Inflammation is an important mechanism for CVD, and increased levels of inflammatory markers are seen in patients with CVD, such as peripheral arterial disease (PAD). Furthermore, the plasma concentrations of acute phase reactants, especially C-reactive protein (CRP) and fibrinogen, are associated with risk of myocardial infarction, ischaemic stroke, PAD and cardiovascular death(Reference Danesh, Wheeler, Hirschfield, Eda, Eiriksdottir, Rumley, Lowe, Pepys and Gudnason1). In addition to being markers of vascular inflammation, it has recently also been suggested that CRP may causally contribute to atherosclerosis(Reference de Maat and Trion2, Reference Pepys, Hirschfield and Tennent3) indicating that reduction in CRP concentration per se may be clinically beneficial. This means that CRP concentrations may be useful measures of the effectiveness of, for instance, cardiovascular risk modifying lifestyle interventions(Reference Ray, Cannon and Cairns4).
Several studies indicate that a high intake of fruit and vegetables is cardioprotective(Reference Joshipura, Hu and Manson5, Reference Hung, Joshipura and Jiang6), an effect that is partly explained by the antioxidative properties of the many bioactive components including their influence on endothelial function(Reference Voetsch, Jin and Loscalzo7). In addition, studies suggest that the fat-soluble antioxidant vitamin E has anti-inflammatory properties. However, the effect of fruit and vegetables on CVD and inflammation(Reference Esmaillzadeh, Kimiagar, Mehrabi, Azadbakht, Hu and Willett8–Reference Gao, Bermudez and Tucker11) cannot be explained by administering a high dose of antioxidants like vitamin C or E as shown in large randomised, placebo-controlled studies such as the Heart Protection Study(12), Women's Antioxidant Cardiovascular Study(Reference Cook, Albert, Gaziano, Zaharris, MacFadyen, Danielson, Buring and Manson13), Heart Outcomes Prevention Evaluation Study(Reference Yusuf, Dagenais, Pogue, Bosch and Sleight14) and HDL-Atherosclerosis Treatment Study(Reference Brown, Zhao and Chait15). These studies emphasise that it is yet too premature to focus on high levels of specific vitamins, minerals or other bioactive substances in the fruits and vegetables.
Since inflammation and endothelial function and activation play key roles in the progression of atherosclerosis(Reference Ross16) therapeutic interventions that influence these processes, such as antioxidant-rich foods, are of special interest. Fruit juices are easily consumed and are, like the original fruits, rich sources of various phenolic substances with antioxidative properties including flavonoids and anthocyanidins as well as vitamin C(Reference Justesen, Knuthsen and Leth17). Therefore, we investigated the effect of orange and blackcurrant juices and low-dose RRR-α-tocopheral (vitamin E) on the low-grade chronic inflammation in patients with PAD during 4 weeks of supplementation. In addition, the effects of juice and vitamin E on markers of endothelial activation and oxidative stress were investigated.
Methods
Patients
One hundred and forty-four patients with PAD were identified from the hospital records from the period 1999–2001 and contacted by mail. Forty-eight patients completed both supplementation periods and were included in this analysis. The participant flow is shown in Fig. 1 and described earlier(Reference Dalgård, Christiansen, Jonung, Mackness, de Maat and Hørder18).

Fig. 1 Participant flow in the study.
The study protocol was approved by the local Ethical Committee of Ringkjøbing, Ribe and South Jutland Counties (M-2242-01).
Study design
The study design has been described in detail previously(Reference Dalgård, Christiansen, Jonung, Mackness, de Maat and Hørder18). Briefly, the study was a block-randomised, 2 × 2 factorial crossover trial with two intervention periods of 4 weeks and one wash-out period of 4 weeks. During the intervention periods, juice (250 ml orange juice and 250 ml blackcurrant juice per day)+vitamin E (15 mg RRR-α-tocopherol/d) (JE); juice+placebo-vitamin E (JP); reference beverage+vitamin E (RE); or reference beverage+placebo-vitamin E (RP), were consumed by the patients. Each patient was allocated to two of the four supplements(Reference Dalgård, Christiansen, Jonung, Mackness, de Maat and Hørder18). Investigators were blinded for allocation and sequence. Patients were blinded for tablet contents.
