Se is an essential micronutrient with a wide range of protective functions, which are exerted through its incorporation into selenoproteins( Reference Duntas and Benvenga 1 ). Inadequate dietary Se intake can compromise human reproduction as Se deficiency has been associated with obstetric and perinatal complications such as male and female infertility, miscarriage, pre-eclampsia, intra-uterine growth restriction, preterm delivery and neural defects in the offspring( Reference Pieczyńska and Grajeta 2 – Reference Rayman, Wijnen and Vader 4 ).
The maternal–fetal transfer mechanisms for Se and selenoproteins have been studied in mice( Reference Burk, Olson and Hill 5 ) and in placental models in vitro ( Reference Nandakumaran, Dashti and Al-Saleh 6 ), leading to the identification of some specific placental transporters( Reference Nandakumaran, Dashti and Al-Saleh 6 , Reference Miyauchi, Srinivas and Fei 7 ). Though a number of studies have measured Se concentrations in mothers and newborns at the time of birth, they have had contradictory results( Reference Jariwala, Suvarna and Kiran Kumar 8 – Reference Özdemir, Karadas and Pappas 10 ). To some extent, these contradictions might be explained by differences in Se status between geographic regions( Reference Rayman 11 ), distribution patterns between Se species and by the reliability of the method of determination of Se( Reference Fairweather-Tait, Collings and Hurst 12 ). Whatever the case, those studies were limited by measurement of only total serum/plasma Se, which does not allow distinction between different species of Se that may be transferred from the mother to the baby by different mechanisms.
In investigating the relative concentrations of Se in mothers and newborns it is important to take account of the different Se components in serum/plasma( Reference Combs, Watts and Jackson 13 ): two selenoproteins (selenoprotein P (SeP)( Reference Gladyshev, Arnér and Berry 14 ) and the extracellular glutathione peroxidase (GPx-3)), Se incorporated non-specifically as selenomethionine (SeMet) in lieu of methionine in albumin (selenoalbumin (SeAlb)) and other plasma proteins, and a small amount of non-protein-bound low molecular weight selenometabolites (SeMetab). SeP is the most abundant selenoprotein in plasma and is a good indicator of Se status in non-Se-replete humans( Reference Burk and Hill 15 ). The activity of GPx-3 in human serum is a complementary marker of Se status. Importantly, GPx-3 has been identified as a key enzyme in the defence against oxidative stress during decidualisation (the postovulatory process of endometrial remodelling in preparation for implantation) by reducing H2O2 in the endometrium( Reference Xu, Leng and Gao 16 ).
We have been able to determine these species in the serum from mother–baby pairs at the time of birth owing to our previous development of a two-dimensional chromatographic method for simultaneous speciation of serum GPx-3, SeP, SeAlb and SeMetab( Reference García-Sevillano, García-Barrera and Gómez-Ariza 17 ). Although the accuracy of this method has been assessed by analysing a commercial human serum (BCR-637), it has not previously been applied in a clinical setting.
The aims of the current study were (i) to simultaneously determine Se, selenoproteins, SeAlb and SeMetab concentrations by this new method in both maternal and cord sera from uncomplicated pregnancies at the time of birth; (ii) to compare maternal and neonatal concentrations in order to elucidate potential maternal–fetal transfer mechanisms; (iii) to assess whether SeP measured by two-dimensional size-exclusion and affinity HPLC (2D/SE-AF-HPLC) corresponded to that measured by ELISA and (iv) to assess the coherence between GPx-3 measured both as a concentration and as an enzyme activity. Our study may also help to assess Se requirements in pregnancy, as recommended by the European Food Safety Authority Panel( 18 ).
Methods
A cross-sectional study was performed on eighty-three healthy mother–baby couples. Participants were recruited at random from pregnant women without any maternal or neonatal risk factors, who gave birth at term, at the Department of Obstetrics and Gynecology, Hospital de Riotinto, during the years 2014–2015. Women with diseases, multiple gestation and with perinatal complications such as obstructed labour, low Apgar score (below 5) or cases where there was suspicion of infant pathology were excluded.
