Dietary fibre is deficient in the diets of modern people. The lack of dietary fibre is viewed as one of the main reason for the current increasing rates of metabolic diseases. Normally, dietary fibre is digested and absorbed in the gastrointestinal tract by complete or partial fermentation to SCFA such as butyric acid( Reference Bergman 1 , Reference Macia, Tan and Vieira 2 ). Considering the fibre effect on the diet of mothers during gestation and lactation, previous studies indicate that a high-fat, Western-style maternal diet, with additional dietary fibre, has a lower risk for developing CHD, hypertension, diabetes, obesity and other metabolic diseases in the offspring( Reference Anderson, Baird and Davis 3 , Reference Lin, Zhuo and Fang 4 ). However, the underlying mechanism is still largely unclear.
In addition to being the energy source for ruminants and monogastric animals( Reference Yen, Nienaber and Hill 5 , Reference Sicilianojones and Murphy 6 ), most previous studies suggest that butyrate, a kind of SCFA, might mediate the effects of gut microbiota and nutrition on immunity, metabolism, as well as the pathogenesis of obesity, diabetes mellitus and inflammatory bowel disease( Reference Meijer, de Vos and Priebe 7 – Reference Henagan, Stefanska and Fang 10 ). Previous studies have suggested that butyrate supplementation in high-fat diets could improve mitochondrial function by promoting a skeletal muscle type 1 fibre phenotype and physiology( Reference Henagan, Stefanska and Fang 10 , Reference Gao, Yin and Zhang 11 ). A few studies have demonstrated that maternal butyrate supplementation can improve offspring growth and antioxidant capacity( Reference Lin, Fang and Che 12 , Reference Lu, Su and Ajuwon 13 ). However, whether maternal butyrate supplementation has an influence on energy metabolism and mitochondrial function in offspring skeletal muscle is still unknown.
Mitochondrial function has an important role in maintaining energy homoeostasis in skeletal muscle by balancing ATP generation and expenditure at the cellular level( Reference Gao, Yin and Zhang 11 , Reference Scheffler, Scheffler and Park 14 ). The functions of the skeletal muscle mostly depend on the highly efficient process of mitochondrial oxidative phosphorylation system (OXPHOS). Previous studies have demonstrated that the enhanced mitochondrial biogenesis could increase OXPHOS with ATP-generating capacity( Reference Wenz, Diaz and Spiegelman 15 , Reference Komen and Thorburn 16 ). Mitochondrial biosynthesis is initiated with increased transcription of both nuclear and mitochondrial DNA (mtDNA; thirteen coding genes). The PPARγ-coactivator (PGC-1α) family of proteins has been identified as the main family of transcriptional co-activators involved in the induction of mitochondrial biogenesis( Reference Baar 17 ). In particular, PGC-1α has been reported to promote mitochondrial biogenesis and fibre-type transformation in skeletal muscle cells( Reference Miura, Kai and Ono 18 , Reference Lin, Wu and Tarr 19 ). In addition, cAMP (cyclic adenosine monophosphate) response element-binding protein (CREB) induces PGC-1α to regulate mitochondrial biogenesis( Reference Than, Lou and Ji 20 ). It has been shown that butyrate can influence body metabolism by activation of the endogenous G-protein-coupled receptors (GPR) 41 and 43( Reference Inoue, Kimura and Wakabayashi 21 , Reference Samuel, Shaito and Motoike 22 ). A previous study demonstrated that SCFA-activated GPR43-stimulated mitochondrial biogenesis in brown adipocytes( Reference Hu, Kyrou and Tan 23 ). If maternal butyrate supplementation influences offspring skeletal muscle energy metabolism, the possible underlying mechanisms need further investigation.
Accordingly, in the present study, we investigated the effects of maternal butyrate supplementation during gestation and lactation on offspring energy metabolism and mitochondrial biogenesis in gastrocnemius muscle at weaning. The results provide important insights into the effects of maternal butyrate supplementation on offspring skeletal muscle metabolism.