The vitamin E dose was selected to be equivalent to the latest US dietary recommendation(Reference Frei and Trabe19). The tablets used were identical in appearance and prepared for this study by Winter Medico A/S (Odense, Denmark). The selected juice dose was based on the assumption that plasma vitamin C may be used as a marker of fruit and vegetable intake(Reference Myint, Luben, Welch, Bingham, Wareham and Khaw20) and that 200 mg, corresponding to an intake of the Danish recommendation of fruit and vegetables(Reference Trolle, Fagt and Ovesen21), results in plasma ascorbate levels(Reference Levine, Wang, Padayatty and Morrow22) that have been shown to be associated with reduced risk of CVD(Reference Khaw, Bingham, Welch, Luben, Wareham, Oakes and Day23). The reference beverage was a sugar-containing beverage with energy content comparable to the juices. The energy and carbohydrate content of the beverages as well as the analysed ascorbic acid content of the juices are shown in Table 1. Subjects were instructed to drink 250 ml juice at breakfast and 250 ml at dinner, and to take the tablet at dinner. After each intervention period, the patients were asked to return their leftovers for estimation of the apparent adherence to treatment.
Table 1 Energy, carbohydrate and vitamin C content in the provided beverages*
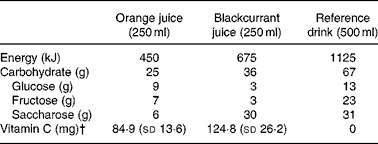
* Values are from the Danish Composition of Foods, 4th edition, and manufacturers' information.
† Six different batches were analysed for each juice.
At baseline, usual intake of fruits, vegetables and juice were assessed by a validated Danish FFQ(Reference Tjonneland, Overvad, Haraldsdottir, Bang, Ewertz and Jensen24). In addition, a self-administered questionnaire was used to collect data on smoking history, physical activity, medication use, pre-study vitamin and/or antioxidant use and family history of CVD. Weight in light clothing was measured before and after each intervention period and height without shoes was measured at baseline(Reference Dalgård, Christiansen, Jonung, Mackness, de Maat and Hørder18).
Blood collection and assessment of biomarkers
Subjects were examined twice, 3 d apart, at baseline and at the end of each intervention period. Venous blood samples were obtained with minimal stasis between 08.30 and 10.00 hours after a light breakfast. Within 1 h the specimens were centrifuged to separate plasma/serum and samples were snap frozen at − 80°C until analysis. All six samples from one subject were analysed within the same run to prevent between-run analytical variation. The specific handling of blood samples has been described previously(Reference Dalgård, Christiansen, Jonung, Mackness, de Maat and Hørder18).
CRP concentrations were determined by particle-enhanced immunonephelometry in accordance with the manufacturer's manual (DadeBehring, Germany). IL-6 concentrations were measured by a high-sensitive enzyme immunoassay (R&D Systems, UK), and functional fibrinogen was measured with the Clauss method, an assay based on the clotting rate(Reference de Maat, Bladbjerg, Drivsholm, Borch-Johnsen, Moller and Jespersen25). von Willebrand factor concentrations were determined by an in-house EIA method (DAKO, Denmark). Plasminogen activator inhibitor-1 and tissue plasminogen activator concentrations were measured with the use of a commercial EIA (Biopool, Umeå, Sweden).
Free F2-isoprostane level was measured as a biomarker of oxidative stress, i.e. lipid peroxidation. We expected that the JE and RP would give the largest difference in this measure of oxidative stress, and due to limited capacity we analysed twenty-three samples that were randomly selected from these two groups. The analyses were performed on GC–MS with negative ion chemical ionisation as previously described(Reference Morrow and Roberts26).
Compliance was assessed by measures of plasma concentrations of α-tocopherol and ascorbate using HPLC with UV(Reference Nielsen27) and coulometric detection(Reference Lykkesfeldt, Loft and Poulsen28), respectively, as well as tablet count. Non-fasting total-cholesterol, HDL and blood-glucose were determined using standard routine methods in the hospital laboratory.
Statistical analyses
Data were checked for Gaussian distribution with the Shapiro–Wilks test and visual inspection of normplots. Non-Gaussian-distributed data (CRP, fibrinogen, IL-6, plasminogen activator inhibitor-1, vitamins C and E, and glucose) are presented as median and the 25th–75th percentile range, whereas Gaussian-distributed data (von Willebrand factor, tissue plasminogen activator, total-cholesterol and HDL) are presented as mean and standard deviation. CRP, fibrinogen, IL-6, and plasminogen activator inhibitor-1 were Gaussian-distributed after logarithmic transformation.
