Congestive heart failure (CHF) is a clinical syndrome that is characterised by dyspnoea, orthopnoea, elevated jugular venous pressure and pulmonary congestion( Reference Kurmani and Squire 1 ). It is caused by a structural and/or functional cardiac abnormality resulting in decreased cardiac output and/or increased intracardiac pressures( Reference Ponikowski, Voors and Anker 2 ). The prevalence of CHF increases with age and is approximately ten per 1000 people for those older than 65 years( Reference Lloyd-Jones, Larson and Leip 3 ). Patients with CHF are more prone to develop type 2 diabetes mellitus (T2DM), and thus approximately 20 to 35 % of enrolled subjects have a clinical history of diabetes mellitus( Reference Horowitz and Kennedy 4 ). Several clinical studies have shown that high levels of oxidative stress markers and inflammatory cytokines may reflect the severity of CHF( Reference Maack and Bohm 5 – Reference Aukrust, Yndestad and Damas 7 ).
A number of studies have documented a relationship between less severe Se deficiency or suboptimal Se levels and heart failure( Reference Cenac, Simonoff and Djibo 8 – Reference Kosar, Sahin and Taskapan 9 ). However, in another study, Se levels in CHF patients were similar to those of controls, and Se levels did not correlate with the degree of left ventricular dysfunction( Reference Ghaemian, Salehifar and Shiraj 10 ). Prior studies have reported that Se deficiency is an accepted cause of reversible CHF( Reference Cenac, Simonoff and Djibo 8 , Reference Ge and Yang 11 ). However, in a Cochrane review( Reference Rees, Hartley and Day 12 ), Se supplementation was associated with a small non-significant increase in diabetes risk. In addition, in the above-mentioned study, there were no statistically significant effects of Se supplementation on all-cause mortality, CVD mortality, non-fatal CVD events or all CVD events( Reference Rees, Hartley and Day 12 ). On the other hand, the results of the selenium and vitamin E cancer prevention trial (SELECT) clearly do not support Se or vitamin E supplementation in adult life for primary prevention of cancer( Reference Lippman, Klein and Goodman 13 ). It has been suggested that Se may be involved in the deconditioning of skeletal and cardiac muscles and in CHF symptoms including fatigue and low exercise tolerance, rather than in ventricular dysfunction( Reference de Lorgeril, Salen and Accominotti 14 , Reference de Lorgeril and Salen 15 ). Some studies have reported the beneficial effects of Se supplementation on glycaemic control and biomarkers of inflammation and oxidative stress in patients with T2DM and CHD( Reference Farrokhian, Bahmani and Taghizadeh 16 – Reference Tabrizi, Akbari and Moosazadeh 18 ).
This evidence may suggest the importance of Se in patients with CHF. However, whether Se has direct benefits on metabolic status in patients with CHF has not yet been assessed. In addition, data on the effects of Se on metabolic profiles in patients without CHF are conflicting. Therefore, the aim of this study was to examine the effects of Se supplementation on metabolic status among subjects with CHF.
Methods
Participants
This study is a randomised, double-blind, placebo-controlled trial, registered in the Iranian registry of clinical trials (http://www.irct.ir: IRCT2017053033941N2), conducted at a cardiology clinic affiliated to Kashan University of Medical Sciences (KaUMS), Kashan, Iran, between June 2017 and September 2017. The subjects were recruited between 1 June 2017 and 15 June 2017 from our Referral centre for CHF in Kashan, Iran. Then, the present trial was conducted among fifty-three participants with CHF from 16 June 2017 to 10 September 2017. Owing to the long duration of the administrative process, the registration number of our study seems retrospective; however, we received the formal ethics approval before beginning our study. Diagnosis of CHF was conducted based on the echocardiography method( Reference Dickstein, Cohen-Solal and Filippatos 19 ). Those consuming Se supplements within the past 3 months, having an acute myocardial infarction within the past 3 months, having cardiac surgery within the past 3 months or significant renal or hepatic failure were not included in this study. This investigation was performed according to the principles of the Declaration of Helsinki, and the study protocol was approved by the ethics committee of KaUMS. All patients were informed about the aims and protocol of the study. Written informed consent was obtained from all subjects before the intervention.
