Antioxidant micronutrients, such as vitamins and carotenoids, exist in abundance in fruit and vegetables and have been known to contribute to the body's defence against reactive oxygen species(Reference Gutteridge1, Reference Rock, Jacob and Bowen2). Numerous epidemiological studies have demonstrated that a high dietary consumption of fruit and vegetables rich in carotenoids or with high serum carotenoid concentrations results in lower risks of certain cancers, diabetes and CVD(3–Reference Ford, Will and Bowman8). These epidemiological studies have suggested that antioxidant carotenoids may have a protective effect against these diseases.
On the other hand, active smokers are exposed to reactive free radicals that are present in cigarette smoke(Reference Church and Pryor9, Reference Pryor and Stone10). Therefore, smoking is a potent oxidative stress in humans(Reference Griendling and FitzGerald11–Reference Chávez, Cano and Souki13). Furthermore, alcohol drinking also induces reactive oxygen species during its metabolism in the liver(Reference Walsh and Alexander14–Reference Koch, Pani and Borrello16). Oxidants and free radicals induced by cigarette smoking and/or alcohol drinking can cause damage to lipids, proteins, DNA, carbohydrates and other biomolecules(Reference Lieber17, Reference Eiserich, van der Vliet and Handelman18). In such circumstances, antioxidant micronutrients, such as carotenoids, may play important roles in defending against oxidative stress by efficiently quenching the production of singlet oxygen and free radicals. Numerous epidemiological studies have shown that serum carotenoid concentrations were low among cigarette smokers and alcohol drinkers(Reference Russell-Briefel, Bates and Kuller19–Reference Gabriel, Liu and Crott31). However, there is limited information about the synergistic interaction of cigarette smoking and alcohol drinking with serum carotenoid concentrations. The major serum carotenoids are lycopene, α-carotene, β-carotene, lutein, β-cryptoxanthin and zeaxanthin, and they account for more than 90 % of the circulating carotenoids in humans(Reference Bieri, Brown and Smith32). However, the differences in the change among these six major serum carotenoid concentrations against oxidative stress induced by cigarette smoking and alcohol drinking have not been thoroughly studied while controlling for dietary carotenoid concentrations.
The present study aimed to investigate the interaction of cigarette smoking and alcohol drinking with the serum concentrations of the following main six carotenoid concentrations, i.e. lutein, lycopene, α-carotene, β-carotene, β-cryptoxanthin and zeaxanthin. The synergistic interaction of cigarette smoking and alcohol drinking with serum carotenoid concentrations was evaluated cross-sectionally.
Subjects and methods
Subjects
Data used in the present study were derived from health examinations of residents of the town of Mikkabi, Shizuoka Prefecture, Japan, aged from 30 to 70 years, in 2003 and 2005. Mikkabi is located in western Shizuoka, and about 40 % of its residents work in agriculture. In 2003, a total of 1979 males and females were subjects for the health examination. As a result, 1448 participants (73·2 % of the total) received a health examination. Informed consent was obtained from the 886 subjects (302 male and 584 female) recruited for the present study. The response rate was 61·2 %. In 2005, a total of 1891 males and females were subjects for the health examinations. As a result, 1369 participants (72·4 % of total subjects) underwent such an examination. Participants who had received the health examination in 2005 were further recruited for the present study, and informed consent was newly obtained from 187 subjects (fifty-five male and 132 female). As a result, a total of 1073 subjects were included in this survey.
In the present study, the following subjects were excluded from the data analysis: (1) those for whom the self-administered questionnaire data were incomplete; and (2) those for whom blood samples for serum carotenoid analysis were not collected. As a result, a total of 354 male and 715 female subjects were included for further data analysis.