Methods
Experimental design and sampling
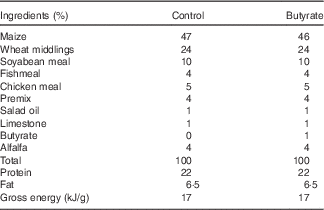
In total, twenty-eight, virgin, female Sprague–Dawley rats were purchased from the Laboratory Animal Center, University of Jiangsu, and housed in individual cages under temperature- and humidity-controlled conditions with a 12 h light–12 h dark cycle. After 7 d of acclimatisation, rats were mated, and conception was confirmed by the presence of spermatozoids in the vaginal wash. Once conception was confirmed, females were randomly assigned to either a control or a butyrate diet (1 % butyrate sodium), with water provided ad libitum. The control diet was prepared following the recommendations of the American Institute of Nutrition for rodent growth (AIN-93G) (Table 1).
Table 1 Compositions of the experimental diets
After delivering, the litter size and litter weight were recorded. The litters were adjusted to eight animals for each dam, maintaining the sex ratio as close as possible to 1:1. During lactation, dams continued to consume their assigned experimental diet. At the end of lactation (21 d), they were killed by exsanguination from the abdominal aorta under anaesthesia with pentobarbital sodium. Blood samples of both mothers and offspring were collected, and serum was obtained by centrifugation at 3500 g for 10 min. The gastrocnemius muscles of offspring were excised, weighed, snap-frozen in liquid N2 and immediately stored at −80°C until analysis.
All animal procedures were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University. The protocol of this study was reviewed and approved with the project number 31572482. All experimental protocols strictly followed the ‘Guidelines on Ethical Treatment of Experimental Animals’ (2006) no. 398 set out by the Ministry of Science and Technology, China, and the Regulation regarding the Management and Treatment of Experimental Animals’ (2008) no. 45 set out by the Jiangsu Provincial People’s Government.
Biochemical analysis
Butyrate levels in the serum of dams were determined by GC. The extraction and gas chromatographic analysis procedures were carried out as described by Zhao et al.( Reference Zhao, Liu and Nyman 24 ) with some modifications. In brief, chromatographic analysis was carried out using a Shimadzu GC-2010 Plus system (Shimadzu) equipped with a flame-ionisation detector (FID). A fused-silica capillary column with a free-fatty acid phase (DB-FFAP 125-3237; J&W Scientific, Agilent Technologies Inc.) of 30 m×0·53-mm I.D. (inner diameter) coated with a 0·50-μm-thickness film was used. He was supplied as the carrier gas at a flow rate of 14·4 ml/min. The initial oven temperature was 100°C; it was maintained at the same level for 0·5 min and then raised to 180°C by 8°C/min and held at the same level 1·0 min; further, the temperature was increased to 200°C by 20°C/min and finally held at 200°C for 5 min. Glass wool (Supelco) was inserted in the glass liner of the splitless injection port. The temperatures of the FID and the injection port were 240 and 200°C, respectively. The flow rates of H2, air and N as makeup gases were 30, 300 and 20 ml/min, respectively. The injected sample volume for GC analysis was 1 μl, and the running time for each analysis was 17·5 min.
RNA isolation and real-time PCR for mRNA quantification
Total RNA was isolated from gastrocnemius muscle tissue with TRIzol reagent (Invitrogen Life Technologies), and reverse transcribed with the PrimeScript 1st Strand cDNA Synthesis Kit (RR048A; Takara) according to the manufacturer’s instructions. Diluted complementary DNA (2 μl, 1:25) was used in each real-time PCR assay with Mx3000P (Stratagene). All primers (Table 2) were synthesised by Generay Biotech. β-Actin was chosen as a reference gene as it is not affected by maternal butyrate supplementation.
Table 2 Primer sequences for quantitative RT-PCR of mRNA

COX1, cytochrome c oxidase subunit 1; COX3,cytochrome c oxidase subunit 3, CYTB, cytochrome b; ND1, NADH dehydrogenase subunit 1; ND2, NADH dehydrogenase subunit 2; ND3, NADH dehydrogenase subunit 3; ND4, NADH dehydrogenase subunit 4; ND4L, NADH dehydrogenase subunit 4L; ND5, NADH dehydrogenase subunit 5; ATP6: ATP synthase F0 subunit 6; ATP8, ATP synthase F0 subunit 8; MYHC, myosin heavy chain.