Study design
At the onset of the study, to decrease potential confounding effects, all participants were subjected to stratified randomisation( Reference Mansournia, Hernan and Greenland 20 ) according to age, BMI, sex and the dosage and kind of medications. Thereafter, the participants in each block were randomly allocated into two treatment groups to take either 200 µg of Se supplements as Se yeast (n 26) or placebo (n 27) per day for 12 weeks. Participants were asked to refrain from all other Se-containing supplements during the trial. Se and its placebos were purchased from Webber Naturals Pharmaceutical Company (lot no. LOT778342) and Barij Essence Pharmaceutical Company, respectively. Both Se supplements and placebo capsules had similar packaging, and patients and researchers were unaware of the content of the package until the end of study. Randomisation assignment was performed using computer-generated random numbers. Randomisation and allocation were concealed from the investigators and participants until the final analyses were completed. The randomised allocation sequence, enrolment of participants and allocation to interventions were conducted by a trained staff member at the cardiology clinic. Compliance with the intake of supplements and placebos was determined by examining the tablet containers. In addition, participants received a daily reminder message on their cell phones to take their supplements regularly. All participants completed 3-d dietary records (2 weekdays and 1 weekend day) at weeks 1, 5, 9 and 12 of the trial. To obtain nutrient intakes of participants according to 3-d food records, we applied the Nutritionist IV software (First Databank) adapted for the Iranian food pattern( Reference Azar and Sarkisian 21 ).
Assessment of anthropometric measures
Weight (Seca) was assessed at baseline and after the 12-week intervention in cardiology clinic by a trained staff member. Height (Seca) was determined by a non-stretched tape measure to the nearest 0·1 cm. BMI was determined as weight in kg divided by height squared in m.
Outcomes
Insulin levels and the homoeostasis model of assessment-insulin resistance (HOMA-IR) were considered as the primary outcome, and lipid profiles, biomarkers of inflammation and oxidative stress, and blood pressures were defined as the secondary outcomes. A volume of 10 ml of fasting blood samples was drawn from the antecubital vein at the beginning and after the 12-week intervention at Kashan reference laboratory, Kashan, Iran. Blood was collected in two separate tubes: (1) one without EDTA to separate the serum, in order to quantify serum insulin, lipid profiles and high-sensitivity C-reactive protein (hs-CRP) concentrations, and (2) another one containing EDTA to examine plasma nitric oxide (NO) and biomarkers of oxidative stress. Fasting plasma glucose (FPG) levels were measured on the day of blood collection. Blood samples were immediately centrifuged (D-78532; Hettich) at 3500 rpm for 10 min to separate the serum. The samples were then stored at −80°C until analysis at the KaUMS reference laboratory. Serum insulin levels were assessed by the use of the ELISA kit (DiaMetra) with inter- and intra-assay CV of 3·2–4·5 %, respectively. HOMA-IR and the quantitative insulin sensitivity check index (QUICKI) were determined according to the standard formula( Reference Pisprasert, Ingram and Lopez-Davila 22 ). Enzymatic kits (Pars Azmun) were applied to evaluate FPG, serum TAG, VLDL-, total-, LDL- and HDL-cholesterol levels. Serum hs-CRP levels were determined using a commercial ELISA kit (LDN) with inter- and intra-assay CV of 4·5–6·5 %, respectively. The plasma NO levels were determined using the Griess method( Reference Tatsch, Bochi and Pereira Rda 23 ). Plasma total antioxidant capacity (TAC) levels by the method of ferric-reducing antioxidant power developed by Benzie & Strain( Reference Benzie and Strain 24 ), total GSH using the method of Beutler & Gelbart( Reference Beutler and Gelbart 25 ) and malondialdehyde (MDA) concentrations by the thiobarbituric acid reactive substances spectrophotometric test( Reference Janero 26 ) were determined. CV for plasma TAC, GSH and MDA were lower than 5 %, respectively. All inter- and intra-assay CV for FPG and lipid values were <5 %. Systolic (SBP) and diastolic blood pressure (DBP) was determined by means of a sphygmomanometer (ALPK2; Ningbo TianHou Import and Export Co., Ltd). Blood pressure values were reported in mmHg.