Measurements
Blood samples were obtained in the morning after overnight fasting. Serum was separated from blood cells by centrifugation and stored at − 80°C until analysis of the serum carotenoid concentrations. The concentrations of six serum carotenoids (lutein, lycopene, α-carotene, β-carotene, β-cryptoxanthin and zeaxanthin) were analysed by reverse-phase HPLC using β-apo-8′-carotenal as an internal standard at the laboratory of Public Health and Environmental Chemistry, Kyoto Biseibutsu Kenkyusho (Kyoto, Japan), as described previously(Reference Sugiura, Nakamura and Ikoma33). The serum total cholesterol was measured using an autoanalyser using a commercial kit (Determiner TC-II C for serum total cholesterol; Kyowa-Medics, Inc., Tokyo, Japan) at the laboratory of the Seirei Preventive Health Care Centre (Shizuoka, Japan). Height and body weight were measured by trained public health nurses. BMI was calculated as the body weight (kg) divided by the height (m2).
Lifestyle assessment and dietary data analysis
A self-administered questionnaire was used to collect lifestyle information, including tobacco use (current smoker, ex-smoker, or non-smoker), exercise (weekly participation), regular alcohol intake (one or more times per week) and dietary habits. The assessment of diet was a modification of the validated self-administered 121-item simple FFQ developed especially for the Japanese by Wakai and colleagues(Reference Wakai, Egami and Kato34, Reference Egami, Wakai and Kato35). Information about alcohol consumption from Japanese sake, beer, shochu, wine and whisky, and the daily intake of eighteen nutrients from foods were estimated from monthly food intake frequencies with either standard portion size (for most types of food) or subject-specified usual portion size (for rice, bread, and alcoholic and non-alcoholic beverages) using the FFQ analysis software package for windows (Food Frequency Questionnaire System; System Supply Co., Ltd, Kanagawa, Japan). This FFQ analysis software computes an individual's food and nutrient intake from FFQ data on the basis of the Standard Tables of Food Composition in Japan(36). The dietary carotenoid intakes of each individual were computed to obtain the amount of six carotenoids (lycopene, α-carotene, β-carotene, lutein, β-cryptoxanthin and zeaxanthin) using a published database of the carotenoid composition of fruit and vegetables(Reference Yano, Kato and Ikoma37, Reference Aizawa and Inakuma38). In our survey, we calculated an individual's carotenoid intake from important sources of carotenoids. In this data analysis, the dietary carotenoid intakes were calculated from the FFQ data of each individual's food items, not dishes. The dietary intakes of six carotenoids and total energy intake of all subjects were used in the present report.
Statistical analysis
The dietary intakes and serum concentrations of carotenoids were skewed toward higher concentrations. These values were log (natural)-transformed to improve the normality of their distribution. The t test was used to compare the means of continuous variables in two groups. The χ2 test was used to compare the rates of categorical variables in two groups. All variables were presented as an original scale. The data are expressed as the mean values and standard deviations, geometric mean values with 95 % CI, range, or percentages. In order to examine the relationship of independent variables with each serum carotenoid concentration, multiple regression analysis was performed with sex, age, BMI, smoking status, ethanol intake, serum total cholesterol, total energy intake excluding ethanol, and dietary intakes of respective carotenoids included always in the models as independent variables. The subjects were divided into three groups stratified by alcohol intake levels defined as non-drinker ( < 1 g ethanol per d), light drinker ( ≥ 1 to < 25 g ethanol per d) and moderate and heavy drinkers ( ≥ 25 g ethanol per d) because one glass of Japanese sake (180 ml) or one bottle of beer (633 ml), which are major alcohol beverages and widely consumed in Japan, commonly contains about 25 g alcohol. In our study population, the number of ex-smokers was ninety-seven (9·1 % among the study population) from the self-administered questionnaire. However, in our survey, we did not collect data on time since last smoking. Therefore, ex-smokers were included in the non-smokers group. All subjects were categorised into six groups according to daily alcohol intake (non-drinkers, < 1 g/d; light drinkers, ≥ 1 to < 25 g/d; moderate-to-heavy drinkers, ≥ 25 g/d) and smoking status (current smokers and non-smokers, including ex-smokers). The multivariate adjusted mean of the dietary intakes and serum concentrations of six carotenoids were calculated after adjusting for confounding factors. Differences in the multivariate adjusted mean of the dietary intakes and serum concentrations of six carotenoids among each group were tested by Bonferroni multiple comparison.
