Milk and dairy product consumption has a long and regular pattern in Europe and North America. Milk protein has been shown to have an insulinotropic effect and to lower glycaemic response to carbohydrate-rich foods. Epidemiological and clinical studies have repeatedly demonstrated an inverse relationship between milk and dairy product consumption and prevalence of obesity and type 2 diabetes (T2D)( Reference Elwood, Pickering and Fehily 1 – Reference Gao, Ning and Wang 3 ). Although the precise mechanism by which milk protein, notably whey protein, stimulates insulin is not known, there is emerging evidence that several amino acids and peptides act as insulinogenic precursors. Moreover, glucose-dependent insulinotropic polypeptide (GIP) has been reported to increase after the consumption of whey protein( Reference Frid, Nilsson and Holst 4 , Reference Nilsson, Stenberg and Frid 5 ). Glucagon-like peptide-1 (GLP-1), similar to GIP, is also known to exhibit insulinotropic response( Reference Asmar and Holst 6 , Reference Gunnerud, Holst and Ostman 7 ). GLP-1 has multiple physiological effects including augmenting insulin secretion( Reference Kreymann, Williams and Ghatei 8 ), inhibiting glucagon secretion and slowing gastric emptying( Reference Layer, Holst and Grandt 9 ).
In most regions of Asia, the putative presumption that lactose intolerance is widespread in people has preluded the consumptions of cows’ milk. Milk consumption therefore in this region remains rather modest. The most popular ‘milk’ here is soya milk. Soya milk has been consumed in China and its surrounding areas for centuries. Vilegas et al.( Reference Villegas, Gao and Yang 10 ) reported that the consumption of soya beverages was significantly associated with lower risk of T2D, whereas Velasquez et al.( Reference Velasquez and Bhathena 11 ) reported that soya proteins have beneficial effects on insulin resistance and obesity. Regular consumption of soya milk has been promulgated as an important component of healthy living.
Given the high prevalence of T2D in this region, diet-based interventions have become an alternative strategy for glycaemic control. It has long been assumed that fasting blood glucose rather than postprandial glucose response is the main arbiter of glycaemic control. However, more recently, it has been recognised that postprandial glucose response is an even more important determinant of glycated Hb (HbA1C)( Reference Ketema and Kibret 12 ). As many of the drugs used for the management of T2D are insulinotropic, this opens up the speculation as to whether proteins may also act as insulinotropics. Therefore, food-based interventions may be used to mimic the pharmacological response of drugs used in managing glycaemia. Milk proteins have been extensively studied, with a few studies examining the effect of soya milk or soya products on glycaemia. It is not clear whether soya milk exerts similar physiological effects as cows’ milk with respect to glycaemic control. In a previous study, we have shown that dairy products and soya milk when consumed together with bread lowered postprandial blood glucose but induced different insulin responses( Reference Sun, Tan and Han 13 ). The aim of the present study was to investigate whether both soya and cows’ milk exert insulinogenic properties and facilitate glycaemic regulation through a mechanism involving elevation of certain amino acids and stimulation of incretins.
Methods
Subjects
A total of twelve lean, Chinese men (age: 21–26 years; body weight: 58–69 kg; BMI: 18·3–24·4 kg/m2) participated in this study. All participants were healthy, and none of them had a family history of either T2D or CVD. None of the subjects smoked or used tobacco products, consumed special diets or took medications known to alter metabolism. All subjects had normal fasting blood glucose (<6·0 mmol/l) and insulin concentrations and no history of food intolerance. The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures were approved by the Domain Specific Review Board of National Healthcare Group, Singapore. Written informed consent was obtained from all subjects before participation.
Study design
This was a randomised, cross-over study with three experimental trials separated by a 1-week washout period to minimise carry-over effects. On the evening before each study day (about 18.30 hours), participants consumed a standardised evening meal consisting of rice, chicken and a non-alcoholic beverage. They were then asked to refrain from consuming any food or beverages except water until they reported to the laboratory the next morning. Participants were also asked to refrain from any vigorous physical activity the day before the study.
On each study day, subjects arrived at the research centre at 08.30 hours following a 10–12-h overnight fast. Upon arrival, they were allowed to rest for about 10 min before any baseline measurements were recorded. An indwelling intravenous catheter was inserted into a vein in the forearm by a registered nurse, and a baseline fasting blood sample was collected. Subsequently, participants consumed the test meal at a comfortable pace (12 min on average). Venous blood samples were collected at 30, 60, 90, 120 and 180 min following the start of the meal. Plasma was isolated and stored until further analysis. Participants remained seated throughout the postprandial period.