Statistical methods
In the present study, we used the formula suggested for randomised clinical trials’ sample size calculation. Type one (α) and type two errors (β) were defined as 0·05 and 0·20 (power=80 %), respectively. According to the previous trial( Reference Bahmani, Kia and Soleimani 27 ), we used 1·4 as the sd and 1·12 as the change in mean (d) of HOMA-IR as a primary outcome in this formula. On the basis of the formula, we needed twenty-five subjects in each group; after allowing for five dropouts in each group, the final sample size was thirty persons in each group.
Multiple linear regression model was used to assess the intention-to-treat effect of treatment on study outcomes after adjusting for random confounding by the baseline values of outcome, age and BMI. Adjustment for age and BMI is necessary for two reasons: (i) to account for residual random confounding by age and BMI as stratified randomisation was only based on broad categories of these variables, and (ii) to obtain correct standard error for treatment effect( Reference Mansournia, Hernan and Greenland 20 , Reference Mansournia and Altman 28 – Reference Greenland and Mansournia 30 ). Normality of residuals was assessed using normal probability plot and Kolmogorov–Smirnov test. Outcome log-transformation was used if model residual has non-normal distribution (QUICKI, NO, TAC, MDA, SBP and DBP). Bootstrapping was also used as a sensitivity analysis. Bonferroni correction (i.e. multiplying P values by the number of tests) was used to account for multiple outcome testing. However, we note that our outcomes and thus statistical tests will tend to be positively correlated, and thus the Bonferroni procedure, which is based on independence of tests, is very conservative. The paired-samples t test was used to detect within-group differences. P values<0·05 were considered significant. All statistical analyses were performed by the Statistical Package for Social Science version 18 (SPSS Inc.).
Results
Among participants, four participants in the Se group (withdrawn owing to personal reasons (n 4)) and three participants in the placebo group (withdrawn owing to personal reasons (n 3)) did not complete the trial (Fig. 1).
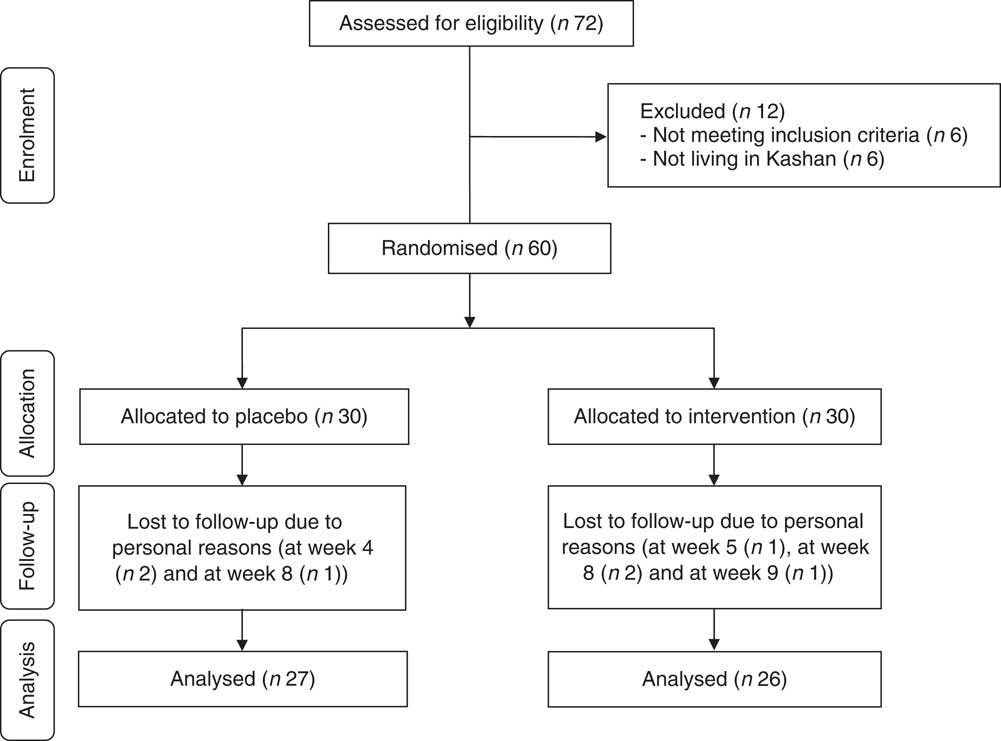
Fig. 1 Summary of patient flow diagram.