The effect of the supplementations was described as the absolute change in the particular variable (post-supplementation value minus baseline values). The change was assessed in an analysis of co-variance model with baseline value, supplementation indicator and period effect as covariates together with the cluster option, which takes into account that data are not independent within clusters (i.e. each patient) due to the cross-over design although independent across clusters. Analyses were performed both for the individual groups (JE, JP, RE, and RP) and for main effects (juice v. reference beverage, and vitamin E v. placebo) in accordance with the 2 × 2 factorial design. Our primary reason for using a factorial design was to make efficient use of the patients' data, and the design also enabled us to estimate interaction.
Within-group changes from baseline to week 4 were analysed by the two-sided paired t test or the Wilcoxon matched pairs signed rank sum test. A value of P < 0·05 (two-tailed) was taken to indicate statistical significance. Stata version 10.0 (Stata Corporation, Texas, USA) software was used for statistical analyses.
We calculated that with forty-eight participants a statistical power of at least 0·75 at significance level 0·05 is obtained testing the global null hypothesis of no difference among all four treatments (JE, JP, RE, RP) and the assumption that in any of the three groups JP, JE, and RE two thirds of the subjects will show a change in the same direction. This corresponds to an expected change of at least 0·43 times the standard deviation of the difference. Furthermore, it is assumed that there is no change in the reference group (i.e. group RP) and that the correlation of repeated measurements within a patient is at least 0·25.
Results
Baseline characteristics of the study population are shown in Table 2. No significant differences were seen in the baseline characteristics among the four groups except for age that was lower in the JP group (Table 2). Body weight, blood lipids and blood glucose did not change during the study.
Table 2 Characteristics of participants at baseline
(Mean values and standard deviation, number and percentage, and median with 27th–75th percentiles as indicated)
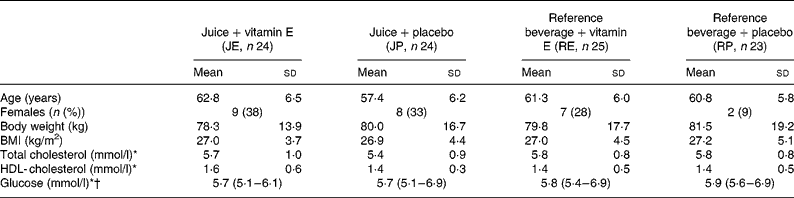
* Non-fasting values.
† Data are presented as median and 25th–75th percentiles due to a non-Gaussian distribution.
Among the forty-eight patients with PAD, 50 % were current smokers, who had initially higher levels of CRP (3·39 v. 2·35 mg/l, P = 0·002), fibrinogen (10·6 v. 9·7 μmol/l, P < 0·001) and IL-6 (3·19 v. 2·44 pg/ml, P = 0·005) compared with non-smokers.
Statins were used by 33 %, aspirin by 77 % and antihypertensives (including angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers) by 33 % of the patients; medication did not change during the study. In addition, levels of inflammation markers were not associated with the use of medication.
Before the juice supplement, the patients had a habitual median fruit and juice intake of 176 g/d, and a median vegetables intake of 164 g/d. This habitual intake did not change during the two intervention periods. At baseline, a relationship (Spearman's rank correlation coefficient) was found between plasma concentrations of CRP and the estimated daily fruit intake (r s − 0·22, P = 0·04). In contrast, no relationship was found between all other risk factors and the daily intake of fruits or vegetables (P>0·2 for all).
Compliance
Forty-eight patients completed both periods. Plasma ascorbate and α-tocopherol concentrations were similar when comparing levels before intervention period 1 with levels before period 2 indicating that the wash-out period was sufficient (Table 3). The observed increases in plasma ascorbate and α-tocopherol concentrations indicate that the participants took their supplementation regularly (Table 3). For α-tocopherol, compliance was 96 % by tablet count.
Table 3 Effects of a 4-week supplementation with orange and blackcurrant juices and vitamin E on plasma ascorbate and α-tocopherol concentrations∥
(Median values with 25th–75th percentiles (%ile))
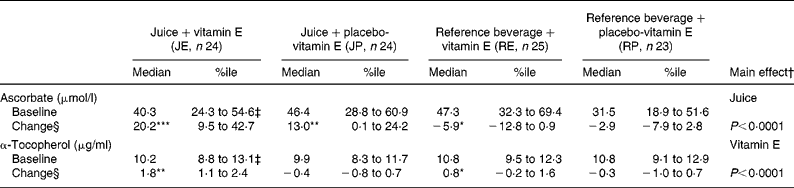
Mean values were significantly different within the group: *P < 0·05, **P < 0·005, ***P < 0·0001, all Wilcoxon matched-pairs signed rank sum test.
† Main effect comparing juice (JP+JE) v. reference drink (RP+RE), independent of vitamin E; or vitamin E (RE+JE) v. placebo (RP+JP), independent of juice.
