Arachidonic acid (ARA) is the common name for all-cis-5,8,11,14-eicosatetraenoic acid (Fig. 1), commonly abbreviated to 20 : 4ω-6 or 20 : 4n-6. ARA is a long-chain PUFA (LC-PUFA) within the n-6 family. ARA is commonly found in cell membranes, particularly in animals. Therefore meat, especially red meat, and also white meat and fish; offal (organ meat) like liver, kidney and brain; and eggs are sources of ARA. A recent study reported estimated dietary intakes for ARA among adults in 47 developed and 128 developing countries(Reference Forsyth, Gautier and Salem1); the study reported that 48 % of the 175 countries have an ARA intake of <150 mg/d. Amongst the developed countries, mean daily ARA intakes were estimated to be between 100 and 350 mg and ARA contributed <0·1 % of total daily energy intake. Data on ARA intake in specific population subgroups are extremely limited(Reference Sioen, van Lieshout and Eilander2). Human milk also contains ARA. Brenna et al.(Reference Brenna, Varamini and Jensen3) reported human milk ARA (as percentage of total fatty acids (FA)) from 106 studies in the range of 0·24–1 % with a median of 0·47 (sd 0·13) %. Many infant formulas are supplemented with ARA at a level of 0·35–0·7 % of total FA.
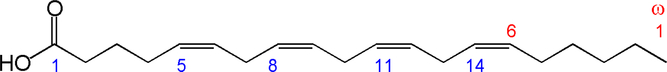
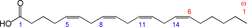
Fig. 1. Structure of arachidonic acid. The numbers 1, 5, 8, 11 and 14 beneath the hydrocarbon chain refer to carbon number counting from the α end of the chain. The numbers 1 and 6 above the hydrocarbon chain refer to carbon number counting from the ω end of the chain.
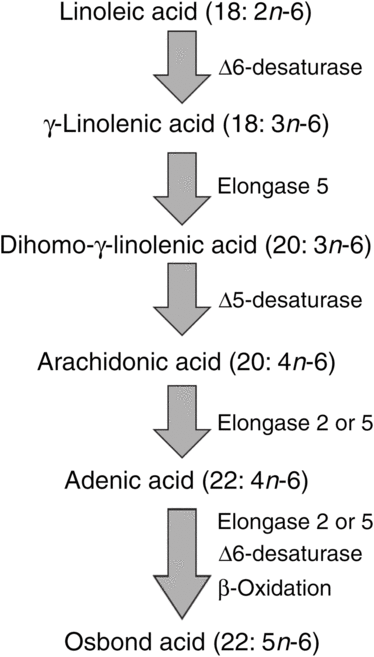
ARA can be synthesised from the essential n-6 PUFA linoleic acid (18 : 2n-6, LA) by a series of desaturation and elongation reactions as shown in Fig. 2. Based upon a study with 2H-labelled LA, Emken et al.(Reference Emken, Adlof and Gulley4) reported that 1–2·2 % of LA is converted to other n-6 PUFA in healthy young adult males with about 0·5 % appearing as ARA.
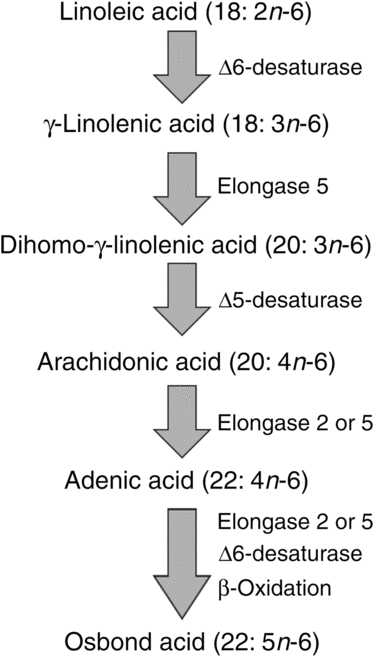
Fig. 2. Outline of the pathway of biosynthesis and further metabolism of arachidonic acid.
Despite its relatively low intake from the diet, ARA makes an important contribution to FA within circulating lipids and in most cells and tissues, particularly to membrane phospholipids (PL). For example, ARA has been reported to contribute an average of 9·6, 6·6, 15·5, 9·5 and 16 % of total FA in plasma phosphatidylcholine, plasma cholesteryl esters (CE), erythrocytes, platelets and blood mononuclear cells in healthy British adults who were non-oily-fish consumers and were consuming typical diets(Reference Walker, West and Browning5). The relatively high content of ARA in these pools, in comparison with its intake from the diet, suggests that synthesis from LA is an important pathway and that metabolic mechanisms might serve to concentrate and retain ARA in cell membranes. ARA has a structural role in the brain(Reference Carlson and Colombo6,Reference Hadley, Ryan and Forsyth7) , free ARA has roles in cell signalling, and ARA-containing PL also have roles in cell signalling and are precursors to endocannabinoids that have a range of biological properties(Reference Witkamp8). A major role of cell membrane ARA is as a substrate for the synthesis of eicosanoids, which include prostaglandins, thromboxanes and leukotrienes. These are formed by metabolism of ARA by cyclo-oxygenase, lipoxygenase and cytochrome P450 pathways(Reference Lewis, Austen and Soberman9–Reference Dennis11). The resulting metabolites have many roles in inflammation and pain, regulation of the immune response, bone turnover, platelet aggregation and blood clotting, smooth muscle contraction and renal function(Reference Lewis, Austen and Soberman9–Reference Dennis11). These well-known functions of ARA-derived metabolites suggest that ARA itself might impact a range of outcomes related to human health. This has been explored in a small number of human studies, frequently using supplements containing ARA. The first such study was by Seyberth et al.(Reference Seyberth, Oelz and Kennedy12). This was a small study involving four healthy men given 6 g of ethyl arachidonate daily for a period of 2–3 weeks. The authors identified an increased content of ARA in plasma TAG, PL and CE and in platelets, and a mean 47 % increase in excretion of the major urinary metabolite of ARA. The threshold of ADP required to induce platelet aggregation was decreased in all four subjects (by 40–90 %), which was interpreted as an enhanced potential for blood clotting. This was considered to be a major health concern and most likely explains why there was no further interest in studies with ARA supplements in humans until the mid-1990s(Reference Nelson, Schmidt and Bartolini13–Reference Ferretti, Nelson and Schmidt17).
The aim of this systematic review is to assess the effects of increasing ARA intake through foods or supplements on ARA levels in different compartments, metabolism and health-related outcomes in humans. All health-related outcomes that have been addressed in randomised controlled trials (RCT) investigating increased ARA intake through foods or supplementation were considered. Research gaps were identified and we assessed whether recommendations for ARA intake can be made for specific health effects.