Finally, fifty-three participants (Se (n 26) and placebo (n 27)) completed the trial. The rate of compliance in our study ranged between 90 and 100 % in both groups. No side effects were reported after Se supplementation in people with CHF throughout the study.
Mean age, height and weight and BMI at baseline and end of trial of study participants were not statistically different between Se and placebo groups (Table 1).
Table 1 General characteristics of study participants (Mean values and standard deviations; numbers and percentages)
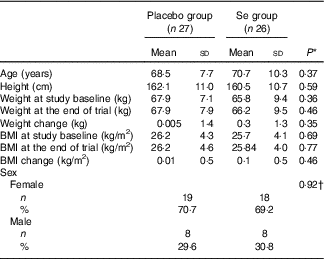
* Obtained from independent t test.
† Obtained from Pearson’s χ 2 test.
On the basis of the 3-d dietary records obtained throughout the intervention, no significant difference was observed between the two groups in terms of micronutrients and macronutrients (Table 2).
After the 12-week intervention, compared with the placebo, Se supplementation led to significant reductions in serum insulin levels (−18·41 (sd 27·53) v. +13·73 (sd 23·63) pmol/l, P<0·001), HOMA-IR (−1·01 (sd 1·61) v. +0·55 (sd 1·20), P<0·001) and a significant increase in QUICKI (+0·007 (sd 0·03) v. −0·01 (sd 0·01), P=0·007) (Table 3). In addition, Se supplementation significantly decreased serum LDL-cholesterol levels (−0·23 (sd 0·29) v. −0·04 (sd 0·28) mmol/l, P=0·03) and total-:HDL-cholesterol ratio (−0·47 (sd 0·31) v. −0·06 (sd 0·42), P<0·001), and significantly increased HDL-cholesterol levels (+0·18 (sd 0·19) v. +0·02 (sd 0·13) mmol/l, P=0·001) compared with the placebo. In addition, taking Se supplements was associated with a significant reduction in hs-CRP (−1880·8 (sd 3437·5) v. +415·3 (sd 2116·5) ng/ml, P=0·01), and a significant elevation in plasma TAC (+30·9 (sd 118·0) v. −187·9 (sd 412·7) mmol/l, P=0·004) and GSH levels (+33·7 (sd 130·4) v. −39·2 (sd 132·8) µmol/l, P=0·003) compared with the placebo. When we applied Bonferroni correction, QUICKI (P=0·11), LDL-cholesterol (P=0·51), hs-CRP (P=0·17), TAC (P=0·06) and GSH (P=0·05) became non-significant, and other metabolic profiles did not alter. Se supplementation did not improve other metabolic profiles. Using bootstrapping analyses, our findings did not change. Within-group changes demonstrated a significant decrease of FPG (P=0·04), serum insulin (P=0·002), HOMA-IR (P=0·004), LDL- (P<0·001), total-:HDL-cholesterol ratio (P<0·001), hs-CRP (P=0.·01) and SBP (P=0·04), and a significant increase of serum HDL-cholesterol levels (P<0·001) in the Se group. In addition, within-group changes revealed a significant increase of serum insulin (P=0·006) and HOMA-IR (P=0·02), and a significant decrease of QUICKI (P=0·001), plasma NO (P=0·02) and TAC levels (P=0·02) in the placebo group.
