 93, while the II values (0–120 min) ranged from 39 to 95 (Table 1).
In the studies that specifically tested or varied the amylose content and its quantitative relationship with glycaemic and insulinaemic responses( Reference Brand Miller, Pang and Bramall 9 , Reference Ranawana, Henry and Lightowler 18 , Reference Li, Piao and Tian 20 , Reference Al-Mssallem, Hampton and Frost 22 , Reference Juliano and Goddard 23 , Reference Kataoka, Venn and Williams 29 – Reference Goddard, Young and Marcus 33 ), the latter measures were significantly inversely associated with the amylose content( Reference Brand Miller, Pang and Bramall 9 , Reference Ranawana, Henry and Lightowler 18 , Reference Li, Piao and Tian 20 , Reference Kataoka, Venn and Williams 29 – Reference Larsen, Christensen and Rasmussen 32 ) (see also online Supplementary Table S2). However, some studies did not find this inverse relationship for all glycaemic parameters( Reference Al-Mssallem, Hampton and Frost 22 , Reference Juliano and Goddard 23 , Reference Goddard, Young and Marcus 33 ). Large differences in amylose content (2 % v. approximately 30 % amylose) were often associated with relatively large glycaemic and insulinaemic effects (approximately 300 % decrease in PPG; approximately 55 % decrease in PPI)( Reference Brand Miller, Pang and Bramall 9 , Reference Ranawana, Henry and Lightowler 18 , Reference Kataoka, Venn and Williams 29 ). However, there were also studies in which this effect was inconsistent( Reference Trinidad, Mallillin and Encabo 30 ) or not observed( Reference Juliano and Goddard23 (Expt 2) , Reference Goddard, Young and Marcus 33 ).
Rice that received post-harvest treatments such as parboiling( Reference Casiraghi, Brighenti and Pellegrini 21 , Reference Kataoka, Venn and Williams 29 , Reference Hettiarachchi, Jiffry and Jansz 34 ) and quick-cooking( Reference Ranawana, Henry and Lightowler 18 , Reference Casiraghi, Brighenti and Pellegrini 21 ) generally gave a lower GI compared with white rice not subjected to these post-harvest treatments (see also online Supplementary Table S3). Larsen et al. ( Reference Larsen, Rasmussen and Rasmussen 28 ) reported that an increased severity of parboiling conditions leads to significant decreases in PPG responses due to the formation of RS. In that study, mild traditional parboiling had no effect on the GI, whereas severely pressure parboiling reduced the GI by almost 30 % compared with non-parboiled rice. However, one study did not show an effect of parboiling( Reference Larsen, Christensen and Rasmussen 32 ), and the reported GI of a thermally treated Indian basmati rice variety (thermal treatment not specified) was 55( Reference Srinivasa, Raman and Meena 35 ), which was in the range between 52 and 59 reported for non-thermally treated Indian basmati rice by Henry et al. ( Reference Henry, Lightowler and Strik 36 ). The influence of another post-harvest treatment, milling, by which brown rice is transformed into white rice, was considered in several studies( Reference Brand Miller, Pang and Bramall 9 , Reference Ranawana, Henry and Lightowler 18 , Reference Panlasigui and Thompson 26 , Reference Trinidad, Mallillin and Encabo 30 , Reference Karupaiah, Aik and Heen 37 ) (see online supplementary Table S4). In those studies where cooking times were identical( Reference Panlasigui and Thompson 26 , Reference Trinidad, Mallillin and Encabo 30 , Reference Karupaiah, Aik and Heen 37 ), brown rice always produced lower PPG and PPI responses. However, when realistic (longer) cooking times were applied to brown rice( Reference Brand Miller, Pang and Bramall 9 , Reference Ranawana, Henry and Lightowler 18 ), the difference between brown and white rice was smaller and inconsistent.
Consumer processing can also make a large contribution to the formation of RS in rice. Chiu & Stewart( Reference Chiu and Stewart 38 ) quantified RS content in four white rice varieties (jasmine, long grain, medium grain and short grain) cooked in three different ways (oven-baked, conventional rice cooker and pressure cooker), and analysed the RS content immediately after preparation or after 3 d of refrigeration at 4°C. Refrigerated long-grain rice cooked in a conventional rice cooker had the highest RS content, while the refrigerated short-grain rice cooked in a pressure cooker had the lowest RS content. However, in this case, the GI values did not differ significantly between the higher-RS and lower-RS rice varieties. Consumer processing can also have a large effect on gelatinisation. Wolever et al. ( Reference Wolever, Jenkins and Kalmusky 39 ) showed that the GI generally increased with cooking time for rice, while Jung et al. ( Reference Jung, Suh and Hong 40 ) showed a marked increase in gelatinisation upon cooking rice and a somewhat higher GI and II.