Test meals
Subjects were given three different meals in random order. All meals contained 50 g of available carbohydrate. The reference meals consisted of white bread (91 g) and water (322 ml). The test meals consisted of white bread (58 g) and isovolumetric amounts of either chilled soya milk or low-fat cows’ milk (322 ml) containing approximately 12 g of soya protein or milk protein. White bread and soya or cows’ milk were purchased from a local supermarket, and the energy and nutrient contents of the test meals were calculated on the basis of the weight of the components and the dietary information provided by the manufacturer. The test foods were freshly prepared in the morning of the test days. The details of meal composition can be obtained from a previous study table 1( Reference Sun, Tan and Han 13 ).
Sample analysis
Venous blood samples were collected in BD Vacutainer® containing spray-dried K2EDTA. A protease inhibitor cocktail (cOmplete™ Mini EDTA-free; Roche) and a DPPIV inhibitor (Ile-Pro-Ile; Sigma) were added into the tubes for active GLP-1 and total GIP analyses. The tubes were centrifuged at 1500 g for 10 min at 4°C within 15 min after sample collection. Plasma samples were aliquoted into Eppendorf tubes and stored at −80°C, until measurement of the concentrations of amino acids, total GIP and active GLP-1.
The amino acid composition of soya and cows’ milk was determined on a HPLC system (Covance Asia Pte Ltd). Plasma amino acids were measured on a Hitachi L-8900A system. TCA 5 % was added to plasma samples and centrifuged for 30 min at 10 000
g
to extract the amino acids. The supernatant was retained and passed through a 0·45-µm filter before analysis. An accurate volume (20 µl) of the sample was injected into the chromatographic column. The column (60×4·6 mm) was a matrix of a high-molecular-mass organic component, consisting of polystyrene cross-linked by divinylbenzene, with sulphone
![]() ${\rm (SO}_{3}^{\;{\minus}} )$
groups as active exchange sites. The elution programme was adapted from that set by Hitachi. Detection was via spectrophotometry at 570 and 440 nm based on the ninhydrin reaction. All buffers and the ninhydrin reagent were supplied by Wako Pure Chemical Industries.
${\rm (SO}_{3}^{\;{\minus}} )$
groups as active exchange sites. The elution programme was adapted from that set by Hitachi. Detection was via spectrophotometry at 570 and 440 nm based on the ninhydrin reaction. All buffers and the ninhydrin reagent were supplied by Wako Pure Chemical Industries.
Plasma total GIP and active GLP-1 concentrations were determined using the human metabolic hormone MILLIPLEX MAP kit (Millipore, Cat. no HMHEMAG-34K). The intra-assay CV for active GLP-1 and GIP were <10 %.
Statistical analysis
Statistical analysis was performed using SPSS software version 23 (IBM/SPSS Inc.). Differences in the concentrations of active GLP-1, GIP and amino acids were evaluated by using repeated-measures two-factor ANOVA, with main effects for milk type (water v. soya milk v. cows’ milk) and time points (over 180 min postprandial). The incremental AUC (iAUC) were used as postprandial summary measures and were calculated using the trapezoidal rule; data were analysed using repeated-measures one-factor ANOVA (milk type) to evaluate differences among trials at each time point and iAUC. For all analyses, Bonferroni’s post hoc tests were used to correct for multiple comparisons. Correlations between change in plasma amino acids, GIP and GLP-1 from 0 to 30 min were determined using Spearman’s bivariate correlation (two-tailed test). Data are presented as mean values with their standard errors, unless otherwise stated. A P-value<0·05 was considered to be statistically significant.
Results
All participants completed the study and consumed the test meals without any problems.
Milk amino acid content and plasma amino acid profile
The total amino acid concentrations in both cows’ milk and soya milk were similar. However, cows’ milk was richer in branched-chain amino acids (BCAA) (approximately 15 % higher concentrations) and essential amino acids (EAA) (approximately 10 % higher concentrations) compared with soya milk (Table 1). Plasma amino acids were measured before (fasting) and 30 min after consumption of each test meal. The changes (30 min minus baseline) that occurred in individual plasma amino acid concentrations following the consumption of the two types of milk reflected the different amino acid composition of cows’ and soya milk (Table 2). The plasma concentrations of valine, methionine and proline as well as total BCAA concentrations were higher after cow milk consumption compared with soya milk-containing meals, whereas the opposite was true for alanine and arginine. Most of the amino acids concentrations were significantly higher in the soya meal compared with the control meal, except for glutamic acid, glycine and proline (Table 2). Cows’ milk meal had higher plasma amino acid concentrations compared with the control meal, except for alanine, cysteine and glycine (Table 2). However, glycine concentration in soya milk was significant higher compared with cows’ milk and the control meal when compared independently (data not shown).