Table 2 Dietary intakes of study participants at weeks 1, 5, 9 and 12 of the study (Mean values and standard deviations)
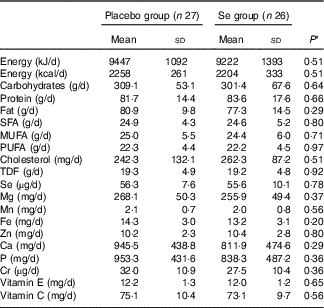
TDF, total dietary fibre.
* Obtained from independent-samples t test.
Table 3 Metabolic profiles, biomarkers of inflammation and oxidative stress at baseline and 12 weeks after the intervention in patients with congestive heart failure (Mean values and standard deviations)
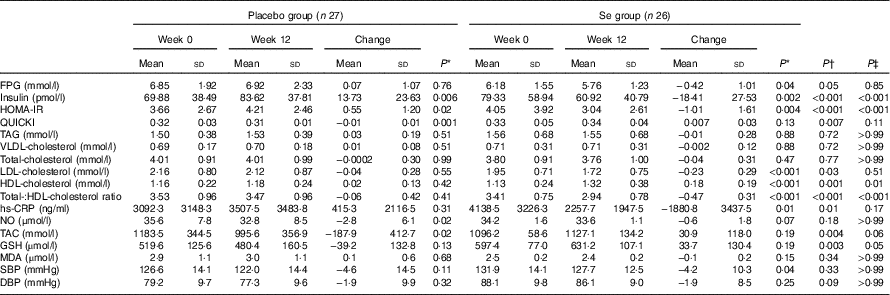
FPG, fasting plasma glucose; HOMA-IR, homoeostasis model of assessment-estimated insulin resistance; QUICKI, quantitative insulin sensitivity check index; hs-CRP, high-sensitivity C-reactive protein; NO, nitric oxide; TAC, total antioxidant capacity; MDA, malondialdehyde; SBP, systolic blood pressure; DBP, diastolic blood pressure.
* Obtained from paired-samples t test.
† Obtained from multiple regression model (adjusted for baseline values of each biochemical variables, age and baseline BMI).
‡ Obtained from multiple regression model and corrected using Bonferroni correction (P value×17).
Discussion
In the present study, which to our knowledge is the first report of Se supplementation in patients with CHF, we evaluated the effects of Se supplementation on markers of insulin metabolism, lipid profiles, biomarkers of inflammation and oxidative stress. The major finding was that Se supplementation improved insulin metabolism, decreased serum LDL-cholesterol, total-/HDL-cholesterol, hs-CRP and increased serum HDL-cholesterol, plasma TAC and GSH concentrations in patients with CHF, but did not improve other metabolic profiles. When we applied Bonferroni correction for multiple outcome testing, QUICKI, LDL-cholesterol, hs-CRP, TAC and GSH became non-significant, and other metabolic profiles did not alter. However, some studies have reported no beneficial effects of Se supplementation on the incidence of diabetes and metabolic profiles in patients with metabolic diseases. Stranges et al.( Reference Stranges, Marshall and Natarajan 31 ) reported that Se supplementation increased T2DM incidence in a randomised controlled trial. In the above-mentioned study, an exposure-response gradient was seen across tertiles of baseline Se levels, with a statistically significantly increased risk for T2DM in the highest tertile of baseline Se levels( Reference Stranges, Marshall and Natarajan 31 ). Such an association has also been documented in a number of observational studies, generally with cross-sectional and prospective design. In a study by Galan-Chilet et al.( Reference Galan–Chilet, Grau-Perez and De Marco 32 ), Se levels were positively associated with prevalent and incident diabetes. Moreover, in US adults, high Se levels were associated with higher prevalence of diabetes and higher FPG and glycosylated Hb levels( Reference Laclaustra, Navas-Acien and Stranges 33 ). The effect of Se supplementation at dosages of 100, 200 and 300 µg/d for 6 months and 5 years to elderly population on plasma cholesterol concentrations or its sub-fractions did not differ significantly from the placebo( Reference Cold, Winther and Pastor-Barriuso 34 ). Se supplementation at a dosage of 100 µg/d as Se yeast in pregnant women for the last 6 months of pregnancy was also associated with increased cord-blood TAG levels, although total-, LDL- and HDL-cholesterol levels did not change significantly( Reference Boskabadi, Maamouri and Rezagholizade Omran 35 ). In addition, the levels found in the previous study were higher than those reported in non-pregnant women from other parts of Iran( Reference Safaralizadeh, Kardar and Pourpak 36 , Reference Rafraf, Mahdavi and Rashidi 37 ). The differences in circulating Se levels are probably owing to the regional and geographic variability in the Se content of soil and plant foods( Reference Rayman 38 ). Different study designs, different dosages of Se used, potential differences in Se status of the participants in different studies