A full history was taken from the enrolled mothers, which included whether or not they took a multivitamin/mineral supplement (a standard formula approved by the Department of Health, containing 200 µg iodide (I–), 55 µg Se, as sodium selenite, 10 mg Zn and 200 mg DHA) during pregnancy. Medical data from clinical examination of mothers and babies were also recorded at birth.
This study was approved by the local ethics committee and written informed consent was obtained from all the participants. As we also collected samples from cord blood, the parents provided separate, signed, informed consent for this specific purpose.
Analytical procedures
Blood samples were collected from mothers during the 24 h before delivery. At birth, a sample of cord blood was obtained and its pH was measured. Serum was separated and frozen at –80°C until further analysis.
Se and selenoprotein concentrations were measured by in-series 2D/SE-AF-HPLC, as previously described; this method overcomes common spectral interferences from chloride and bromide( Reference García-Sevillano, García-Barrera and Gómez-Ariza 17 ). In brief, plasma and serum samples were filtered before injecting on to the chromatographic platform, which connected two in-series, stacked, 5 ml HiTrap® Desalting columns (for size exclusion separation; HiTrap-Desalting-Sephadex G-25 Superfine; GE Health Bio-Science AB) and a dual-affinity column arrangement comprising a 1 ml heparin–sepharose column (HEP-HP; able to retain selectively SeP; GE Health Bio-Science AB) and a 1 ml blue-sepharose column (BLU-HP; that retains both SeP and SeAlb; GE Health Bio-Science AB) interconnected by a six-port column-switching valve, which were coupled in series to an inductively coupled plasma MS system (ICP-MS Agilent 7500ce; Agilent Technologies) by means of a 30-cm polyether ether ketone (PEEK) tubing (0·6 mm i.d.) for Se detection. Ammonium acetate 0·05 m (at pH 7·5), phase A and 1·5 m phase B were successively used as the mobile phase. Postcolumn isotope dilution analysis was performed by the introduction of 74Se via a T-connector.
Chromatographic performance was checked regularly by measuring control standards to ensure enough separation between species and to ensure method sensitivity after a considerable number of analyses. The proposed speciation method has been validated using a CRM of human serum (BCR-637) certified for total Se content (Se total=81 (sd 7) ng/ml). The concentration of different Se species in BCR-637 obtained with this method has been compared with previous results for this CRM from Jitaru et al. ( Reference Jitaru, Roman and Barbante 19 ). The relative standard deviation (RSD, %) and the detection limits (DL, ng/g of Se) are as follows: GPx-3, RSD 21 and DL 0·2; SeP, RSD 12 and DL 0·7; SeAlb, RSD 9 and DL 0·9.
SeP concentration was also analysed by ELISA (USCN Business Co., Ltd), and GPx-3 activity was analysed by a commercial kit (Cayman Chemical).
Statistical analysis
Sample size calculation: with thirty-eight subjects per mother–baby group, and a sd of 15 µg/l of Se in mean serum Se concentration of 71 µg/l (obtained from a recent study of Spanish pregnant women (71·2 (sd 14·9)))( Reference Bermúdez, García-Vicent and López 20 ), our study had 80 % power to detect a difference of at least 10 µg/l in total serum Se in mother–baby groups at a two-sided, 5 % significance level. We included eighty-three healthy mother–baby couples.
As our sample included women who took a multivitamin/mineral supplement and women who did not, a comparison of means between these two groups was made to see whether this supplementation had a significant effect. Assuming a two-sided hypothesis, the conventional α and β levels, and a proportion of 3:1 for the studied groups (the use of multivitamin/mineral supplements in our population barely reached a quarter of pregnant women), the sample size calculated was nineteen women for the supplemented group and fifty-seven pregnant women for the non-supplemented group. We included twenty-one and sixty-two women, respectively.
Data are presented as medians and standard deviations for continuous variables and as percentages for categorical variables. The contrast hypothesis for two samples was evaluated using Fisher’s exact test for categorised variables and Student’s t test for continuous variables. The Wilcoxon signed-rank test was used to compare matched samples. The correlation between variables was determined using the Spearman test, designing multiple linear regression models in those cases where it was desired to predict the variance adjusted for other variables, besides the main variable. The contrast hypothesis for more than two samples was determined with an ANOVA. All P values were two-sided, and statistical significance was declared at P<0·05. All data were analysed using SPSS 20.0 (IBM SPSS Statistics).