In our study population, the subjects who take carotenoid supplements were only 0·2 % among the study population. Therefore, we did not take account of carotenoid intake from supplements in the data analysis. The detection limit for the serum lycopene concentration for the method used in the study was 0·04 μg/ml (0·075 μmol/l), and values below the limit of detection of the assay were marked as 0·03 μg/ml (0·056 μmol/l) in the analysis. Each of the detection limits of other carotenoids was 0·02 μg/ml. In our survey, except for lycopene, there was no subject whose serum concentration of carotenoid was under the limit of detection. All statistical analyses were performed using the statistical software package SPSS for Windows (version 12.0J; SPSS Inc., Chicago, IL, USA) on a personal computer.
Ethical approval
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the ethics committee of the National Institute of Fruit Tree Science and the Hamamatsu University School of Medicine. Written informed consent was obtained from all subjects.
Results
Table 1 shows the characteristics of the study subjects stratified by sex. The dietary carotenoid intakes, excluding β-cryptoxanthin, were significantly higher in female than in male subjects. The serum carotenoid concentrations, excluding zeaxanthin, were significantly higher in female than in male subjects. The rates of current smokers and regular alcohol drinkers were higher in male than in female subjects.
Table 1 Characteristics of the study subjects
(Geometric means and 95 % confidence intervals, mean values and standard deviations or percentages)
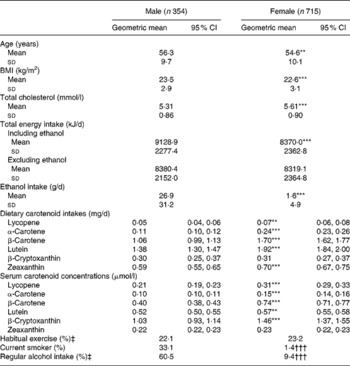
Mean value was significantly different from that for the male subjects: ** P < 0·01, *** P < 0·001 (Student's t test).
††† Percentage was significantly different from that for the male subjects (P < 0·001; χ2 test).
‡ One or more times/week.
Next, in order to examine the relationship of independent variables with each serum carotenoid concentration, multiple regression analysis was performed; sex, age, BMI, smoking status, ethanol intake, serum total cholesterol, total energy intake excluding ethanol, and dietary intakes of carotenoids were always included in the models as independent variables (Table 2). All six serum carotenoid concentrations were significantly associated with the dietary intakes of carotenoids. The strongest correlation between dietary intake and serum concentration was observed in β-cryptoxanthin. The serum total cholesterol was a significant positive predictor of the serum carotenoid concentration. The BMI was a significant negative predictor of serum carotenoid concentrations excluding β-cryptoxanthin. Smoking status was a significant negative predictor of the serum carotenoid concentration except for zeaxanthin. Ethanol intake was a significant negative predictor of the concentrations of serum lycopene, α-carotene, β-carotene and β-cryptoxanthin. Neither smoking status nor ethanol intake was associated with the serum zeaxanthin concentration.
Table 2 Standard regression coefficients of each serum carotenoid concentrations with contributory factors*
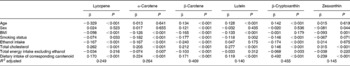
* Standard regression coefficients of each serum carotenoid concentrations with independent variables were calculated by multiple linear regression analysis included always in the models as independent variables.
Table 3 shows the results of the dietary carotenoid intakes in six groups stratified by daily alcohol intake and smoking status. The dietary intakes of α- and β-carotene in current smokers and/or in regular alcohol drinkers (>1 g/d) were significantly lower than those in non-smokers among non-drinkers. In addition, the dietary intake of lutein was significantly lower in moderate-to-heavy drinkers (more than 25 g/d) and in light drinkers (1–25 g/d) among current smokers than it was in non-smokers among non-drinkers. The dietary intake of β-cryptoxanthin was significantly lower in light drinkers (1–25 g/d) among current smokers than it was in non-smokers among non-drinkers. However, after adjusting for age and sex, these significantly lower dietary intakes of carotenoids were non-significant.