along with characteristics of study participants might provide some reasons for discrepant findings. In addition, we could not explore the possibility that the different results might relate to genetic differences. Alternatively, there may have been differences in the intake of dietary macronutrients and micronutrients between different populations that could have modified the effects of additional Se on metabolic profiles. It must be kept in mind that there is a small possibility of selection bias in our study. To decrease potential confounding effects, all participants were categorised according to age, BMI, sex and the dosage and kind of medications at the onset of the study. Then, participants in each category were randomly allocated into two treatment groups to take either Se supplements or placebo. We believe that further studies are needed to confirm our findings.
The present study evidenced that Se supplementation for 12 weeks to patients with CHF resulted in a significant decrease in serum insulin concentrations and HOMA-IR, LDL-cholesterol, total-:HDL-cholesterol and a significant elevation in serum HDL-cholesterol and QUICKI compared with the placebo, but had no significant effect on FPG and other lipid concentrations. When we applied Bonferroni correction for multiple outcome testing, QUICKI and LDL-cholesterol became non-significant, and other metabolic profiles did not alter. Earlier, few studies have reported that CHF was correlated with lower Se and Zn concentrations( Reference Kosar, Sahin and Taskapan 9 , Reference de Lorgeril, Salen and Accominotti 14 ). It has been suggested that Se element may be involved in the cardiac muscles and in CHF symptoms including fatigue and low exercise tolerance, rather than in ventricular dysfunction( Reference de Lorgeril, Salen and Accominotti 14 , Reference de Lorgeril and Salen 15 ). Some observational studies, as well as Se supplementation trials, in which the association between Se levels/Se intake and the risk of diabetes or glycaemic control has been evaluated, generated inconsistent results( Reference Gao, Hagg and Sjogren 39 , Reference Wei, Zeng and Gong 40 ). On the other hand, animal studies demonstrated that Se supplementation may lead to hyperinsulinaemia and glucose intolerance( Reference Zhou, Huang and Lei 41 ). Few studies have assessed the effects of Se supplementation on glycaemic control and lipid profiles in patients with T2DM, CHD and metabolic diseases. In a meta-analysis study, we have previously demonstrated that Se administration to patients with metabolic diseases improved insulin levels and QUICKI, but did not influence HOMA-IR and lipid profiles( Reference Tabrizi, Akbari and Moosazadeh 18 ). Taking Se supplements at a dose of 200 µg/d for 6 weeks resulted in a significant reduction in serum insulin levels and HOMA-IR in subjects with central obesity( Reference Alizadeh, Safaeiyan and Ostadrahimi 42 ). Se supplementation for 4 months to Sprague–Dawley rats lowered TAG levels, whereas other lipid profiles remained unchanged( Reference Kaur and Bansal 43 ). Insulin resistance and hyperinsulinaemia were correlated with inflammation, oxidative stress, cardiac remodelling and endothelial dysfunction that lead to decreasing endothelial NO synthase expression, which in turn result in an increase in vascular tone( Reference Kuboki, Jiang and Takahara 44 ). Furthermore, lipid accumulation in the heart, by the production of toxic intermediate products and derangement of insulin and oxidative pathways, determines conditions known as lipotoxicity and lipoapoptosis that impair cardiac function and promote CHF( Reference van Herpen and Schrauwen-Hinderling 45 ). Se administration may improve insulin metabolism by inhibiting the expression of cyclo-oxygenase-2 and P-selectin( Reference Li, Han and Jiang 46 ) and suppressing the production of inflammatory markers including TNF-α and IL( Reference Brigelius-Flohe, Banning and Kny 47 ).