Results
Maternal and neonatal characteristics
Table 1 shows maternal and neonatal characteristics. As exclusively healthy pregnant women were recruited, the caesarean section rate was only 7·5 % (compared with the usual caesarean section rate at our centre of about 16 %) and perinatal outcomes were good (there were no admissions to the Neonatal Intensive Care Unit).
Table 1 Main characteristics of participants (Mean values and standard deviations)

No correlations were found between maternal age, parity, BMI or level of education and maternal Se concentration, nor were any correlations found between neonatal weight at birth or gestational age with maternal or neonatal serum Se concentrations. However, there was a negative correlation between hours of fasting before blood drawing and total Se concentration in maternal serum (r −0·30; P<0·05).
Women who did not take multivitamin/mineral supplements (containing 55 µg Se) were significantly younger than women who did take them (29·42 (sd 5·35) v. 31·16 (sd 4·80) years, respectively; P<0·05). No significant differences in parity, BMI or level of education were found between women who took multivitamin/mineral supplements and those who did not take them (data not shown).
Selenium and selenoprotein concentrations by two-dimensional size-exclusion and affinity HPLC
Total Se and selenoprotein concentrations in maternal and cord sera are summarised in Table 2. Total Se, GPx-3 and SeP concentrations were significantly higher in maternal serum than in cord serum, whereas SeAlb was significantly more concentrated in cord serum. There was no significant difference in the SeMetab concentration between maternal and cord sera. The chromatograms of maternal and umbilical cord sera are shown in Fig. 1.

Fig. 1 Selenoprotein chromatograms in (a) maternal and (b) umbilical cord sera. GPx-3, extracellular glutathione peroxidase; SeP, selenoprotein P; SeAlb, selenoalbumin.
Table 2 Comparisons between concentrations of total selenium, selenoproteins, selenoalbumin (SeAlb) and selenometabolites (SeMetab) in maternal and cord sera measured by two-dimensional size-exclusion and affinity HPLC (2D/SE-AF-HPLC) (Medians and standard deviations)
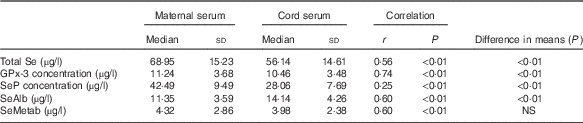
GPx-3, extracellular glutathione peroxidase; SeP, selenoprotein P.
The correlations between maternal and cord Se species were significant in all cases, reaching the highest value for GPx-3 and the lowest for SeP.
The proportion of Se species in maternal and cord sera was different; whereas SeP accounted for 65 % of total Se in maternal serum, it only represented 50 % of total Se in cord serum. By contrast, SeAlb constituted 15 % of total Se in maternal serum but 28 % in cord serum. The percentages for GPx-3 and SeMetab were similar in maternal and cord sera: 14 % for GPx-3 in both, and 7 and 8 % for SeMetab in maternal and cord sera, respectively (Fig. 2(a)). When the Se species concentrations are represented graphically, the different pattern in maternal and cord sera is apparent (Fig. 2(b)).

Fig. 2 Comparison between (a) percentage of selenoproteins and selenometabolites (SeMetab) in maternal and cord sera and (b) concentrations of total selenium, selenoproteins and SeMetab in maternal and cord sera (µg/l). (a): ![]() , selenoprotein P (SeP);
, selenoprotein P (SeP); ![]() , extracellular glutathione peroxidase (GPx-3);
, extracellular glutathione peroxidase (GPx-3); ![]() , selenoalbumin (SeAlb);
, selenoalbumin (SeAlb); ![]() , SeMetab. (b):
, SeMetab. (b): ![]() , cord serum;
, cord serum; ![]() , maternal serum.
, maternal serum.
There were no significant differences in the concentrations of Se species in maternal or cord sera between women who took multivitamins/minerals containing Se and those who did not (Table 3).