Table 3 Unadjusted and adjusted dietary carotenoid intakes stratified by smoking status and daily alcohol intake
(Geometric means and 95 % confidence intervals, mean values and standard deviations or percentages)
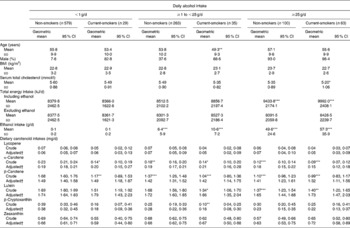
Mean value was significantly different from that of non-smoking non-drinkers: * P < 0·05, ** P < 0·01, *** P < 0·001 (Bonferroni multiple comparison test).
† Age and sex were adjusted.
The unadjusted and adjusted means of the serum carotenoid concentration among the six groups are shown in Table 4. After adjusting for age and sex, the serum lutein and zeaxanthin concentrations were not different among the six groups stratified by daily alcohol intake and smoking status. In contrast, the serum lycopene concentration in moderate-to-heavy drinkers was significantly lower than that in non-drinkers. Although this lower serum lycopene concentration in moderate-to-heavy drinkers was observed in both non-smokers and current smokers, no significant difference was observed between non-smokers and current smokers. On the other hand, the concentrations of serum α-carotene, β-carotene and β-cryptoxanthin were significantly lower than those with increased alcohol intake. Furthermore, the serum α-carotene, β-carotene and β-cryptoxanthin of current smokers among regular alcohol drinkers (more than 1 g/d) were significantly lower than those of non-smokers who have the same alcohol intake. The lower serum carotenoid concentrations among regular alcohol drinkers were more evident in current smokers than in non-smokers. After further adjusting for BMI, total cholesterol, total energy intake excluding alcohol, and dietary intake of respective carotenoids, no obvious change in these associations of cigarette smoking and alcohol drinking with the serum carotenoid concentration was observed.
Table 4 Unadjusted and adjusted serum carotenoid concentrations stratified by smoking status and daily alcohol intake
(Geometric means and 95 % confidence intervals)
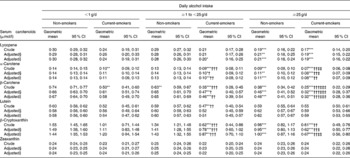
Mean value was significantly different from that of non-smoking non-drinkers: * P < 0·05, ** P < 0·01, *** P < 0·001 (Bonferroni multiple comparison test).
Mean value was significantly different from that of non-smokers who have the same alcohol intake: † P < 0·05, †† P < 0·01, ††† P < 0·001 (Bonferroni multiple comparison test).
Mean value was significantly different from that of non-drinking current smokers: ‡ P < 0·05, ‡‡ P < 0·01, ‡‡‡ P < 0·001 (Bonferroni multiple comparison test).
§ Age and sex were adjusted.
∥ Age, sex, BMI, total cholesterol, total energy intake excluding alcohol, and dietary intake of corresponding carotenoid were adjusted.
Furthermore, after multivariate adjusting, no obvious change of the present results about the differences in the changes among the serum concentrations of six carotenoids stratified by cigarette smoking and alcohol drinking habits were observed, even though female subjects were excluded (data not shown).
Discussion
The original FFQ we used in the present survey is not able to estimate the amount of carotenoid intake from foods because all six carotenoid contents in each food are not assessed in standard tables of food composition in Japan. However, recently, a database of the carotenoid composition of fruit and vegetables was published(Reference Yano, Kato and Ikoma37, Reference Aizawa and Inakuma38). In the present study, we adapted FFQ analysis software package to calculate an individual's carotenoid intake from important sources of carotenoids, and significant relationships between dietary intake of each carotenoid and its serum levels were observed. This is the first experiment to estimate six dietary carotenoids in Japan.
The present investigation is the first-reported cross-sectional study to examine the synergistic interaction of cigarette smoking and alcohol drinking with the six major serum carotenoids while controlling for dietary carotenoid intakes. The results indicated that daily alcohol intake may reduce the serum concentrations of lycopene, α-carotene, β-carotene and β-cryptoxanthin in a dose-dependent manner. Furthermore, these alcohol-related lower serum carotenoid concentrations, except for lycopene, were more evident in current smokers than in non-smokers. The serum lycopene concentration seems to be influenced by alcohol drinking and not by smoking. In addition, neither smoking nor alcohol drinking affected the serum lutein and zeaxanthin concentrations.