Methods
Details of the methods used for the systematic review are published in the PROSPERO international prospective register of systematic reviews (registration number: CRD42017076493).
Criteria for considering studies in this review
Types of study
RCT in humans were included if they measured any human health-related outcome. Observational and non-randomised interventional studies were excluded.
Types of intervention
Studies were included if they increased intake of ARA through a food or supplement and had a control group with either a lower ARA intake or no ARA supplementation. We excluded studies assessing the effect of the combination of DHA and ARA or the combination of any other FA with ARA.
Types of population
Studies of human subjects (infants, children and adults) conducted in any country were included, without restriction on age or health status. We excluded animal and in vitro studies.
Minimum duration of intervention
Duration of ARA intervention and follow-up varied depending on the study design and health outcome measured; we did not place a restriction on this.
Types of outcome measure
All types of health outcomes, including risk markers of disease, were included.
Date of publication: There was no restriction on study inclusion according to the year of publication.
Search methods for identification of studies
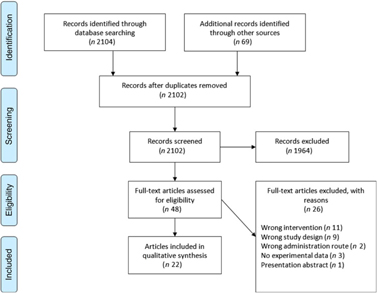
The Cochrane Central Register of Controlled Trials database was searched on 23 August 2017 and again on 23 March 2018 in an update search, using text words with appropriate truncation and relevant indexing terms (Table 1). The keywords for the search were different synonyms related to the intervention (i.e. ARA). The systematic review software Covidence (www.covidence.org) was used to facilitate screening of the literature. Titles and/or abstracts of studies retrieved using the search strategy were screened independently by two review authors (a combination of S. L., A. S., R. P. M., M. F. and B. S.) to identify studies that met the inclusion criteria. The full texts of the potentially eligible studies were retrieved and independently assessed for eligibility by two review team members (a combination of S. L., A. S., R. P. M. and B. S.) with final agreement by all authors. Any disagreement between them over the eligibility of particular studies was resolved through discussion between the two relevant reviewers or the whole group. A standardised, pre-piloted form was used to extract data from the included studies for assessment of study quality and evidence synthesis. Extracted information included study setting; study population and participant demographics and baseline characteristics; details of the intervention and control conditions; study methodology; recruitment and study completion rates; outcomes and times of measurement; and funding. One of the authors (B. v. d. H., R. P. M., M. F. or B. S.) extracted data and the data were double-checked by another member in the team. A flow chart of the literature identification can be found in Fig. 3.
Table 1. Search terms used for the Cochrane Central Database search
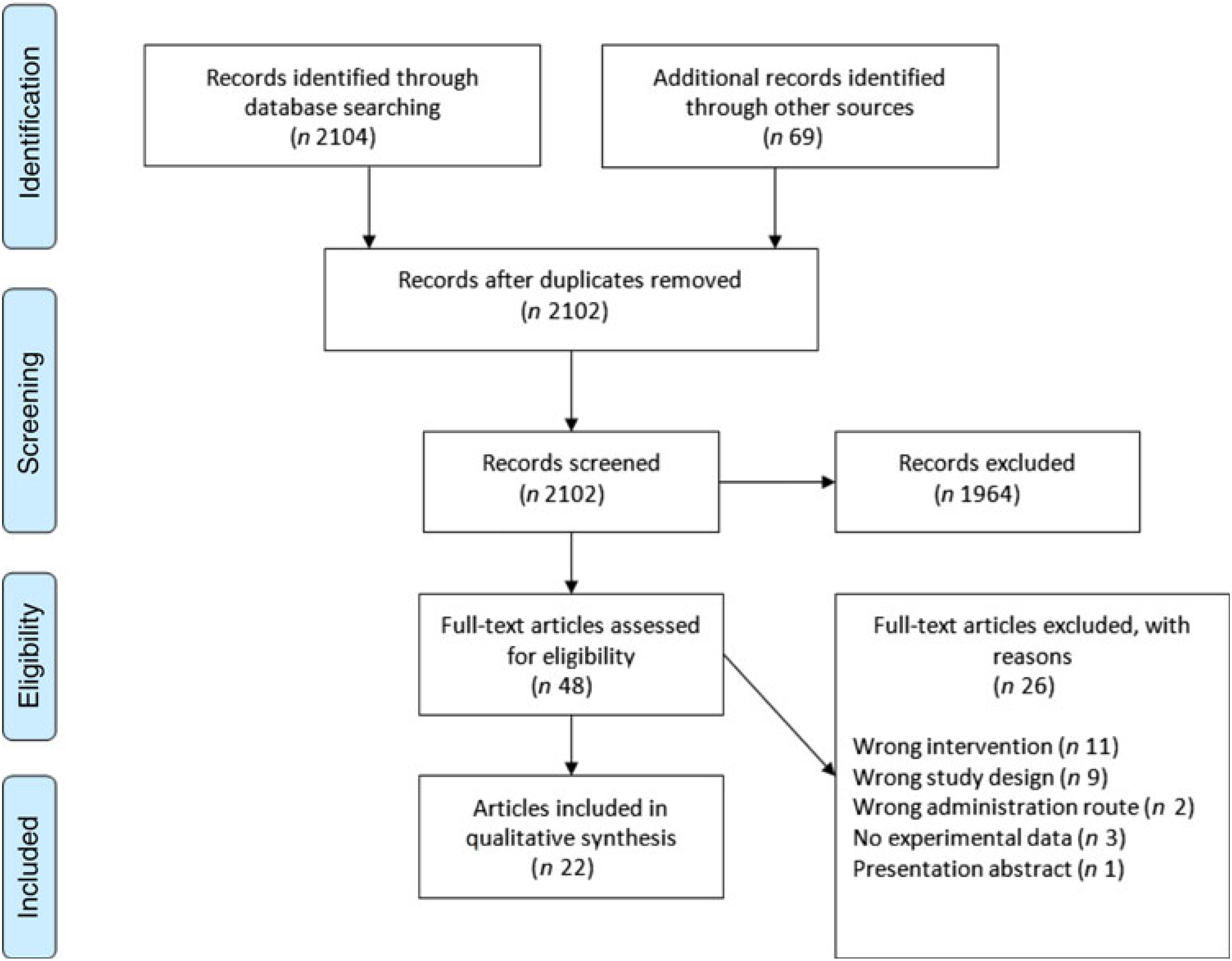
Fig. 3. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart of study selection.