Table 3 Concentration of various selenium species in maternal and cord sera in women who took multivitamin/mineral supplement containing selenium during pregnancy and those who did not take such supplements (Medians and standard deviations)
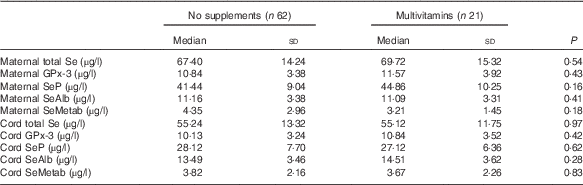
GPx-3, extracellular glutathione peroxidase; SeP, selenoprotein P; SeAlb, selenoalbumin; SeMetab, Selenium metabolites.
Maternal total serum Se was strongly correlated with all the Se species in maternal serum, that is, GPx-3 (r 0·71; P<0·1), SeP (r 0·89; P<0·01), SeAlb (r 0·80; P<0·01) and SeMetab (r 0·42; P<0·01), and moderately correlated with the concentrations of the Se species in cord serum, that is, total Se (r 0·56; P<0·01), GPx-3 (r 0·47; P<0·01), SeP (r 0·43; P<0·01), SeAlb (r 0·45; P<0·01) and SeMetab (r 0·48; P<0·01).
Total Se in cord serum was correlated with all the Se species studied in cord serum, that is, GPx-3 (r 0·78; P<0·1), SeP (r 0·89; P<0·01), SeAlb (r 0·80; P<0·01) and SeMetab (r 0·60; P<0·01), and moderately correlated with the concentration of the Se species in maternal serum, that is, GPx-3 (r 0·64; P<0·01), SeP (r 0·32; P<0·01), SeAlb (r 0·61; P<0·01) and SeMetab (r 0·66; P<0·01).
Negative correlations were found between umbilical cord pH and maternal SeAlb (r −0·33; P=0·01), cord total Se (r −0·30; P=0·04) and cord SeAlb (r −0·30; P=0·02).
Extracellular glutathione peroxidase activity
GPx-3 activity was significantly higher in maternal serum than in cord serum (112·87 (sd 68·91) and 38·67 (sd 25·03) nmol/min per ml, respectively; P<0·01), and there was no correlation between the activities (r 0·12; P=0·48). There was a significant correlation between GPx-3 concentration and its activity in maternal serum (r 0·33; P=0·04), but this correlation was not apparent in cord serum (data not shown). Maternal GPx-3 activity also correlated significantly with total Se concentration in maternal serum (r 0·36; P=0·03) and with SeMetab concentration in cord serum (r 0·50; P<0·01), but not with other Se species in either maternal or cord sera (data not shown). GPx-3 activity in cord serum was significantly correlated with birth weight (r 0·39; P=0·02) and gestational age (r 0·43; P<0·01). This last correlation remained significant after adjusting for birth weight and height (R 2 0·23; P=0·04). However, GPx-3 activity in cord serum did not correlate with total Se or Se species either in cord serum or maternal serum (data not shown).
Selenoprotein P measured by ELISA
SeP concentration determined by ELISA was significantly different in maternal and cord sera (38·27 (sd 12·34) and 1·93 (sd 0·98) µg/l, respectively; P<0·01). No correlation was found between SeP in maternal and cord sera when measured by ELISA. There was no correlation between SeP concentrations measured by HPLC and ELISA in either maternal serum or cord serum (data not shown). Although SeP concentration in maternal serum measured by HPLC and ELISA was not significantly different (42·49 (sd 9·49) and 38·27 (sd 12·34) µg/l, respectively; P=0·10), there was a significant difference between the concentrations obtained by these two methods of determination in cord serum (28·06 (sd 7·69) µg/l by HPLC and 1·93 (sd 0·98) µg/l by ELISA; P<0·01). Maternal SeP concentration measured by ELISA correlated significantly with GPx-3 concentration in cord serum (r 0·33; P=0·04).
Discussion
This is the first study to simultaneously analyse total Se and Se species in maternal and cord sera at the time of birth using a new optimised HPLC method. Although some of these factors have previously been measured at delivery, the interrelationships between the different Se species in maternal and cord sera are largely unknown, as is the biological significance of such relationships. Our findings indicate that, at birth, total Se, GPx-3 and SeP concentrations are significantly higher in maternal serum than in cord serum, whereas the opposite is the case for SeAlb. In general, simple diffusion cannot explain the maternal–neonatal transfer of Se and Se species about the time of birth.