Although many epidemiological studies have indicated that the serum carotenoid concentrations were low among current smokers and/or regular alcohol drinkers(Reference Russell-Briefel, Bates and Kuller19–Reference Gabriel, Liu and Crott31), most of these previous studies evaluated the effect of these lifestyle factors on the serum carotenoid concentrations separately. It is widely known that the dietary intake of carotenoids is lower in smokers than in non-smokers, in alcohol drinkers than in non-drinkers, and in males than in females(Reference Stryker, Kaplan and Stein21). In addition, the consumption of alcohol among smokers is higher than that among non-smokers. Therefore, the possibility that the correlation between cigarette smoking and alcohol drinking with the serum carotenoid concentrations observed in these previous reports would be an artifact of the strong correlation of cigarette smoking and alcohol drinking could not be ruled out. In our data analysis, the subjects were stratified into six groups according to their smoking status and the amount of daily alcohol intake. Thus, we believe that the influence of the association among cigarette smoking and alcohol drinking can be completely eliminated in the analysis of these six subgroups.
Previously, seven cross-sectional studies have reported the possibility that cigarette smoking and alcohol drinking might synergistically enhance the depletion of serum carotenoid concentrations(Reference Russell-Briefel, Bates and Kuller19–Reference Tanabe, Toyoshima and Hayashi23, Reference Tsubono, Tsugane and Gey25, Reference Fukao, Tsubono and Kawamura26). However, these studies did not take into account the dietary intakes of carotenoids; therefore, it is not clear whether the low serum carotenoid concentration was due to the low dietary intake of carotenoids or whether cigarette smoking and alcohol drinking would cause deterioration in the serum carotenoid concentrations. To our knowledge, there have been no reports about the synergistic interaction of cigarette smoking and alcohol drinking with the serum carotenoid concentrations after taking into account the dietary carotenoid intakes. Furthermore, there is limited information about the differences in the changes among the serum concentrations of the six major carotenoids against oxidative stress induced by cigarette smoking and alcohol drinking. In our data analysis, the dietary intakes of α-carotene, β-carotene and lutein in current smokers and/or regular alcohol drinkers were significantly lower than those in non-smokers among non-drinkers. However, these significantly lower dietary intakes became insignificant after adjusting for age and sex. Furthermore, a significant synergistic interaction of cigarette smoking and alcohol drinking with the serum carotenoid concentrations was observed after further adjusting for the dietary intakes of respective carotenoids. Therefore, it seems that the significant differences of the multivariate adjusted means of the serum carotenoid concentrations among the six groups stratified by lifestyle factors might be caused by the depletion of serum carotenoids and not by differences in dietary carotenoid intakes.
Recently, some animal experiments have been reported concerning the synergistic effect of cigarette and alcohol on the antioxidant defence system in several tissues(Reference Sandhir, Subramanian and Koul39–Reference Hartwig, Werner and Ryschich41). These animal experiments show the possibility that combination of alcohol plus cigarette smoke induces the excessive generation of reactive oxygen species and free radicals to a greater extent than that from alcohol or cigarette smoke alone. Thus, the combination of cigarette smoking and alcohol drinking might induce the excessive generation of free radicals and cause the marked depletion of serum carotenoid concentrations of regular alcohol drinkers among current smokers than those of non-smokers who have the same alcohol intake.
It is also known that a transforming reaction from pro-vitamin A to retinol is induced by smoking(Reference Goodman42). On the other hand, alcohol is known to promote increased oxidation of vitamin A compounds and reduced liver stores(Reference Leo and Lieber43). It can be postulated that alcohol intake may also accelerate the conversion of pro-vitamin A to retinol(Reference During and Harrison44). From among the six major serum carotenoids we measured, α-carotene, β-carotene and β-cryptoxanthin are pro-vitamin A. These three carotenoids are converted to retinol in the body. Therefore, the serum concentrations of α-carotene, β-carotene and β-cryptoxanthin might be more easily influenced by cigarette smoking and alcohol drinking than lycopene.