Western blotting analysis
Total cellular proteins were extracted from 100 mg of frozen gastrocnemius muscle tissue samples as previously described( Reference Liu, Wang and Li 25 ). Nuclear protein extracts were used to detect the expression of PGC-1α. Protein concentration was measured with a Pierce BCA Protein Assay kit (no. 23225; Thermo Scientific). Western blot analysis of the target proteins was carried out according to the protocols provided by the manufacturer. The sources of primary antibodies used in the Western blot analysis are as follows: anti-GPR41 (no. sc-98332; Santa Cruz Biotechnology), anti-GPR43 (no. sc-32906; Santa Cruz Biotechnology), anti-mitochondrial transcription factor A (TFAM, no. ab176558; Abcam), anti-MYHC1 (no. 05716; Millipore), anti-cytochrome c oxidase subunit 1 (COX1; no. BS1636; Bioworld Technology), anti-COX4 (no. AP0705; Bioworld Technology), anti-phospho-ser133 CREB (p-CREB, no. BS6345; Bioworld Technology), anti-CREB (no. BS6230; Bioworld Technology), anti-PGC-1α (no. sc-13067; Santa Cruz Biotechnology) and anti-uncoupling protein 3 (UCP3, no. BS2849; Bioworld Technology). Lamin A/C (no. BS3774; Bioworld Technology) was used to verify the purity of nuclear proteins. β-Actin (no. AP0060; Bioworld Technology), GAPDH (glyceraldehyde 3-phosphate dehydrogenase; MB001H; Bioworld Technology) and α-tubulin (no. BS1699; Bioworld Technology) were used to verify total cellular proteins.
Determination of AMP, ADP, ATP and NAD concentrations in gastrocnemius muscle tissue
The concentrations of AMP (adenosine monophosphate), ADP, ATP and NAD (aldehyde dehydrogenase) in gastrocnemius muscle were determined according to a previous study( Reference Jia, Li and Cong 26 ). Concentrations were determined by HPLC with a reverse-phase column (C18, 5 μm, 250×4·6 mm; Dikma Technologies Inc.). Next, using a dual λ absorbance detector (Waters Corporation), the detection wavelength of the HPLC was found to be 260 nm. The mobile phase contained phosphate buffer (50-mmol/l potassium dihydrogen orthophosphate, 50-mmol/l dipotassium hydrogen orthophosphate, pH=5·4), 2·3-mm tetrabutylammonium hydrogen sulphate and 1 % acetonitrile. The adenine nucleotides were eluted after 30 min of isocratic elution at a flow rate of 1 ml/min. Energy charge (EC) was calculated using the following formula: EC=([ATP]+1/2[ADP])/([ATP]+[ADP]+[AMP]).
Determination of mitochondrial DNA copy number in gastrocnemius muscle tissue
mtDNA copy number was measured in skeletal muscle of weaning offspring. In brief, total genomic DNA was isolated from skeletal muscle, and the mtDNA copy number was determined using real-time PCR as previously described(
Reference Jia, Li and He
27
). Primers specific for the coding region of mtDNA (ATP synthase F0 subunit 8 (ATP8)) were used for the quantification of the mtDNA copy number, whereas primers specific for the 18S nuclear gene were used for standardisation. The relative mtDNA copy number was calculated using the
![]() $2^{{{\minus}\Delta \Delta C_{t} }} $
method.
$2^{{{\minus}\Delta \Delta C_{t} }} $
method.
Statistical analysis
Numerical data are expressed as mean values with their standard errors. All statistical analyses were performed using Statistical Program for Social Sciences (SPSS) software 18.0 for Windows (SPSS Inc.). The
![]() $2^{{{\minus}\Delta \Delta C_{t} }} $
method was used to analyse the real-time PCR data. The differences were analysed using independent t test with SPSS 18.0 for Windows. Differences were considered significant at P<0·05.