‡ One blood sample for vitamins E and C analyses after supplementation was lost thus values represent n 23 subjects.
§ Change indicates difference between baseline and treatment in absolute values.
∥ For details of supplementation see Methods.
Inflammation and endothelial cell markers
The multivariate analysis showed significant differences only for CRP changes among the four groups, i.e. the changes in groups JE and JP were significantly different from the changes in the reference group (i.e. RP). There were no significant differences in analysis of interaction effects (P>0·05) and results were independent of the vitamin E supplementation (results not shown).
The multivariate analysis of the main effects, i.e. juice v. reference beverage showed significant differences in CRP and fibrinogen only. Thus, after 4 weeks of orange and blackcurrant juice supplementation, changes in plasma CRP and fibrinogen concentrations were significantly different from changes in subjects who received the reference beverage (CRP: − 11 % v. +13 %, P < 0·008; fibrinogen: − 3 % v. +2 %, P < 0·002). We observed no significant effect on IL-6 or the endothelial cell markers (Table 4). We observed no significant differences in any variable in patients who received vitamin E compared with those who did not receive vitamin E (Table 4).
Table 4 Main effects of 4-weeks supplementation with orange and blackcurrant juices or vitamin E on markers of inflammation and endothelial function. The data show baseline values and changes (Δ) between baseline and treatment in absolute values†
(Values are median and 25th–75th percentile (%ile) or mean and 95 % CI)

CRP, C-reactive protein; Fbg, fibrinogen; vWF, von Willebrand factor; tPAag, tissue plasminogen activator antigen; PAI-1, plasminogen activator inhibitor type 1.
* P-values are for main effect of differences in changes.
† For details of supplementation see Methods.
Significant relationship was seen between changes in CRP and changes in fibrinogen (r s 0·63, P < 0·0001). No relationships were observed between all other biomarkers and changes in plasma vitamins.
F2-isoprostane
Median percentage changes in plasma F2-isoprostane concentrations among patients in the reference+placebo group (RP) (16 % (4–28 %)) were significantly greater than those of patients in the juice+vitamin E group (JE) ( − 10 % ( − 17 to 17 %)) (P = 0·04, Mann–Whitney U test).
Discussion
In the present study in patients with PAD, CRP levels were reduced by 11 % after juice supplementation and increased by 13 % after supplementation with a reference beverage that contained a similar amount of carbohydrates but no bioactive components. Minor, but significant changes were also observed for fibrinogen. Furthermore, our study confirms that the plasma CRP concentration is inversely associated with the intake of fruit in these patients.
Inflammation has a central role, not only in the initiation but also in the progression of the atherosclerotic lesion and the stability of the fibrous cap. CRP has been shown to be a sensitive marker of the inflammatory activation of the vessel wall and furthermore to associate well with cardiovascular-event risk(Reference Libby29). CRP is also related to inflammation in other tissues including adipose tissue(Reference Mehta and Farmer30). However, in the present study, there were no indications of other underlying diseases or changes in environment and lifestyle, including body weight during the study period(Reference Dalgård, Christiansen, Jonung, Mackness, de Maat and Hørder18) that could affect levels of the measured variables. Therefore, the observed reduction in CRP may reflect a subdued inflammatory signalling that eventually may slow down or stop the atherosclerotic progression(Reference Libby29, Reference Wilson, Swan and Ding31). Furthermore, the changes in fibrinogen were highly correlated with the CRP changes, which further supports that the supplementation affects a common underlying inflammatory process. In contrast, we did not observe any association between changes in blood levels of vitamin C (or vitamin E) and CRP (or fibrinogen) which support the perception that it is too early to ascribe the observed effects to single nutrients.
CRP concentrations below 2 mg/l are associated with 30 % lower risk of cardiovascular events compared with higher CRP values(Reference Ridker, Cannon, Morrow, Rifai, Rose, McCabe, Pfeffer and Braunwald32) and in our study juice supplementation increased the number of patients having a CRP concentration below 2 mg/l from 38 to 50 %.