Reference lists of all the eligible papers and relevant systematic reviews were searched for additional studies.
Risk of bias assessment
Three authors independently (R. P. M., M. F. and B. S.) assessed the risk of bias in included studies by following the Cochrane Risk of Bias guidelines(Reference Higgins, Altman and Sterne18), and any disagreement between them was resolved through discussion.
Results
CENTRAL search
A total of 2104 titles and abstracts were identified via the electronic search from which 1255 duplicates were removed. Additional references (n 69) were found via reference screening of review papers found in the electronic search, of which thirty-six were duplicates. In total, 1964 titles were excluded as irrelevant based on title and abstract considering the inclusion and exclusion criteria. The remaining forty-eight papers were screened based on full text, and twenty-two were considered as relevant for review inclusion. A flow chart of the literature identification process can be found in Fig. 3. The twenty-two articles included in the systematic review(Reference Nelson, Schmidt and Bartolini13–Reference Ferretti, Nelson and Schmidt17,Reference Barakat, Abou El-Ela and Sharaf19–Reference Thies, Miles and Nebe-von-Caron35) came from fourteen individual studies. Table 2 summarises these studies. Most studies were conducted in healthy young or older adults and several were restricted to men. One study was conducted in breast-feeding women, one in patients with liver cirrhosis and two in children with parasitic worm infestation. Studies in adults used between 80 and 2000 mg ARA per d, were of 1–8 weeks duration and usually used ARA as a supplement.
Table 2. Characteristics of the included articles

ARA, arachidonic acid.
Risk of bias assessment
The overall risk of bias analysis shows that for many studies bias is unclear due to insufficient reporting of required details essential for an informed decision (Fig. 4).
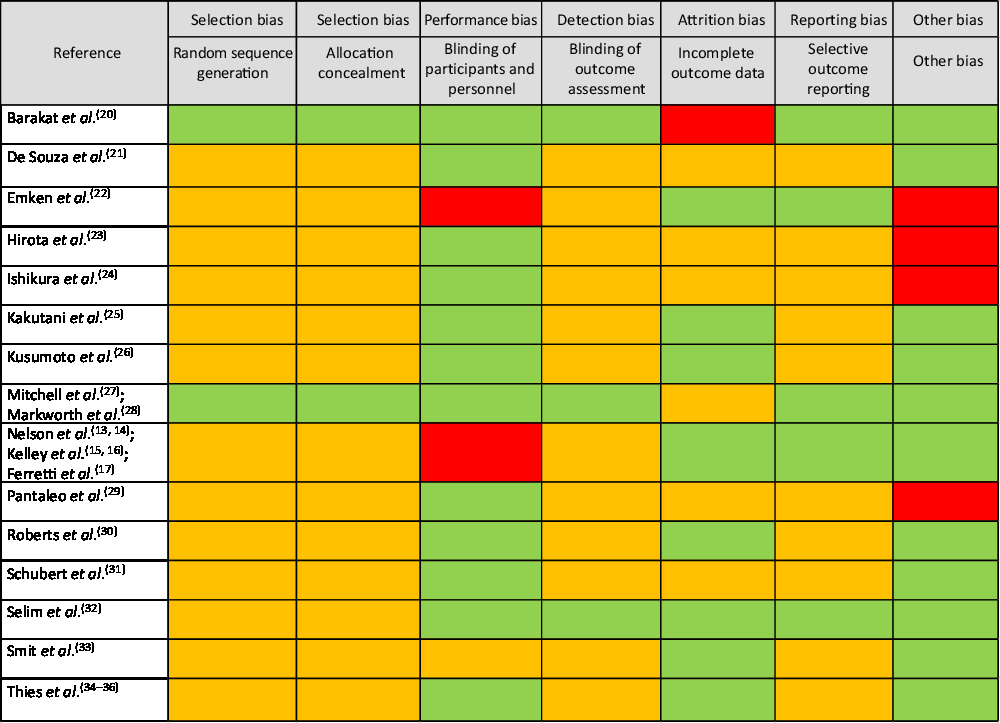
Fig. 4. Risk of bias analyses. Under ‘other bias’ power analyses, statistical shortcomings (e.g. only within-treatment comparisons) and experimental design issues were considered. Legend: green: low risk of bias; red: high risk of bias; orange: unclear risk of bias.
Fatty acid composition in different compartments
A number of included studies reported the effect of increased intake of ARA on the FA composition of one or more blood pools.
Nelson and colleagues(Reference Nelson, Schmidt and Bartolini13–Reference Ferretti, Nelson and Schmidt17) performed a placebo-controlled, random order, cross-over study in twelve healthy male participants housed in a metabolic unit. Participants consumed a stabilisation diet that contained about 200 mg/d ARA for 15 d, and then either continued on the stabilisation diet for 50 d or consumed a diet that provided 1·7 g/d ARA for 50 d. After that, the participants crossed over to the other diet for 50 d, followed by 15 d of the stabilisation diet. Ten participants completed the study. The FA compositions of plasma, plasma TAG, CE, PL and NEFA, erythrocytes, adipose tissue(Reference Nelson, Schmidt and Bartolini14) and platelets(Reference Nelson, Schmidt and Bartolini13) were reported. There was a near doubling of plasma ARA when ARA was consumed (approximately from 8 to 16 % of total FA) which was accompanied by a decrease in LA. ARA increased in plasma TAG, CE (approximately from 7·5 to 15 % of FA) and PL (approximately from 10 to 19 % of FA) again mainly at the expense of LA. There was a small increase in ARA in erythrocytes and platelets but no change in adipose tissue ARA. Increases in ARA content were not reflected in any changes in EPA or DHA, except in erythrocytes where DHA content decreased.