$2^{{{\minus}\Delta \Delta C_{t} }} $
method was used to analyse the real-time PCR data. The differences were analysed using independent t test with SPSS 18.0 for Windows. Differences were considered significant at P<0·05.
Results
Performance of mother and offspring and serum butyrate content of dams
Food intake of dams per week during gestation and lactation in the butyrate group did not change significantly compared with the control group (223·69 (se 5·74) v. 224·89 (se 6·16) g, P>0·05). Body weight of dams showed no significant difference between the butyrate group and the control group( Reference Zhou, Gao and Chen 28 ). Maternal butyrate supplementation demonstrated a trend towards increasing litter weight, litter size( Reference Zhou, Gao and Chen 28 ) and the ratio of muscle weight:body weight (7·27 (se 0·16) v. 6·79 (se 0·19) g, P<0·1) as well as enhanced muscle weight of the offspring (0·54 (se 0·01) v. 0·48 (se 0·02) g, P<0·05) compared with the control group. The average weight of newborns (7·04 (se 0·14) v. 7·18 (se 0·12) g, P>0·05) and the body weight of weaning rats( Reference Zhou, Gao and Chen 28 ) showed no significant differences between the two groups.
Butyrate levels in the serum of supplemented dams were significantly higher compared with the control group (Fig. 1(a)). Our previous study has been reported that serum concentrations of total cholesterol and TGA in dams and weaned offspring did not change with butyrate supplementation( Reference Zhou, Gao and Chen 28 ).

Fig. 1 Butyrate concentration in the serum of dams after weaning (a: □, control; ■, butyrate); maternal butyrate supplementation throughout gestation and lactation increased G-protein-coupled receptors (GPR) 43 (b) and GPR41 (c) protein expressions in gastrocnemius muscle of weaning rats (n 7 per group). β-Actin was used as the internal standard for Western blotting. Values are means with their standard errors. Con, control. *P<0·05, **P<0·01, compared with control.
Protein levels of G-protein-coupled receptor 43 and 41 in gastrocnemius muscle
The expressions of GPR43 and 41 proteins were determined in gastrocnemius muscle. Both GPR43 and GPR41 protein levels were significantly higher (P<0·05) in offspring gastrocnemius muscle in the treated group compared with the control group (Fig. 1(b) and (c)).
Contents of ATP, ADP, AMP and NAD in gastrocnemius muscle
Gastrocnemius muscle ATP concentration was significantly higher (P<0·05) in the butyrate group compared with the control group. However, the concentrations of ADP, AMP and NAD exhibited no obvious differences between the control and butyrate groups (Fig. 2(a)). EC of skeletal muscle in the butyrate group was higher (P<0·01) than the control group (Fig. 2(b)).

Fig. 2 Effect of maternal butyrate supplementation throughout gestation and lactation on gastrocnemius muscle ATP, ADP, AMP, NAD contents (a) and muscle energy charge (EC) (b) in weaning rats (n 5 per group); mitochondrial DNA-encoded mRNA expression (c) (n 8 per group) and cytochrome c oxidase (COX) protein expression (d, e) (n 7 per group) in gastrocnemius muscle; mitochondrial DNA copy number in gastrocnemius muscle in weaning rats (f); EC was calculated using the following formula: EC=([ATP]+1/2[ADP])/([ATP]+[ADP]+[AMP]). β-Actin and α-tubulin were used as internal standards. Values are means with their standard errors. Con, control; COX1, cytochrome c oxidase subunit 1; COX3,cytochrome c oxidase subunit 3, CYTB, cytochrome b; ND1, NADH dehydrogenase subunit 1; ND2, NADH dehydrogenase subunit 2; ND3, NADH dehydrogenase subunit 3; ND4, NADH dehydrogenase subunit 4; ND4L, NADH dehydrogenase subunit 4L; ND5, NADH dehydrogenase subunit 5; ATP6: ATP synthase F0 subunit 6; ATP8, ATP synthase F0 subunit 8. a, c, e, f: □, Control; ■, butyrate. *P<0·05, **P<0·01, compared with control.