Table 1 Amino acid composition of soya and cows’ milk (mg/100 ml)
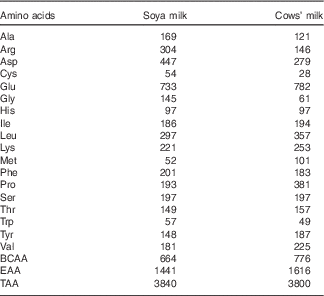
BCAA, branched-chain amino acids; EAA, essential amino acids; TAA, total amino acids.
Table 2 Incremental changes in the concentrations of individual plasma amino acids, total amino acids, branched-chain amino acids (BCAA) and essential amino acids (EAA) 30 min after ingestion of mixed meals containing water, soya milk or cows’ milk (Mean values with their standard errors)
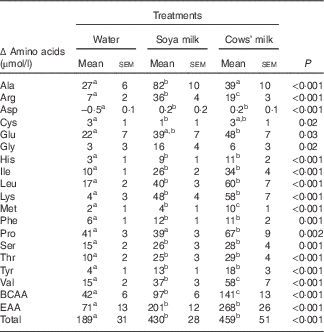
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Postprandial glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide responses
For both incretins, the two-way time×milk-type interaction was statistically significant, indicating that there were significant main effects for milk type (water v. soya v. cows’ milk). These results indicate that the magnitude of postprandial GLP-1 and GIP responses is affected by the type of milk. Postprandial active GLP-1 concentrations were greater after ingesting cows’ milk compared with soya milk without significant difference with respect to control meal (Fig. 1(A)), whereas the opposite was true for GIP concentrations, which were greater after soya milk consumption compared with cows’ milk and water without difference between cows’ milk and control meal (Fig. 1(B)). GLP-1 peaked at approximately 30 min after consumption of all three meals – it returned to baseline levels 90 min after soya milk ingestion and control meal but remained elevated after cows’ milk ingestion (Fig. 1(A)). GIP peaked at approximately 60 min after soya milk and control water ingestion but much later (90–120 min) after cows’ milk ingestion (Fig. 1(B)).
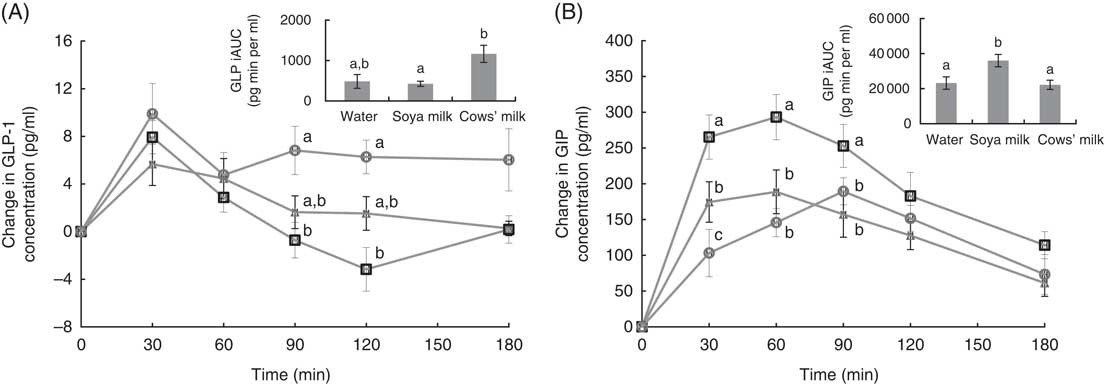
Fig. 1 Postprandial, plasma active glucagon-like peptide-1 (GLP-1) concentrations (A), glucose-dependent insulinotropic polypeptide (GIP) concentrations (B). a,b,c Mean values with unlike letters were significantly different at each measured time point (P<0·05). Values are means (n 12), with their standard errors. iAUC, incremental AUC. ![]() , Water;
, Water; ![]() , soya milk;
, soya milk; ![]() , cows’ milk.
, cows’ milk.
Correlations between parameters
Positive correlations were found between change in alanine and arginine and the corresponding change in GIP for the first 30 min (P<0·05). In addition, GLP-1 correlated positively with valine, isoleucine, leucine and BCAA, although the values did not reach statistical significance (P>0·05) (Table 3).