Thies et al.(Reference Thies, Nebe-von-Caron and Powell33–Reference Thies, Miles and Nebe-von-Caron35) performed a placebo-controlled, randomised, parallel study in healthy older British subjects: eight participants received a blend of palm and sunflower seed oils as control, while eight received approximately 700 mg ARA (from ARASCO) daily for 12 weeks. Participant’s habitual intake of ARA was about 150 mg/d. Blood samples were collected at baseline and at 4, 8 and 12 weeks supplementation and then after 4 weeks washout. The FA compositions of plasma PL(Reference Thies, Nebe-von-Caron and Powell33) and blood mononuclear cells(Reference Thies, Nebe-von-Caron and Powell34) were reported. In plasma PL, ARA increased from 9·3 to 16 % of FA by 4 weeks and did not increase further, but returned to baseline levels after 4 weeks washout(Reference Thies, Nebe-von-Caron and Powell33). Plasma PL ARA levels were significantly higher in the ARA group than in the control group at weeks 4, 8 and 12. The increase in ARA in plasma PL did not significantly affect LA, EPA and DHA levels. In mononuclear cells, ARA increased significantly from approximately 20 % of total FA at baseline to approximately 23 % at 8 and 12 weeks(Reference Thies, Nebe-von-Caron and Powell34). This increase was mainly compensated for by a decrease in LA. EPA and DHA levels in mononuclear cells were not affected by ARA supplementation. ARA levels in mononuclear cells were not significantly different between the ARA and control groups.
Pantaleo et al.(Reference Pantaleo, Marra and Vizzutti28) evaluated if ARA supplementation could increase ARA levels in plasma and erythrocyte lipids in Italian patients with liver cirrhosis. Patients received either 2 g ARA (as 4 g ARASCO) daily or oleic acid as control for 8 weeks. ARA supplementation for 8 weeks significantly increased ARA levels in plasma total lipids, plasma PL and erythrocytes. This increase was transitory, since ARA levels returned to baseline 4 weeks after cessation of supplementation. No changes in ARA levels were observed in the control group. There was no significant effect of ARA on LA in plasma total lipids, plasma PL or erythrocytes, although LA was numerically lower in all three pools after ARA compared with before. No results were given for n-3 FA levels, and no between-group comparison was made.
Schubert et al.(Reference Schubert, Kitz and Beermann30) compared the effect of two different fat blends on the FA status of thirty healthy German adults. Participants were randomly distributed into two groups receiving for 2 weeks either an oil blend providing 240 mg/d of α-linolenic acid, 120 mg/d of EPA, 49 mg/d of stearidonic acid and 73 mg/d of γ-linolenic acid in three capsules per d or an oil providing 40 mg ARA per d in one capsule per d plus two olive oil capsules per d. Results showed that 40 mg/d ARA supplementation for 2 weeks did not increase plasma or erythrocyte ARA. This lack of change is most likely because of the low amount of ARA provided. Between-group comparison, done at each time point, showed that EPA was higher in plasma lipids after the 2 weeks supplementation period in the group receiving the blend containing EPA than in the ARA group.
Kusumoto et al.(Reference Kusumoto, Ishikura and Kawashima25) carried out a double-blind, placebo-controlled study in healthy Japanese men consuming a high fish diet. One group of men received capsules providing 838 mg ARA incorporated into a TAG derived from Mortierella alpina (SUNTGA40S) daily for 4 weeks, while another group received capsules with olive oil as control. FA in serum PL and TAG were measured in fasted blood at four time points: baseline, after 2 and 4 weeks of supplementation and 4 weeks after the end of supplementation. Supplementation with ARA increased ARA content of serum PL from 9·6 to 13·7 (after 2 weeks) and 13·9 % (after 4 weeks) of total FA, which was significantly different from baseline, and then ARA content decreased to a level close to that of baseline 4 weeks after the end of supplementation. Serum PL ARA did not change in the control group. The same pattern was observed in serum TAG, although levels of ARA were lower in this lipid class than in PL. Between-group comparison showed a significantly higher ARA content in serum PL, but not in serum TAG, in the group supplemented with ARA than in the control group, after 2 and 4 weeks supplementation. In serum PL, LA was significantly lower than baseline after 2 and 4 weeks ARA supplementation, while it was unchanged in the control group and in serum TAG. Between-group comparison showed no significant difference in LA in serum PL or TAG. There was no significant change in plasma PL or TAG EPA and DHA. This study shows that in a healthy male adult population with high fish intake (approximately 860 mg EPA+DHA intake per d) and a basal intake of approximately 177 mg ARA per d, supplementation with 838 mg/d is able to increase ARA levels in serum PL without compromising EPA and DHA levels.
Ishikura et al.(Reference Ishikura, Ikeda and Akimoto23) supplemented the habitual diet of Japanese elderly men with three capsules per d providing either 240 mg ARA in the form of ARA-enriched TAG (SUNTGA40S; 600 mg oil) or control (600 mg olive oil per d) for 1 month. ARA supplementation significantly increased ARA content in serum PL (from 140 μg/ml serum to 175 μg/ml serum; this was equivalent to an increase from 8·7 to 10·7 % of FA), and significantly decreased EPA but did not affect DHA. In the control group, there was no change in ARA, EPA or DHA levels. No between-group comparison was made.
Hirota et al.(Reference Hirota, Adachi and Gomyo22) performed a double-blind, random order cross-over, placebo-controlled intervention study with twenty-three young Japanese women aged 18–23 years. The subjects received one 200 mg capsule daily, either containing a low dose of ARA (approximately 80 mg per d in 200 mg ARA enriched-TAG) or olive oil as control. The study duration was 12 weeks in total, including four successive periods of 3 weeks: 3 weeks washout, 3 weeks with treatment 1, 3 weeks washout and 3 weeks with treatment 2. The participants were asked to limit their fish consumption to a maximum of four times per week. They recorded their food intake throughout the study, allowing their FA intake at the beginning and at the end of the two treatment periods to be estimated. Fasted blood was taken at the beginning (baseline value) and at the end of the two 3-week treatment periods, and FA were analysed in erythrocyte and plasma PL, TAG and CE. ARA supplementation significantly increased ARA and total n-6 LC-PUFA levels in the four blood pools compared with olive oil, without decreasing n-3 LC-PUFA, except for a significant decrease in n-3 LC-PUFA in plasma CE. This study shows that in a female population with relatively high fish intake (460–560 mg EPA + DHA intake per d) and a basal intake of approximately 150 mg ARA per d, supplementation with a small dose of ARA (approximately 80 mg/d) is able to significantly increase ARA levels in erythrocyte and plasma fractions without compromising n-3 LC-PUFA levels.