Mitochondrial DNA-encoded mRNA expressions, cytochrome c oxidase protein expression and mitochondrial DNA copy number in gastrocnemius muscle
The mRNA abundance of twelve genes involved in gastrocnemius muscle OXPHOS was assessed. Among these genes, COX3, NADH dehydrogenase subunit 5 (ND5), ATP synthase F0 subunit 6 (ATP6) and ATP8 were significantly up-regulated in gastrocnemius muscle of the butyrate group compared with the control group (P<0·05) (Fig. 2(c)). Consistent with the abundance of the aforementioned mRNA, the protein expressions of mitochondrial COX1 and COX4 were significantly increased in weaning rats born to butyrate-supplemented mothers compared with the control group (P<0·05) (Fig. 2(d) and (e)). mtDNA copy number was also increased significantly in offspring skeletal muscle with maternal butyrate supplementation (P<0·01) (Fig. 2(f)).
Energy metabolism-related protein content in gastrocnemius muscle
Maternal butyrate supplementation significantly increased the protein expression of TFAM in gastrocnemius muscle of offspring rats (Fig. 3(a)). Maternal butyrate supplementation significantly increased the level of total CREB and p-CREB (P<0·05), although the ratio did not change in gastrocnemius muscle of offspring rats (Fig. 3(b)). Compared with the control group, the levels of total PGC-1α, nuclear PGC-1α and UCP3 (P<0·05) proteins in weaning rats of the treated group were significantly enhanced compared with control group (Fig. 3(c) and (d)).

Fig. 3 Mitochondrial transcription factor A (TFAM) (a), phosphorylated cAMP response element-binding protein (CREB) and total CREB (b), total and nuclear PPAR-coactivator-1α (PGC-1α) (c) and uncoupling protein 3 (UCP3) expressions (d) in gastrocnemius muscle of weaning rats (n 7 per group). β-Actin, α-tubulin, GAPDH and lamin A/C were used as internal standards for Western blotting. Values are means with their standard errors. *P<0·05, compared with control. □, Control; ■, butyrate: Con, control; p-CREB, anti-phospho-ser133 CREB.
mRNA and protein expressions of myosin heavy chain in gastrocnemius muscle
The type 1 myosin heavy chain (MYHC1) mRNA and protein expressions in the butyrate group were significantly higher than that of the control group (P<0·05). The mRNA expressions of type 2 variants, including MYHC2A, MYHC2B and MYHC2X, showed not significant changes between the two groups (Fig. 4). Similarly, mRNA expressions of MYOD (myogenic differentiation) and myogenin, myogenic regulatory factors and myoglobin did not change compared with the control group. The mRNA expression of mTOR (mechanistic target of rapamycin), which could regulate protein synthesis in muscle, was also not altered with maternal butyrate supplementation (Fig. 4).

Fig. 4 Effect of maternal butyrate supplementation throughout gestation and lactation on mRNA expressions of the type of myosin heavy chain (MYHC) and MYOD, mTOR, myogenin and myoglobin in offspring gastrocnemius muscle ((a), n 8 per group); MYHC protein expression in offspring gastrocnemius muscle (b). α-Tubulin was used as the internal standard for Western blotting. Values are means with their standard errors. *P<0·05, compared with control. □, Control; ■, butyrate.
Discussion
Numerous studies have shown that maternal nutrients influence the growth performance of their offspring( Reference Pantaleao, Teodoro and Torres-Leal 29 , Reference Max, Brandsch and Schumann 30 ). Butyrate supplementation to gestating sows has been reported to enhance post-weaning growth performance of offspring( Reference Lu, Su and Ajuwon 13 ), although supplementation with sodium butyrate after farrowing did not have a significant effect on weaning body weight( Reference Wang, Yang and Xu 31 ). It has also been shown that butyrate supplementation of high-fat diet-fed mothers increased the number of fetuses compared with the control group( Reference Lin, Fang and Che 12 ). Butyrate can act as a growth promoter in milk replacement formula for young calves( Reference Guilloteau, Zabielski and David 32 ). Our previous study showed that maternal sodium butyrate supplementation elevated lipolysis in adipose tissue in dams and led to lipid metabolism changes in offspring liver of weaning-age rats( Reference Zhou, Gao and Chen 28 ). The present results show that maternal butyrate supplementation increased ATP content and EC in gastrocnemius muscle. This investigation provides evidence that maternal butyrate supplementation throughout gestation and lactation had effects on energy metabolism in offspring skeletal muscle.