Table 3 Spearman’s correlations between changes in plasma amino acids, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) (0–30 min)

BCAA, branched-chain amino acids.
Discussion
In a previous study, we observed that postprandial glucose concentrations were attenuated to a similar extent after soya and cows’ milk ingestion together with bread compared with controls, with different insulin responses( Reference Sun, Tan and Han 13 ). We hypothesised that soya and cows’ milk could facilitate glycaemic regulation through two different mechanisms. These mechanisms may be different from the conventional ones such as insulin response and may include others such as gastric emptying and gut hormones. Therefore, the aim of this study was to examine the effect of soya and cows’ milk, ingested together with white bread, on gut-related hormones (GLP-1 and GIP) and plasma amino acids to better understand the role of milk consumption on glycaemic control.
We found that the two types of milk affect plasma amino acid profiles in a manner consistent with their protein amino acid composition. They have diametrically opposite effects on the two major incretin hormones. The mechanism for soya milk-induced hyperinsulinaemia seems to be linked to the higher amino acids (alanine and arginine), which then stimulate the secretion of GIP. However, GIP is not a major mediator of reduced glycaemia after cows’ milk consumption. Cows’ milk with higher BCAA concentrations led to a greater GLP-1 response, which may in turn be responsible for the lower glycaemia without elevating insulin response as observed previously( Reference Sun, Tan and Han 13 ).
In an earlier study, we found that the consumption of soya milk led to higher insulin response compared with controls. This suggests that the reduction in glycaemic response can be explained by an insulin-dependent mechanism. Soya protein contains higher levels of arginine, glycine and alanine compared with cow milk protein (Table 1). Several amino acids are potent stimulators of insulin release. Arginine has been suggested to have the most potent insulinotropic effects( Reference Floyd, Fajans and Conn 14 , Reference Sener, Blachier and Rasschaert 15 ). Close associations have been observed between insulin and amino acid responses, particularly phenylalanine, arginine and BCAA( Reference Calbet and MacLean 16 ). In our study, we found that plasma alanine concentrations were two times greater after soya milk consumption compared with cows’ milk. Alanine may also have insulinotropic effects, which may account for the higher insulin response after soya milk-containing meals compared with cows’ milk-containing meals( Reference Sener and Malaisse 17 ). These findings suggest that the postprandial amino acid pattern may be an important factor for the insulinogenic effect of soya milk.
Milk products are an important part of breakfast and are regarded as having low glycaemic index( Reference Foster-Powell, Holt and Brand-Miller 18 ). Earlier studies have demonstrated that milk products are powerful acute stimulants of insulin secretion and enhance insulin responses when consumed with a mixed meal( Reference Östman, Liljeberg Elmståhl and Björck 19 – Reference Liljeberg Elmstahl and Bjorck 21 ). However, in our earlier study, we did not find significant higher insulin response after consumption of cows’ milk with white bread( Reference Sun, Tan and Han 13 ). The improved postprandial glycaemia may be explained by insulin-independent mechanisms such as the release of gut hormones (incretins) that delay stomach emptying and increase insulin efficacy( Reference Jakubowicz and Froy 22 ). Whey protein has been shown to increase GLP-1( Reference Hall, Millward and Long 23 ) and delay gastric emptying when compared with carbohydrates alone( Reference Ma, Stevens and Cukier 24 ). GLP-1 inhibits gut motility, slows down gastric emptying and rapidly crosses the blood–brain barrier to directly transmit signals that inhibit gastric emptying( Reference Nauck, Niedereichholz and Ettler 25 , Reference Akhavan, Luhovyy and Panahi 26 ). GLP-1 inhibits gastric emptying and may outweigh its insulinotropic effects in healthy humans( Reference Nauck, Niedereichholz and Ettler 25 ). This corresponds to our results in a previous study where gastric emptying was shown to be significantly slower after cows’ milk consumption compared with controls( Reference Sun, Tan and Han 13 ).