Kakutani et al.(Reference Kakutani, Ishikura and Tateishi24) performed a double-blind, parallel, placebo-controlled intervention study in 118 healthy Japanese elderly who were not supplement consumers. They received ten 170 mg capsules daily, either containing a low dose or a high dose of ARA (total of approximately 240 mg or approximately 720 mg/d) as an ARA enriched-TAG (SUNTGA40S) or olive oil as control for 4 weeks, followed by a 4-week washout period. The participants recorded their food intake throughout the study, allowing to calculate their FA intake at the baseline and after 4 weeks of supplementation. FA in plasma PL were measured in fasted blood at four time points: baseline, after 2 and 4 weeks of supplementation and 4 weeks after the end of supplementation. Supplementation with 240 and 720 mg/d of ARA increased ARA content of plasma PL by 2·5 % and 5·6 % of total FA, respectively, which was significantly different from baseline, and then ARA decreased to a level close to that of baseline 4 weeks after the end of supplementation. Plasma ARA did not increase in the control group. Between-group comparison was done but not clearly reported. The ARA increase in plasma PL was dose dependent, at least up to 720 mg/d. In the high ARA group, plasma PL LA was significantly lower than at baseline after 2 and 4 weeks supplementation, while it was unchanged in the low ARA and the control groups. There was no significant change in plasma PL EPA and DHA. This study shows that in an elderly population with high fish intake (853–1176 mg EPA + DHA intake per d) and a basal intake of approximately 170 mg ARA per d, supplementation with a 240 or 720 mg/d is able to dose-dependently increase ARA levels in plasma PL without compromising EPA and DHA levels.
Recently Markworth et al.(Reference Markworth, Mitchell and D’souza27) reported from a RCT of 1500 mg ARA per d for 4 weeks in young men participating in a resistance training programme. FA were measured in plasma and in skeletal muscle at the start and end of the supplementation period. Plasma ARA increased from 8·4 to 16·2 % of total FA and was higher in the ARA group than in the control group at the end of the intervention. Plasma LA decreased from 25 to 14 % of total FA in the ARA group. Plasma EPA decreased slightly in the ARA group but was not different between groups at week 4. Plasma DHA was not significantly altered. Skeletal muscle ARA increased from 12 to 14·6 % of total FA but this was not a significant effect.
Smit et al.(Reference Smit, Koopmann and Boersma32) performed a randomised, open intervention study in twenty breast-feeding Israeli women. The women’s mean age was 23 years and the lactation duration ranged between 3 and 10 months. The ARA group received 284 mg ARA per d (0·8 ml ARA-rich oil), while the control group did not receive any supplement. The study duration was 1 week, during which three milk samples were taken (before, after 1 d and after 7 d supplementation). Milk FA were analysed and the FA composition of the two groups was compared at the three time-points. It was found that women in the ARA group had the same content of ARA in their milk as women who did not receive any supplement. Other long chain n-6 PUFA and DHA did not differ either, while EPA was significantly lower in the ARA group at day 7. This small study suggests that in a population of lactating women with a habitual estimated ARA intake of approximately 200 mg/d, a supplement of approximately 300 mg ARA per d does not significantly affect milk ARA (or DHA), but does lower milk EPA. This effect can be observed after 1 week of ARA supplementation.
In Egyptian schoolchildren infected with Schistosoma mansoni, ARA (10 mg/kg body weight per d for 5 d in each of 3 weeks) significantly increased plasma ARA from 7·9 to 12·1 % of total FA, with no significant effect on LA(Reference Barakat, Abou El-Ela and Sharaf19).
In summary, increased ARA intake significantly increases the ARA content in different blood fractions, including erythrocytes, platelets and mononuclear cells, with doses as low as 80 mg/d being effective. The low dose of 40 mg ARA per d did not affect ARA level in plasma or erythrocytes of healthy adults. It is likely that enrichment of ARA in different compartments and pools is dose dependent. Fig. 5 shows data for serum and plasma PL from seven studies and indicates a near linear dose-dependent increase in ARA content up to an intake of about 1000 mg/d. Lack of studies using higher intakes of ARA and the short duration of most studies performed to date limit a full understanding of this dose–response relationship. A study specifically designed to assess the dose–response relationship is required to be certain about the exact nature of this relationship. EPA was decreased in several studies (human milk with 300 mg/d ARA, plasma CE with 80 mg/d ARA, serum PL with 240 mg/d ARA and plasma with 1500 mg/d ARA) and one study reported a decrease in DHA content of erythrocytes when a high dose (1·7 g/d) of ARA was consumed for 50 d.

Fig. 5. Relationship between arachidonic acid (ARA) intake (mg/d) and increment in ARA in serum or plasma phospholipids (as percentage of total fatty acids). Data are taken from Hirota et al.(Reference Hirota, Adachi and Gomyo22), Ishikura et al.(Reference Ishikura, Ikeda and Akimoto23), Kakutani et al.(Reference Kakutani, Ishikura and Tateishi24) (two doses of ARA used), Kusumoto et al.(Reference Kusumoto, Ishikura and Kawashima25), Nelson et al.(Reference Nelson, Schmidt and Bartolini14), Pantaleo et al.(Reference Pantaleo, Marra and Vizzutti28) and Thies et al.(Reference Thies, Nebe-von-Caron and Powell33). The line of best fit for these data points is shown.
Effect of arachidonic acid on PUFA metabolism
A small sub-study(Reference Emken, Adlof and Duval21) within the larger study by Nelson et al.(Reference Nelson, Schmidt and Bartolini13,Reference Nelson, Schmidt and Bartolini14) investigated the effect of ARA on Δ5-desaturation and incorporation of 2H-labelled dihomo-γ-linolenic acid (DGLA; 20 : 3n-6) into plasma lipids. Adult male subjects (n 4) were given diets containing either 1·7 g/d ARA or 0·21 g/d ARA for 50 d. After 50 d, subjects were dosed with a mixture containing 2H-labelled ethyl esters of DGLA[d4] and oleic acid[d2], and a series of blood samples were sequentially drawn over a 72-h period. The estimated conversion of DGLA[d4] to ARA[d4] was 17·7 ± 0·8 % when subjects had been on the high ARA intake and 2·1 ± 1·4 % when subjects had been on the low ARA intake. The concentrations of ARA[d4] in total plasma lipids from subjects after the high and low ARA periods were 2·1 ± 0·6 and 0·3 ± 0·2 μmol/ml plasma/mmol of DGLA[d4] fed per kg of body weight. These data indicate that conversion of DGLA[d4] to ARA[d4] was stimulated 7- to 8-fold by the high ARA intake, although a decrease in turnover of ARA in the high ARA group cannot be excluded.
Effect of arachidonic acid on blood lipid concentrations
Roberts et al.(Reference Roberts, Iosia and Kerksick29) conducted a study in resistance-trained male subjects and found that serum cholesterol concentrations were not changed after ARA supplementation (1 g/d for 50 d). Likewise, there were no changes in serum concentrations of total, LDL- and HDL-cholesterol, TAG, apo AI, apo B or lipoprotein (a) after ARA supplementation in different study designs (see Table 2) in adults(Reference Nelson, Schmidt and Bartolini14,Reference Kusumoto, Ishikura and Kawashima25,Reference Markworth, Mitchell and D’souza27) and schoolchildren(Reference Barakat, Abou El-Ela and Sharaf19,Reference Selim, El Sagheer and El Amir31) .