Discussion
The literature reveals considerable variation in the glycaemic or insulin response to rice. This is largely attributable to (1) starch characteristics, (2) post-harvest processing (particularly parboiling and to a much lesser extent dehulling and milling) and (3) consumer processing (cooking, storage and reheating). The relationships among rice characteristics and processing factors, and their physico-chemical effects and impact on glycaemic responses are qualitatively shown in Fig. 3.

Fig. 3 Relationship between rice characteristics, processing factors, physico-chemical processes and glycaemic response (+ indicates increased effect; − indicates decreased effect). This is a general figure, depending on specific processes, e.g. conditions of parboiling; the effects may differ. PPG, postprandial glucose response.
Influence of the composition and processing of rice
The most consistently important source of variation in PPG responses to rice is amylose content. The amylose content of rice varies between 0 % (waxy rice) and 30 % (Doongara)( Reference Brand Miller, Pang and Bramall 9 ), with basmati having an intermediate value (20–25 % amylose( Reference Bhattacharjee, Singhal and Kulkarni 41 )). One of the reasons for the lower PPG responses to high amylose varieties is incomplete gelatinisation of amylose under normal cooking conditions, while amylopectin is fully gelatinised under these conditions( Reference Björck, Granfeldt and Liljeberg 42 ). Gelatinisation temperature is known to be positively correlated with amylose content( Reference Fredriksson, Silverio and Andersson 43 ), implying that rice with a higher amylose content requires a higher gelatinisation temperature due to restrained swelling by amylose, resulting in a longer required cooking time( Reference Fitzgerald, Rahman and Resurreccion 44 ). The formation of complexes between amylose and lipids upon heating further contributes to reduced access to starch by gut enzymes( Reference Goddard, Young and Marcus 33 ). These complexes with lipids are only found in association with amylose; therefore, rice with the highest amylose content would have more lipid–amylose complexes( Reference Goddard, Young and Marcus 33 ). In addition, a higher amylose content (after cooking and cooling) leads to a greater degree of retrogradation( Reference Ranawana, Henry and Lightowler 18 ). A recent study found the major gene associated with the variation in the GI was the waxy gene( Reference Fitzgerald, Rahman and Resurreccion 44 ), which codes for different structures of amylose within the grain and leads to different retrogradation rates( Reference Tran, Daygon and Resurreccion 45 ).
The in vitro literature showed that the rice cultivar, clustered as Indica, Japonica and Hybrid rice type, plays a pivotal role in the rate and degree of starch digestion: low-amylose Indica showed a faster and higher degree of digestion than low-amylose Japonica, while a high-amylose Japonica was faster and more completely digested (reflected by a higher content of rapidly digestible starch and a lower content of slowly digestible starch and RS) than high-amylose Indica( Reference Hu, Zhao and Duan 11 ). In addition, Benmoussa et al. ( Reference Benmoussa, Moldenhauer and Hamaker 46 ) showed that amylopectin fine structure in rice cultivars affects starch digestion properties in vitro: cultivars with the highest amount of slowly digestible starch contained mainly long-chain amylopectin.
Post-harvest treatments such as parboiling( Reference Casiraghi, Brighenti and Pellegrini 21 , Reference Kataoka, Venn and Williams 29 , Reference Hettiarachchi, Jiffry and Jansz 34 ) and quick-cooking( Reference Ranawana, Henry and Lightowler 18 , Reference Casiraghi, Brighenti and Pellegrini 21 ) also have a large influence on the GI (see online Supplementary Table S3). Gelatinisation and re-crystallisation are the major changes that occur in rice starch during parboiling( Reference Oli, Ward and Adhikari 47 ). The parboiling process increases the gelatinisation temperature of rice that is proportional to the severity of the heat treatment( Reference Islam, Shimizu and Kimura 48 ). This is probably the reason why pressure parboiling lowers the GI to such a large extent, especially of high-amylose starches( Reference Zavareze, Storck and de Castro 49 ). The pressure parboiling process increases gelatinisation temperature due to the formation of retrograded amylose and amylopectin. Wet heating and subsequent drying during these processes result in the gelatinisation of starch, followed by retrogradation of amylose and amylopectin( Reference Ranawana, Henry and Lightowler 18 ) leading to higher levels of RS. It is possible that amylopectin crystallites (part of RS) retain some of the associating forces during reheating, and are partly responsible for the low glucose response observed during pressure parboiling. The amylose–lipid complexes have a melting temperature above 100°C and are not melted during the cooking process, resulting in higher levels of RS( Reference Larsen, Rasmussen and Rasmussen 28 ).