In the present study, serum lutein and zeaxanthin concentrations were not influenced not only by alcohol drinking but also by cigarette smoking. We have no clear explanation for these inconsistencies with previous results(Reference Tsubono, Tsugane and Gey25, Reference Brady, Mares-Perlman and Bowen27–Reference Dietrich, Block and Norkus29, Reference Gabriel, Liu and Crott31). We concluded that lutein and zeaxanthin might be difficult to be exposed to oxidative stress or that the differences among the six serum carotenoids observed occurred by chance. One possible explanation is that the differences in the associations of the six serum carotenoids with cigarette smoking and alcohol drinking might be attributed to the polar characteristics of each carotenoid. It is conceivable that the tissue distribution and localisation in the cell membranes of carotenoids differ in each carotenoid. Especially, the chemical structure of a carotenoid may determine its localisation in a cell membrane. Hydrocarbon carotenoids, such as lycopene, α-carotene and β-carotene, are located within the hydrophobic membrane core with multiple orientations, whereas xanthophylls, such as lutein and zeaxanthin, have a more rigid membrane-spanning orientation(Reference Chaudière and Ferrari-Iliou45). Therefore, we believe that the antioxidant defence system by carotenoids against lipid peroxidation in a cell membrane depends on the polar characteristics of each carotenoid. Alternatively, as another one possible explanation for the differences in the associations of the six serum carotenoids with cigarette smoking and alcohol drinking, we consider the differences of carotenoid distribution in lipoproteins. Lipid-soluble carotenoids are carried by lipoproteins from the liver into the blood circulation. Hydrocarbon carotenoids, such as lycopene, α-carotene and β-carotene, are mainly found in LDL, whereas xanthophylls are equally found in HDL and LDL(Reference Romanchik, Morel and Harrison46). Recent studies indicate that oxidised LDL increases in smokers and/or heavy alcohol drinkers(Reference Alho, Sillanaukee and Kalela47, Reference Yamaguchi, Haginaka and Morimoto48). Therefore, it is conceivable that the plasma clearance rate of carotenoids might be influenced by oxidised LDL induced by cigarette smoking and alcohol drinking.
The present study had some limitations. First, we could not evaluate the association of blood concentrations of vitamins C and E with cigarette smoking and alcohol drinking. It would be necessary to measure the blood concentrations of vitamins C and E in order to examine the associations of these antioxidant vitamin concentrations with these lifestyle factors. Second, the data obtained here consisted of cross-sectional analyses. Therefore, only limited inferences can be made regarding temporality and causation. Third, in the present study, the sample size of current smokers was not particularly large. Therefore, it was impossible to examine the quantitative effects of cigarette smoking on the serum carotenoid concentration. Further studies on a large scale will be required. Fourth, seasonal influence was not considered in the present study, but it could be important to consider this influence in future studies. Last, our findings might be specific to middle-aged Japanese. Further studies in other races and/or regions will be required.
Acknowledgements
The present study was supported by a grant from the Ministry of Agriculture, Forestry, and Fisheries (MAFF) for a food research project titled ‘Integrated Research on Safety and Physiological Function of Food’ and a grant from the Council for the advancement of Fruit Tree Science. We are grateful to the participants in our survey and to the staff of the health examination programme for residents of the town of Mikkabi, Shizuoka, Japan. We are also grateful to the staff of the Seirei Preventive Health Care Centre (Shizuoka, Japan).
M. S. was responsible for study design, data collection, and data management and carried out the data analysis and wrote the manuscript. M. N. was responsible for study design, data collection, and data management and assisted in manuscript preparation. K. O., Y. I., H. M., F. A., H. S. and M. Y. were involved in the data collection and assisted in manuscript preparation. All the authors provided suggestions during the preparation of the manuscript and approved the final version submitted for publication.
None of the authors had any personal or financial conflict of interest.