Thus, from the available evidence it appears that increasing ARA intake even up to 1·7 g/d does not affect blood lipid concentrations. However, there are few such studies and the effect of ARA on blood lipids in dyslipidaemic subjects has not been investigated.
Effect of arachidonic acid on blood pressure
In the study of Kusumoto et al.(Reference Kusumoto, Ishikura and Kawashima25), blood pressure was not affected by increasing ARA intake to 838 mg/d. Participants in this study were normotensive and there are no studies of increasing ARA intake on blood pressure in hypertensive subjects.
Effect of arachidonic acid on platelet aggregation, bleeding and haematological markers
In a randomised double-blind trial in Italian patients with liver cirrhosis, Pantaleo et al.(Reference Pantaleo, Marra and Vizzutti28) compared the effects of 2 g/d ARA from ARASCO for 8 weeks with those of oleic acid on collagen-induced aggregation of platelet-rich plasma. Compared with pre-study levels, platelet aggregation was significantly increased in the ARA group, but not in the oleic acid group.
Kusumoto et al.(Reference Kusumoto, Ishikura and Kawashima25) carried out a double-blind, placebo-controlled study in healthy Japanese men consuming a high fish diet. One group of men received capsules providing 838 mg ARA incorporated into TAG (SUNTGA40S) daily for 4 weeks, while another group received capsules with olive oil as control. Collagen-, ARA- and ADP-induced aggregation of platelet-rich plasma were not affected by ARA supplementation. Also, haematological parameters (Hb concentration, packed cell volume, erythrocyte numbers, total leucocytes, thrombocytes and mean of corpuscular volume) and coagulation parameters (prothrombin time and antithrombin III activity) remained unchanged.
In a single-blind cross-over study, healthy male volunteers received 1·7 g/d or 0·2 g/d of ARA for 50 d(Reference Nelson, Schmidt and Bartolini13). Aggregation of platelet-rich plasma was induced using ADP, collagen, and ARA. No effects of increased ARA intake were observed. Also, platelet count, bleeding time, partial thromboplastin time and antithrombin III concentrations remained unchanged. Prothrombin time, however, was significantly lowered by about 10 % after ARA intake.
In a randomised double-blind study, resistance-trained male subjects received either ARA (1 g/d) or maize oil as placebo for 50 d(Reference Roberts, Iosia and Kerksick29). There was no effect on erythrocyte numbers.
Most haematological parameters were not altered by 1500 mg/d ARA for 4 weeks in healthy young men(Reference Markworth, Mitchell and D’souza27).
Haematological parameters (Hb concentration, packed cell volume, erythrocyte numbers, numbers of segmented neutrophils, eosinophils, basophils and platelets) were not changed in a study with Egyptian schoolchildren who received ARA (10 mg/kg body weight per d) for 15 d (5 d over each of 3 consecutive weeks) with or without praziquantel, a medication used to treat certain types of parasitic worm infection(Reference Barakat, Abou El-Ela and Sharaf19). Several coagulation parameters (prothrombin concentration, international normalised ratio and partial thromboplastin time) were also not changed, although both prothrombin time and clotting time were significantly shorter, although only by <2 %(Reference Barakat, Abou El-Ela and Sharaf19). Comparable results were found in a smaller study in older schoolchildren using a similar design(Reference Selim, El Sagheer and El Amir31).
In summary, most studies report no effect of increased ARA intake on platelet aggregation or coagulation parameters, and no studies report effects on bleeding time. However, the study that used the highest intake of ARA(Reference Pantaleo, Marra and Vizzutti28) reported enhanced platelet aggregation. The early study of Seyberth et al.(Reference Seyberth, Oelz and Kennedy12) using 6 g ARA per d also saw this effect. The effect of doses of ARA of 2 g/d or more on platelet aggregation requires further investigation.
Effect of arachidonic acid on biomarkers of immunity and inflammation
Kelley et al.(Reference Kelley, Taylor and Nelson15,Reference Kelley, Taylor and Nelson16) reported data from a controlled, cross-over trial in ten healthy adult men in the USA; this is the same study as Nelson et al.(Reference Nelson, Schmidt and Bartolini13,Reference Nelson, Schmidt and Bartolini14) . The participants consumed a standard diet providing 0·21 g ARA per d or an intervention diet providing 1·7 g ARA per d for 50 d. ARA did not influence ex vivo lymphocyte proliferation in response to several agents or ex vivo natural killer cell activity(Reference Kelley, Taylor and Nelson15). Participants received the measles/mumps/rubella and influenza vaccines: the ex vivo proliferation of lymphocytes in response to influenza vaccine was about 4-fold higher in the group receiving 1·7 g ARA per d than in the low ARA group(Reference Kelley, Taylor and Nelson15). However, serum antibody titres against the influenza vaccine were not affected by high ARA(Reference Kelley, Taylor and Nelson16). Although the total number of leucocytes was not affected, there were significantly more blood granulocytes (mainly neutrophils) when participants received 1·7 g ARA per d(Reference Kelley, Taylor and Nelson15). However, blood lymphocyte and monocyte numbers were not affected by ARA(Reference Kelley, Taylor and Nelson16). Ex vivo production of several inflammatory cytokines in response to lipopolysaccharide was not different between low and high ARA treatment(Reference Kelley, Taylor and Nelson16). However, lipopolysaccharide-stimulated production of PGE2 and leukotriene B4 was significantly higher after high ARA than after the standard diet(Reference Kelley, Taylor and Nelson16). This probably reflects the higher ARA content of the cells involved, although this was not reported.
Thies et al.(Reference Thies, Nebe-von-Caron and Powell33–Reference Thies, Miles and Nebe-von-Caron35) reported results from a placebo-controlled, randomised, parallel study in healthy older British subjects: eight participants received a blend of palm and sunflower seed oils as control, while eight received approximately 700 mg ARA (from ARASCO) daily for 12 weeks. Participant’s habitual intake of ARA was about 150 mg/d. Blood samples were collected at baseline and at 4, 8 and 12 weeks supplementation and then after 4 weeks washout. There was no effect of increased intake of ARA on the numbers or percentages of different types of immune cells in the blood, on ex vivo blood lymphocyte proliferation in response to mitogenic stimulation, on ex vivo production of T-cell derived cytokines or lipopolysaccharide-stimulated cytokines, on ex vivo natural killer cell activity, on phagocytic activity and respiratory burst of neutrophils and monocytes or on the plasma concentrations of three different adhesion molecules(Reference Thies, Nebe-von-Caron and Powell33–Reference Thies, Miles and Nebe-von-Caron35). This study clearly demonstrates that there is no strong impact of approximately 700 mg/d ARA on blood immune cell numbers, on ex vivo markers of the immune response or on markers of inflammation in healthy older subjects. The study did not measure concentrations or production of ARA-derived lipid mediators like prostaglandins.