Another way of achieving a high RS content is to apply multiple heating/cooling cycles( Reference Yadav, Sharma and Yadav 50 ). After three heating/cooling cycles, the RS content of legumes, cereals and tubers increased from 4·18, 1·86 and 1·51 % to 8·16, 3·25 and 2·51 %, respectively, on a DM basis. However, a ten times greater RS content in rice varieties had no effect on the GI( Reference Chiu and Stewart 38 ). It is possible that the tested range of difference in RS content in that study was not sufficient to observe a change in the GI( Reference Chiu and Stewart 38 ), which is confirmed by the fact that only large differences in amylose content (leading to high RS content after cooking and cooling) lead to relatively large effects on the GI( Reference Brand Miller, Pang and Bramall 9 ).
Another final process shown to have a major influence on the PPG response is the gelatinisation process during cooking, which needs moisture and a high temperature (above gelatinisation temperature) for a particular period of time. Using different rice types with the same high amylose content, Panlasigui et al. ( Reference Panlasigui, Thompson and Juliano 25 ) reported that PPG responses differed between rice types when a fixed cooking time was used; however, these differences disappeared when the minimum cooking time for each particular rice type was used. This is likely attributed to other physico-chemical properties of rice types. Physico-chemical parameters that predict lower blood glucose responses are high gelatinisation temperature, high minimum cooking time, lower viscosity measured by amylograph consistency (amylograph is an instrument for measuring gelatinisation temperature and viscosity of flour and starch pastes), and low volume expansion upon cooking, all parameters relating to lower gelatinisation( Reference Panlasigui, Thompson and Juliano 25 ). Steaming also gave a larger PPG response than boiling and simmering( Reference Parastouei, Shahaboddin and Motalebi 51 ), which may reflect greater gelatinisation by steaming.
A factor that has a relatively less impact on PPG responses is physical size and form of the whole kernel rice, probably due to the fact that size is minimised by chewing( Reference Ranawana, Henry and Pratt 52 ). Particle size only plays a major role when the rice is milled to rice flour, resulting in the higher surface area:starch ratio that leads to an increased rate of digestion( Reference Chang, Hong, Jung, Watson, Preedy and Zibadi 53 ). In addition, the effect of brown rice v. white rice on glycaemic and insulinaemic responses shows a clear difference( Reference Panlasigui and Thompson 26 , Reference Trinidad, Mallillin and Encabo 30 , Reference Karupaiah, Aik and Heen 37 ) when compared at identical cooking times: for instance, brown rice always gives a lower PPG and PPI response (see online Supplementary Table S4). However, in reality, consumers cook brown rice longer than white rice, resulting in a mixed outcome: in some cases, white rice was found to have a higher glycaemic response( Reference Brand Miller, Pang and Bramall 9 ) (for Pelde), or a neutral effect( Reference Brand Miller, Pang and Bramall 9 ) (for Doongara and Calrose) or even a lower response than brown rice( Reference Ranawana, Henry and Lightowler 18 ). In most of these studies( Reference Brand Miller, Pang and Bramall 9 , Reference Ranawana, Henry and Lightowler 18 , Reference Trinidad, Mallillin and Encabo 30 ) commercially available white rice was taken at random and not milled from the same batch of brown rice. Therefore, the variety and physico-chemical properties of rice samples may have differed( Reference Chang, Hong, Jung, Watson, Preedy and Zibadi 53 ). Only two studies( Reference Panlasigui and Thompson 26 , Reference Karupaiah, Aik and Heen 37 ) used white and brown rice from the same batch. However, a recent longer-term study showed that the iAUC over 5 d consumption was 19·8 % lower for a group eating brown v. white rice, as measured with a continuous glucose monitoring device( Reference Mohan, Spiegelman and Sudha 54 ). However, it is not clear whether brown rice and white rice were of the same rice variety. Therefore, the results cannot clearly be attributed to the milling process alone. It is possible that the dietary fibre-rich bran fraction in brown rice can continue to serve as a barrier to digestive enzymes( Reference Chang, Hong, Jung, Watson, Preedy and Zibadi 53 ), but several other modes of action are also possible. The magnitude of the effect of milling and polishing could also be somewhat dependent on the rice strain and cooking conditions( Reference Ranawana, Henry and Lightowler 18 ). White rice has a shorter minimum cooking time and higher volume expansion than brown rice, indicating that white rice is more easily hydrated and gelatinised compared with brown rice, and therefore more readily digested resulting in a higher PPG response( Reference Chang, Hong, Jung, Watson, Preedy and Zibadi 53 ) when cooked under the same conditions.