Amino acids may affect insulin secretion in two ways: either directly by acting on the pancreatic β-cells or indirectly by promoting incretin release during digestion in the small intestine. Gannon et al.( Reference Gannon, Nuttall and Neil 27 ) also suggested that the major stimulus for insulin secretion is the increase in incretin hormones in response to the presence of protein or digestion in the intestine. Both GLP-1 and GIP have been identified as strong insulinotropic agents( Reference Asmar and Holst 6 ). GIP is probably predominantly secreted in the upper small intestine, whereas the density of L-cells, responsible for the secretion of GLP-1, is the highest in the lower small intestine. Therefore, smaller loads of rapidly absorbable nutrients would preferentially activate the upper gut incretin – that is, GIP. On the other hand, ingestion of larger meals containing more complex nutrients that require more extensive digestive processing would activate the distal gut incretin – that is, GLP-1( Reference Vilsboll and Holst 28 ). In the present study, we found that incretins were uniquely affected by the different types of milk, with cows’ milk augmenting more GLP-1 release and soya milk augmenting more GIP release. Whey and casein proteins have been shown to increase GLP-1 and GIP responses( Reference Gunnerud, Holst and Ostman 7 ). Soya protein, which is classified as a fast digesting protein, compared with casein, a slow digesting protein, may stimulate the earlier release of GIP. A positive correlation was found between early GLP-1 response (change in 0–30 min) and postprandial BCAA release 30 min after the meal, which indicate that BCAA may facilitate GLP-1 release from intestinal cells, consistent with previous reports( Reference Chen and Reimer 29 ). Veldhorst et al.( Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 30 ) found that whey protein triggered stronger GLP-1 responses compared with casein or soya proteins, which is in agreement with our results. We found a positive strong correlation between alanine, arginine and GIP changes at 30 min, which suggests that alanine and arginine may be important for GIP release to modulate postprandial glycaemia after soya milk ingestion. However, besides amino acids, the sugar composition of the two types of milk may be involved in the different incretin responses; sucrose is present in soya milk (as a sweetener), whereas lactose is present in cows’ milk. In contrast to cows’ milk, soya milk resulted in higher insulin( Reference Sun, Tan and Han 13 ) and GIP responses. Soya milk is a mixture of soya protein and sucrose, whereas cows’ milk is a complex mixture of proteins (whey protein:casein in the ratio of 20:80) and lactose. GIP and insulin responses were augmented by an acute increase in sucrose intake( Reference Mazzaferri, Starich and St Jeor 31 ). However, further investigation is required to determine whether sucrose intake resulted in higher insulin and GIP levels compared with lactose. Therefore, differences in the pattern of incretin secretion and the digestion or absorption of soya milk and cows’ milk might contribute to the differences in insulin responses, but similar lower glycaemia responses were found previously( Reference Sun, Tan and Han 13 ).
The main strength of our study is the use of real-life foods. Previous studies have used pure soya, whey and casein to evaluate the effects of different types of protein on glucose, insulin, and incretin responses. However, people do not consume pure protein beverages, but rather drink whole milk. In addition, soya milk is a popular beverage in Asia. Taking these factors into account, in this study, we used whole cows’ milk and soya milk, making sure the two types of milk were isoenergetic and contained the same amount of protein and carbohydrate. This reflects the local diet and provides a more realistic insight into glycaemic control. Our study, however, is not without limitations. First, besides amino acids, the sugar composition of the two types of milk could be involved in the observed differences – sucrose is present in soya milk (as a sweetener), whereas lactose is present in cows’ milk. We were unable to isolate the effect of amino acids and incretins from that of sugar. Second, owing to the high cost of amino acid analysis, we only measured two time points for amino acids. Third, this study recruited only healthy, Chinese, male participants, and therefore we cannot be certain that our results are applicable for other populations as well.
We conclude that soya milk and cows’ milk have different effects on early postprandial concentrations of amino acids and incretin hormones. The results from the present study indicate that cows’ milk ingestion leads to greater BCAA and GLP-1 concentrations in the blood stream than soya milk, whereas soya milk leads to greater concentrations of non-EAA (alanine, arginine and glycine) and GIP than cows’ milk. When the results are considered as a whole, it can be concluded that soya milk is a good alternative to cows’ milk for glycaemic regulation, with different mechanisms involved.
Acknowledgements
The authors extend their appreciation to Faidon Magkos for his intellectual contribution to the revision of the manuscript. The authors thank the volunteers who participated in this trial. The study was supported by the Singapore Institute for Clinical Sciences, A*STAR.
The authors’ responsibilities were as follows: C. J. H. and L. S. were responsible for the study concept and design; L. S., K. W. J. T. and P. C. S. were responsible for study conduct, data collection and data analysis. L. S. and K. W. J. T. were responsible for the first draft of the manuscript. C. J. H. was responsible for the critical revision of the manuscript for important intellectual content.
None of the authors has any conflicts of interest to declare.