Roberts et al.(Reference Roberts, Iosia and Kerksick29) saw no effect of 1 g ARA per d for 50 d on total blood leucocyte numbers or types in resistance trained men.
Schubert et al.(Reference Schubert, Kitz and Beermann30) performed a study comparing a mix of FA considered to be anti-inflammatory with ARA (40 mg/d) for 2 weeks in thirty healthy participants; an additional blood sample was collected 2 weeks after stopping the intervention. Whole blood was stimulated ex vivo with lipopolysaccharide and appearance of PGE1 (produced from DGLA), PGE2, leukotriene B4, TNF, IL-8 and IL-10 were measured. There were no significant differences in any of these at the end of supplementation compared with baseline in either group, and there were no significant differences between the two groups at the end of the supplementation period. However, some differences were seen between groups 2 weeks after stopping the supplementation; these are difficult to interpret.
Kakutani et al.(Reference Kakutani, Ishikura and Tateishi24) performed a double-blinded, parallel, placebo-controlled intervention study with 118 healthy Japanese elderly subjects. They received olive oil as control or capsules providing approximately 240 mg or approximately 720 mg ARA per d for 4 weeks, followed by a 4-week washout period. There was no impact of ARA on circulating concentrations of C-reactive protein, IgE, two pro-inflammatory cytokines (TNF and IL-6), PGE2 or lipoxin A4. The authors concluded that there was no impact of 240 or 720 mg ARA daily on inflammation.
In Egyptian schoolchildren infected with S. mansoni, ARA (10 mg/kg body weight per d for 5 d in each of 3 weeks) significantly decreased plasma IL-10 and interferon-γ concentrations compared with study entry(Reference Barakat, Abou El-Ela and Sharaf19). However, these findings are difficult to interpret because ARA was able to effectively treat the parasitic infection, and the altered plasma cytokines may simply reflect reduced pathogen burden.
In summary, the available evidence suggests little or no impact of increasing ARA intake by as much as 1·5 g/d on immune function or on markers of inflammation, apart from a small increase in ARA-derived eicosanoid production when cells are stimulated. However, the health impact of the latter response is not known.
Effect of arachidonic acid on cognitive function
Despite ARA having an important structural and functional role in the brain(Reference Hadley, Ryan and Forsyth7), there are very few RCT of cognitive function in humans where ARA is the sole intervention. Most intervention studies involving ARA have been in combination with DHA and have been undertaken during infancy.
Ishikura et al.(Reference Ishikura, Ikeda and Akimoto23) investigated the effects of ARA on age-related event-related potentials in twenty-five healthy elderly Japanese men. The study was performed using a double-blind crossover design and the subjects were administered 240 mg/d of ARA from an ARA-enriched TAG (SUNTGA40S) in capsules or the same amount of olive oil in capsules as placebo for 1 month. Event-related potentials, which included P300 latency and amplitude, were measured before capsule administration and after 1 month of administration. In subjects administered ARA, P300 latency was significantly shorter, and P300 amplitude was significantly higher than in those administered olive oil capsules. It was concluded that supplementation of ARA may reduce age-related decline in cognitive function and learning ability. However, this is based upon a single small study and more research is needed in this important area.
Effect of arachidonic acid on body composition, muscle function and physical performance
Three double-blind RCT have reported outcomes of the effect of ARA on body composition, muscle function and physical performance(Reference De Souza, Lowery and Wilson20,Reference Mitchell, D’souza and Figueiredo26,Reference Roberts, Iosia and Kerksick29) . In the first study, thirty-one males from the USA were randomly assigned to receive capsules providing either 1000 mg ARA or maize oil per d for 50 d. No significant effects were found on body weight, fat free mass, fat mass, anabolic hormones or intramuscular markers of muscle hypertrophy(Reference Roberts, Iosia and Kerksick29). However, compared with baseline, ARA supplementation increased anaerobic peak power by 8·5 % at day 50. On day 25, the ARA supplemented group had attenuated serum IL-6 levels whereas levels of serum PGE2, a potential ergogenic factor, tended towards an increase. The authors suggested that ARA supplementation would decrease inflammation (lower IL-6), thus making intense training more tolerable. No support was found for ARA to stimulate muscle hypertrophy, which would lead to a greater strength gain and/or muscle mass due to training.
In the second study, thirty males from the USA were randomised to receive either 600 mg ARA (from 1·5 g ARASYN oil) or maize oil daily during an 8-week training programme. The ARA group showed a significant increase in lean body mass (2·9 %), upper body strength (8·7 %) and peak power (12·7 %)(Reference De Souza, Lowery and Wilson20). ARA supplementation was suggested to increase post-exercise anabolic signalling rather than protein synthesis in skeletal muscle.
Markworth et al.(Reference Markworth, Mitchell and D’souza27) conducted a 4-week RCT of 1500 mg/d ARA in nineteen young males involved in a resistance training programme. There was a significant reduction in fat mass (–0·33 kg or –1·7 %) in the ARA group compared with the control group (+0·49 kg or +3·8 %). There was no effect of ARA on lean mass and effects of ARA on leg muscle volume were small. Other measurements in these individuals were reported by Mitchell et al.(Reference Mitchell, D’souza and Figueiredo26): prior ARA supplementation did not alter the acute anabolic response (i.e. muscle protein synthesis and anabolic signalling in muscle) to resistance exercise in these trained men, and the authors concluded that there is no link between ARA and short-term anabolism. However, some muscle changes were seen 48 h after completing the resistance exercise in men in the ARA group.
In summary, two of these studies suggest that ARA can improve peak power and may have an effect on lean body mass, while a third study suggests that ARA has an effect on late responses to resistance exercise. These effects and the underlying mechanisms require further exploration.