CRP and other acute phase proteins like fibrinogen are synthesised in hepatocytes mediated by the action of the pro-inflammatory cytokines IL-6, IL-1 and TNF, whose expression is regulated by the activation of the pro-inflammatory transcription factor NF-κB(Reference de Winther, Kanters, Kraal and Hofker33). Studies have shown that this activation is redox sensitive(Reference Barnes and Karin34) and therefore is activated by an increased level of reactive oxygen species. In the present study, it is possible that the intake of either beverage (both with high level of sugars) leads to an increased intracellular concentration of glucose which is followed by an increased reactive oxygen species production that affects the level of inflammation(Reference Esposito, Nappo, Marfella, Giugliano, Giugliano, Ciotola, Quagliaro, Ceriello and Giugliano35, Reference Yano, Hasegawa, Ishii, Yamasaki, Fukui, Nakamura and Yoshikawa36). In contrast, the presence of various bioactive molecules in the juices only (like the antioxidants which are known to be bioavailable(Reference Manach, Williamson, Morand, Scalbert and Remesy37)) may counterbalance the potential reactive oxygen species production. The results from the present study support this notion, as we observed an increased level of F2-isoprostane after sugar beverage+placebo-vitamin E; a change that was significantly different from the change after juice+vitamin E. However, although observed previously in female rats(Reference Breinholt, Nielsen, Knuthsen, Lauridsen, Daneshvar and Sorensen38), a pro-inflammatory effect of a sugar beverage was not confirmed in a smaller study in obese but otherwise healthy human subjects(Reference Sorensen, Raben, Stender and Astrup39). Thus, this observation warrants further examination.
It may seem surprising that CPR and fibrinogen change while IL-6 did not change, as IL-6 is the main up-stream determinant of the acute phase reactants(Reference Gabay and Kushner40). However, IL-6 is not the only determinant of the plasma levels of CRP and fibrinogen(Reference Weinhold and Rüther41), and taking into consideration the short IL-6 plasma half-life of about 3 min(Reference Castell, Geiger, Gross, Andus, Walter, Hirano, Kishimoto and Heinrich42), the large variability in circulating IL-6 levels(Reference Dibbs, Thornby, White and Mann43) and the presence of the soluble form of the IL-6 receptor which binds the IL-6(Reference Rose-John, Scheller, Elson and Jones44), these factors may together with the small sample size and IL-6 assay characteristics make it difficult to observe significant changes.
We also investigated the effect of 15 mg α-tocopherol/d on the selected biomarkers. The dose selected for the study corresponds to the latest US recommendation(Reference Frei and Trabe19) and it is even higher than the Nordic recommendation(45). However, we observed no effect on either the inflammatory or the endothelial cell markers and the results thus supports the notion that only very high un-physiological doses of vitamin E may affect inflammation(Reference Azzi, Gysin, Kempna, Munteanu, Villacorta, Visarius and Zingg46).
In this study population, 33–70 % of the patients received drugs that are known to have anti-inflammatory as well as antioxidative capabilities. This has been clearly demonstrated for statins(Reference Bermudez and Ridker47–Reference De Caterina, Cipollone and Filardo50), but also for several of the antihypertensive drugs have anti-inflammatory effects been demonstrated(Reference Palmas, Ma, Psaty, Goff, Darwin and Barr51). However, the patients did not change their medication during the study. Still, an interaction between medication and juice or vitamin E cannot be excluded since our patient groups were too small to determine this.
The habitual diet of the patients was supplemented with more than 1 MJ from the beverages, but their body weight did not increase, which suggests that the patients may have changed their diet and this may have influenced our results. However, we do not expect that this would induce differences in the effect on inflammatory responses between groups because so far there is no evidence that juice and sugar beverages induce different satiating effects.
Our study was a relatively small intervention study and results should be replicated in larger study groups preferentially with clinically relevant endpoints.
In summary, the use of blackcurrant and orange juices reduces the level of the inflammatory markers CRP and fibrinogen in patients with PAD compared to a sugar-containing beverage while no effect of vitamin E was observed. The study supports the view that increased intake of fruit and vegetables, or products thereof, decreases the cardiovascular risk.
Acknowledgements
The study was supported by The Regional Institute for Health Sciences and the Institute of Clinical Research at University of Southern Denmark; The Counties of Ribe and Funen; the Foundation of Carpenter A. Andersen and Wife; and the Foundation of Sawmill owner Jeppe Juhl and Wife. J. D. M. was supported by NIH grants GM15431, CA77839, RR00095 and DK48831. Measures of vitamin C concentration in the juice were kindly provided free of charge by Rynkeby A/S, Denmark. Winter Medico A/S, Odense, Denmark, has provided the study tablets free of charge.
We thank our participants and K. Overgaard, A. Larsen, and A. Nørregaard for technical assistance, A. Mains for blood sampling and C. Skoubo for blood sample preparation. Furthermore, Dr N. Rohr identified the patients from the Odense Registry for which he is thanked. Professor W. Vach is thanked for valuable statistical assistance.
Authors report no potential conflict of interest relevant to this article.