We found that Se supplementation in patients with CHF resulted in a significant decrease in serum hs-CRP, and a significant increase in plasma TAC and GSH levels, but did not change plasma NO and MDA levels compared with the placebo. When we applied Bonferroni correction for multiple outcome testing, hs-CRP, TAC and GSH became non-significant, and other metabolic profiles did not alter. Supplementation with combined Se and coenzyme Q10 for 48 months to elderly individuals decreased CRP and sP-selectin levels( Reference Alehagen, Lindahl and Aaseth 48 ). Previous studies in comparable groups have demonstrated that Se administration decreased NF-κB activation, and down-regulated gene expression related to inflammatory cytokines in macrophages( Reference Vunta, Davis and Palempalli 49 , Reference Vunta, Belda and Arner 50 ). High-dose Se supplementation for 14 d to sepsis patients did not decrease CRP levels, but Se levels correlated with glutathione peroxidase and prealbumin concentrations( Reference Brodska, Valenta and Malickova 51 ). In another study, Se intake significantly increased MDA and hydroxyl radical levels in the lens of naphthalene-treated rats( Reference Zhu and Lu 52 ). Increased systemic inflammation is a critical element underlying the pathophysiology of CHF, contributing to myocardial remodelling and peripheral vascular damage( Reference Gullestad and Aukrust 53 ). Moreover, increased oxidative stress markers correlate positively with clinical parameters of CHF and their high concentrations are a poor prognostic factor in patients with CHF( Reference Amir, Paz and Rogowski 54 ). Therefore, decreased biomarkers of inflammation and oxidative stress by Se may decrease complications related to CHF.
The present study had a few limitations. For shortage of funding, we did not verify plasma or urine Se levels in the supplement and placebo groups. Whether Se supplementation is beneficial or detrimental depends on Se status before supplementation of this element; Se status was not measured in this study. Few studies have reported that high Se levels were associated with low overall mortality( Reference Bleys, Navas-Acien and Guallar 55 , Reference Akbaraly, Arnaud and Hininger-Favier 56 ). Increasing serum Se levels up to about 130 μg/l were associated with reduced mortality( Reference Bleys, Navas-Acien and Guallar 55 ). In the 9-year longitudinal Epidemiology of Vascular Ageing (EVA) study, plasma Se levels at baseline (mean 87 μg/l) were associated with increased overall and cancer mortality( Reference Akbaraly, Arnaud and Hininger-Favier 56 ). In the Baltimore Women’s Health and Aging Study, low Se level was a significant independent predictor of all-cause 5-year mortality in older women living in the community( Reference Ray, Semba and Walston 57 ). Therefore, this should be considered in our interpretation. In addition, we did not evaluate gene expression related to insulin, lipid and inflammation.
Overall, our study supported that Se supplementation for 12 weeks to patients with CHF had beneficial effects on insulin metabolism and few markers of cardio-metabolic risk.
Acknowledgements
The authors would like to thank the staff of Shahid Beheshti Clinic for their assistance in this project.
The present study was funded by a grant from the Vice-chancellor for Research, KaUMS, Iran.
Z. A. contributed in conception, design, statistical analysis and drafting of the manuscript. F. R., M. B., V. O., F. B., M. A. M. and F. K. contributed in data collection and manuscript drafting. Z. A. supervised the study.
The authors declare that there are no conflicts of interest.