Effect of arachidonic acid on urinary metabolites
Ferretti et al.(Reference Ferretti, Nelson and Schmidt17) reported increased urinary levels of ARA metabolites (11-dehydro-thromboxane B2 and 2,3-dinor-6-oxo-PGF1) following 1·7 g ARA per d for 50 d. Kidney diuretic function, both normal and diuretic-stimulated, was not compromised in patients with liver cirrhosis (aged >60 years) who consumed 2 g/d ARA for 8 weeks: urinary sodium and ARA metabolites (i.e. PGE2, 6-keto-PGF1, 8-epi-PGF2 and 11-dehydro-thromboxane B2) were similar to those observed in the placebo group(Reference Pantaleo, Marra and Vizzutti28). More recently, with smaller dosages (240 or 720 mg ARA per d), for a shorter duration (4 weeks) and on a background of high DHA and EPA intake levels, ARA metabolite (11-dehydro-thromboxane B2, 2,3-dinor-6-keto-PGF1α and 9,15-dioxo-11α-hydroxy-13,14-dihydro-2,3,4,5-tetranor-prostan-1,20-dioic acid),) excretion in healthy Japanese elderly (>55 years of age) was also found not to be affected compared with the placebo (olive oil) group(Reference Kakutani, Ishikura and Tateishi24).
In summary, most studies report no effect of increased ARA intake on urinary excretion of ARA metabolites, and one study reports no effect of ARA on renal function in cirrhotic patients.
Summary and conclusions
The literature search identified twenty-two articles from fourteen RCT of increasing ARA intake in humans. These studies were published between 1997 and 2018. Most were conducted in adults. Studies in adults used between 40 and 2000 mg ARA per d, were of 1–12 weeks duration and usually used ARA as a supplement. Most studies used ARA intakes of 240–1000 mg/d. Only one study investigated more than one dose of ARA(Reference Kakutani, Ishikura and Tateishi24). Many studies were conducted in healthy young or older subjects, and several were restricted to men. One study was conducted in breast-feeding women and one in patients with liver cirrhosis. Few studies controlled the diet of the subjects under study and few studies assessed background diet. A number of studies reported the effect of ARA on FA composition of blood pools like lipids, erythrocytes and mononuclear cells, and one study reported on breast milk FA and another on skeletal muscle FA. Given the role of ARA-derived eicosanoids in regulating inflammation, immune function, platelet aggregation and blood clotting, it is not surprising that several studies investigated the effect of ARA on these outcomes. In contrast, there have been only few studies investigating effects on blood lipids, blood pressure and cognition. In most of these areas, there are too few studies to draw firm conclusions on the impact of ARA. Furthermore, the risk of bias was unclear for many of the studies, limiting the robustness of their findings.
It is clear from the existing studies that ARA supplements significantly increase the content of ARA in different blood fractions with doses as low as 80 mg/d being effective. The low dose of 40 mg ARA per d did not affect ARA level in plasma and erythrocytes. It is likely that enrichment of ARA in different compartments and pools is dose dependent, but may become saturated at higher intakes. EPA was decreased in several studies but DHA was usually not affected by ARA supplementation, even at the highest doses tested. Often incorporation of ARA was at the expense of LA. From the available evidence, it appears that increasing ARA intake does not affect blood lipid concentrations or blood pressure. However, there are few such studies and the effect of ARA on blood lipids in dyslipidaemic subjects or on blood pressure in hypertensive subjects has not been investigated. Furthermore, most studies have reported no effect of increased ARA intake on platelet aggregation or coagulation parameters and no studies have seen effects on bleeding time. However, one study that used the highest intake of ARA(Reference Pantaleo, Marra and Vizzutti28) reported enhanced platelet aggregation and this requires further investigation. The available evidence from rather detailed studies suggests little or no impact of increasing ARA intake by as much as 1·5 g/d on immune function or on markers of inflammation, apart from a small increase in ARA-derived eicosanoid production when immune cells are stimulated. However, the effect of the latter response is not known. Several studies report no effect of increased ARA intake on urinary excretion of ARA metabolites. One study concluded that supplementation with ARA may reduce age-related decline in cognitive function and learning ability. This could be an important effect and more research should be conducted in this area. Another interesting observation is the improvement in peak power and lean body mass seen in young adults undergoing exercise training. Again these effects need further investigation along with exploration of the likely mechanisms. It is important to note that none of the studies included here was of more than 12 weeks duration, and most were shorter than this; this may be insufficient time to affect several of the outcomes assessed such as cognitive function, body composition and physical performance.
Thus, overall there seem to be few marked benefits of increasing ARA intake from the typical intake of 100–200 mg/d to as much as 1000 mg/d or perhaps even more. However, the suggested impacts on cognitive and muscle function may both be important, particularly in the ageing population, and therefore the effect of higher intakes of ARA is deserving of further study. The studies reviewed herein suggest no adverse effects of increased ARA intake up to at least 1000–1500 mg/d on blood lipids, platelet aggregation and blood clotting, immune function, inflammation or urinary excretion of ARA metabolites. However, in many areas there are insufficient studies to make firm conclusions. Based on the RCT reviewed, there are not enough data to make any recommendations for specific health effects of ARA intake.
Acknowledgements
This work was conducted by an expert group of the European branch of the International Life Sciences Institute (ILSI Europe). The expert group received funding from the ILSI Europe’s Nutrient Intake Optimisation and Early Nutrition & Long-Term Health Task Forces. Industry members of these task forces are listed on the ILSI Europe website at http://ilsi.eu/task-forces/. Experts were not paid for the time spent on this work; however, the non-industry members within the expert group received a small compensatory sum (honorarium) and travel support from the Functional Foods Task Force to attend meetings to discuss the review. The expert group carried out the work, that is, collecting/analysing data/information and writing the scientific paper separate to other activities of the task force. The research reported is the result of a scientific evaluation in line with ILSI Europe’s framework to provide a precompetitive setting for public–private partnership (PPP). For further information about ILSI Europe, please email at [email protected] or call +32 2 771 00 14. The opinions expressed herein and the conclusions of this publication are those of the authors and do not represent the views of ILSI Europe nor those of its member companies.
ILSI Europe facilitated scientific meetings and coordinated the overall project management and administrative tasks relating to the completion of this work.
All authors conceived the research, selected papers for inclusion, carried out data extraction and assessment of papers and contributed to drafting of the manuscript. S. L. and A. S. conducted the literature search. P. C. C. had responsibility for final preparation of the manuscript. All authors read and approved the final version of the manuscript.
A. E. works for Unilever; M. F. works for Nestlé; P.-O. L. works for BASF; B. v. d. H. works for Danone; S. F. consults for DSM; P. C. C. consults for DSM, Danone/Nutricia, Cargill, BASF, FrieslandCampina and Smartfish; ILSI Europe is funded by its industry members.