Mitochondria use substrates and oxygen to produce ATP through the highly efficient process of OXPHOS in skeletal muscle. The biogenesis of mitochondria can prevent metabolic imbalances and promote healthy skeletal muscle( Reference Patti, Butte and Crunkhorn 33 , Reference Joseph, Joanisse and Baillot 34 ). In the present study, mitochondria-encoded genes such as COX3, ND5, ATP6 and ATP8 showed significant increases in offspring skeletal muscle of the butyrate group. In addition, COX1 and COX4 protein expressions also increased. Meanwhile, increased amount of mtDNA copy number was detected in offspring skeletal muscle of the butyrate group. The present results show that mitochondrial biogenesis was enhanced in offspring skeletal muscle with butyrate supplementation. The ATP demand required for muscle development is accommodated by elevations in mitochondrial biogenesis. TFAM is an important transcriptional activator of mitochondrial biogenesis, which allows for the nuclear control of mtDNA gene transcription and replication. As a previous study reported, accumulation of TFAM protein could increase the expressions of mtDNA and COX1( Reference Wen, Gao and Zhang 35 ). Our present results indicate that TFAM protein expression increased, which may enhance mitochondrial biogenesis by enhancing the expressions of mtDNA and COX1 in offspring skeletal muscle of the butyrate group.
We measured serum butyrate contents in both mother and offspring. The results showed that maternal serum butyrate content significantly increased in the treated group. The serum butyrate content in the offspring was too low to perform statistical analysis (data not shown). Several studies have established that through activation of their receptors, such as GPR43 and GPR41, SCFA regulate important signalling pathways involved in energy metabolism and homoeostasis( Reference Macia, Tan and Vieira 2 , Reference Jia, Li and Cong 26 ). In the present study, significant increases in GPR41 and GPR43 protein expressions were observed in offspring skeletal muscle of the butyrate group. The effect of butyrate on cAMP production and signalling pathway can be regulated by Gai-coupled GPR41 and GPR43( Reference Le Poul, Loison and Struyf 36 ). In ruminants, the SCFA could be acted as an external milk energy source. After birth, butyrate can be directly ingested by the neonates( Reference Castro, Gomez and White 37 ). For butyrate crossing the placenta, previous studies have shown that multiple subtypes of maternal monocarboxylate transporters (MCT) were expressed( Reference Wanaga and Kishimoto 38 ) in the placenta, and maternal high-fat diets with butyrate could increase MCT mRNA expression in the placenta( Reference Lin, Fang and Che 12 ). Thus, our present study speculated that maternal butyrate supplementation during gestation and lactation could cross the placental barrier of dams and pass through milk of dams to offspring to influence offspring skeletal muscle metabolism. Further investigation will be needed to determine the effects of maternal butyrate on MCT expression in the placenta and the content in milk.
A previous study demonstrated that mitochondrial biogenesis could be promoted through activation of p-CREB expression, accompanied by increased levels of ATP( Reference Zuo, Li and Sun 39 ). In the present study, an increase in both total and p-CREB content was observed in the treated group, although the ratio did not change. Further, we detected in offspring skeletal muscle, a significant increase in protein expression of PGC-1α, a major factor for the regulation of mitochondrial biogenesis( Reference Gleyzer, Vercauteren and Scarpulla 40 ). CREB has been identified as an activator of PGC-1α transcription by binding to the PGC-1α promoter( Reference Chowanadisai, Bauerly and Tchaparian 41 ). Previous studies have demonstrated that p-CREB mediates mitochondrial biogenesis probably through PGC-1α ( Reference Mehta, Mendelev and Kumari 42 , Reference Kim, Song and Lee 43 ). The present results indicated that mitochondria biogenesis in offspring skeletal muscle might be mediated by PGC-1α with enhanced GPR. Thus, we speculated that maternal butyrate supplementation enhanced mitochondrial biogenesis in offspring skeletal muscle by increasing the expressions of TFAM and COX and mtDNA copy number through the GPR and PGC-1α pathways (Fig. 5).