In addition to the rice source and processing, there is an inter-individual variation observed in PPG (iAUC and peak blood glucose) responses to carbohydrate-rich foods. This was reported to account for at least 20 % of the total variation in PPG responses( Reference Vega-Lopez, Ausman and Griffith 55 ). One of the factors that could be responsible for the inter-individual variation in PPG responses to rice could be ethnicity. The PPG (+iAUC) response was 60 % greater for five rice varieties and 39 % greater for glucose among the Chinese population compared with Europeans( Reference Kataoka, Venn and Williams 29 ) (Table 1). The most likely explanation for these ethnic differences is that the Chinese population are more likely to become insulin resistant than Europeans of the same or higher relative body weight and waist circumference( Reference Dickinson, Colagiuri and Faramus 56 ). Truong et al. ( Reference Truong, Yuet and Hall 57 ) also observed that Asian Americans on average exhibited higher levels of blood glucose than Caucasians after consumption of a control food with 50 g carbohydrates. Therefore, when comparing the results across studies, ethnicity of the subjects should be taken into account: i.e. Asian people typically have a higher PPG response than Caucasians, which may also increase the apparent magnitude of differences between rice types and characteristics.
A final factor contributing towards the inter-individual variation in PPG responses is the degree of habitual mastication( Reference Ranawana, Henry and Pratt 52 ). The latter may be a considerable contributor, especially to foods consisting of intact grains (such as rice) that rely on mechanical breakdown for carbohydrate release. Indeed, a recent study( Reference Ranawana, Leow and Henry 58 ) showed that rice chewed fifteen times produced a PPG, peak PPG and GI response significantly lower than that when chewed thirty times.
Conclusions
While rice as a total category may be a major global contributor to dietary glycaemic load, there is a wide variation in glycaemic and insulinaemic responses to rice as consumed. This can be largely attributed to the inherent starch characteristics of specific cultivars; however, within a given rice type, the mode of post-harvesting processing and ‘at-home’ preparation can also have a large influence. A reduced glycaemic impact is mediated mainly by the relative content of amylose (v. amylopectin), reduction in gelatinisation, or the facilitation of retrogradation. Perhaps, surprisingly, milling and polishing (thus white v. brown rice) has been found to have inconsistent impacts on acute glycaemic responses when compared at realistic cooking times that are longer for brown rice. The glycaemic response to rice can be further influenced by individual characteristics of the consumer, such as chewing habit and ethnicity. In order to interpret and compare the reported PPG responses between different studies in rice, the rice cultivar, amylose:amylopectin ratio, post-harvest processing parameters and cooking conditions should be considered. In addition, a lower PPG response to rice can be achieved by choosing right conditions, for example high amylose content, minimised cooking times (or pressure parboiled) and cooled before consumption. The opposite effect (a higher PPG response) can be achieved by selecting for low-amylose (waxy) white rice, with a long cooking time, and consuming directly after cooking.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114515001841
Acknowledgements
The present study was not supported by any external funding.
The authors' contributions are as follows: H. M. B. carried out the systematic review; H. M. B. and D. J. M. extracted the data from the articles; H. M. B. wrote the manuscript with significant contributions from D. J. M. and J. S. t. H.
H. M. B., D. J. M. and J. S. t. H. are employees of Unilever. Unilever manufactures and markets consumer food products, including products used for the preparation of rice-based dishes.