Fig. 5 Proposed mechanisms of maternal butyrate supplementation effect on mitochondrial biogenesis in offspring skeletal muscle. COX, cytochrome c oxidase; GPR, G-protein-coupled receptors; MCT, monocarboxylate transporters; mtDNA, mitochondrial DNA; PGC-1α, PPARγ-coactivator-1α; TFAM, mitochondrial transcription factor A.
As a mitochondrial transport protein, UCP3 was associated with the uncoupling of substrate oxidation( Reference Boss, Hagen and Lowell 44 – Reference Nabben and Hoeks 46 ). Mitochondrial UCP3 was shown to play an important role in regulating the energy balance of skeletal muscle under fasting and high-fat feeding conditions( Reference Nabben and Hoeks 46 ). In addition, a previous study suggested that UCP3 could be regulated by p-CREB and participate in the regulation of fuel oxidation pathways( Reference Dorsa, dos Santos and da Silva 47 ). Our study also demonstrated that maternal butyrate supplementation increased UCP3 protein expression in offspring skeletal muscle.
Differences in skeletal muscle fibre type are indicative of the oxidative capacity and mitochondrial content of skeletal muscle. Type 1 myofibres are highly oxidative and exhibit an increased number of mitochondria compared with type 2 fibres. It has been shown that butyrate supplementation in high-fat diets can promote type 1 fibre ratio in muscles associated with improved mitochondrial function( Reference Henagan, Stefanska and Fang 10 , Reference Gao, Yin and Zhang 11 ). In the present study, increased MYHC1 mRNA and protein expressions were observed in offspring skeletal muscles of the maternal butyrate group. The present study is the first to suggest that maternal butyrate supplementation could affect the myofibre type of offspring skeletal muscle.
The average daily food intake and weight gain of the dams showed no significant differences between the two groups. The body weight of weaning rats also did not change. The detected serum metabolic indices in mother and offspring showed no obvious changes, except for an increase in total cholesterol and LDL-cholesterol in offspring( Reference Zhou, Gao and Chen 28 ). However, the data presented here show that maternal butyrate supplementation did not alter the expressions of MYOD, mTOR, myogenin and myoglobin in offspring skeletal muscle, which are important genes that regulate myofibre growth and differentiation. In our study, we added 1 % butyrate into maternal diets of the butyrate group during gestation and lactation. We also added 4 % alfalfa to the experimental diets of both groups. It could be digested and absorbed in the gastrointestinal tract by complete or partial fermentation to SCFA such as butyrate. Further investigations need to be carried out to determine the effects of the composition of the experiment diets without alfalfa on mitochondrial biogenesis.
In conclusion, the results of our study for the first time indicate that maternal butyrate supplementation during gestation and lactation significantly influence offspring skeletal muscle energy metabolism and mitochondria biogenesis at weaning. These effects may be mediated by the GPR and PGC-1α pathways. However, follow-up studies are necessary to further clarify the detailed mechanisms on how this action is transferred through the placenta or milk to offspring from mothers supplemented with butyrate.
Acknowledgements
The authors gratefully acknowledge Dr Yue Li for her assistance in the animal experiment. This study was supported by the National Natural Science Foundation of China (31572482), the National Key Research and Development Program of China (2016YFD0500502) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
The authors’ contributions were as follows: all authors contributed to the study conception and design; Y. H. performed the experiments, analysed the data and wrote the draft manuscript; S. G. and G. J. maintained the animals; R. Z. and X. Y. provided scientific direction and interpreted the results; X. Y. revised and finalised the manuscript. All authors provided final approval of the manuscript. X. Y. is responsible for the integrity of the work as a whole.
The authors declare that they have no conflicts of interest.