Oxidative stress is tightly related to perinatal morbidity and mortality; therefore, the antioxidant selenoproteins may have a central role in protecting against adverse pregnancy outcomes( Reference Mariath, Bergamaschi and Rondó 21 ). Thus, total Se concentration and GPx-3 activity in cord serum were found to increase with advancing pregnancy( Reference Makhoul, Sammour and Diamond 22 ), whereas oxidative stress during labour was associated with elevated fetal GPx-3 activity( Reference Katzer, Mueller and Welzing 23 ). A study in rats showed that a sufficient Se supply was required to ensure antioxidative protection to the fetus during the oxygen transformations that take place during delivery and early postnatal life( Reference Nogales, Ojeda and Fenutría 24 ). By contrast, in a UK population of slightly higher Se status than ours, maternal blood Se concentrations fell from 12 to 35 weeks of gestation (believed to be partly due to the expansion of plasma volume and partly due to receptor-mediated transfer of SeP from mother to fetus) and maternal GPx-3 activity did not change( Reference Rayman, Bath and Westaway 25 ).
Our study found lower Se concentrations than those reported in other studies of Spanish pregnant women( Reference Bermúdez, García-Vicent and López 20 , Reference Izquierdo Alvarez and Castañón 26 ). Using the most restrictive cut-off point of 70 µg/l of total serum Se concentration required to optimise GPx-3 activity( Reference Combs 27 ), fifty-seven of eighty-three (69 %) pregnant women in our study had insufficient serum Se to fulfil this requirement. However, based on alternative estimates of the range of serum Se concentration required for optimal GPx-3 activity, that is, 89–114 µg/l( Reference Rayman 11 ), 90 % of the women in our sample did not reach this optimal status.
The dietary intake of Se is the major determining factor for serum Se concentration. As we used data from medical records, information on dietary Se intake was not available. However, other factors have been reported to be associated with Se status such as sex( Reference Méplan, Crosley and Nicol 28 , Reference Millán-Adame, Florea and Sáez Pérez 29 ), age, BMI( Reference Méplan, Crosley and Nicol 28 ), education( Reference Stranges, Laclaustra and Ji 30 ), socio-economic status( Reference Méplan, Crosley and Nicol 28 , Reference Stranges, Laclaustra and Ji 30 ) or physical activity( Reference Millán-Adame, Florea and Sáez Pérez 29 ), as well as location( Reference Matos-Reyes, Cervera and Campos 31 ). In this regard, our patients came from a non-urban area in southern Spain where Se intake is traditionally low( Reference Matos-Reyes, Cervera and Campos 31 ). Furthermore, most of them were overweight or obese, had a low level of education and had never worked or were unemployed. All these factors lead us to speculate that they were unlikely to eat the traditional diet with high consumption of vegetables and cereals( Reference Sánchez, López-Jurado and Aranda 32 ).
It might have been expected that pregnant women who took multivitamins/multiminerals containing sodium selenite would have had significantly higher concentrations of at least some Se species than those who did not, as sodium selenite can be used for the synthesis of selenoproteins such as GPx-3 or SeP (though it will not increase SeAlb)( Reference Fairweather-Tait, Collings and Hurst 12 ). However, the absence of higher concentrations in the women on supplements is not altogether surprising as the Se dose was relatively low (55 µg/d); only 25 % of the women took such supplements and we have no data on their compliance. Furthermore, in vivo human studies showed that selenite is only moderately absorbed (between 56 and 85 %)( Reference Moreda-Piñeiro, Moreda-Piñeiro and Bermejo-Barrera 33 ).
In recent years, Se status in maternal and cord blood at delivery has been determined in a number of studies, though with varied outcomes( Reference Al-Saleh, Al-Rouqi and Angela 34 – Reference Yang, Bao and Fu 36 ). Al-Saleh et al. ( Reference Al-Saleh, Al-Rouqi and Angela 34 ) examined the role of Se in reducing oxidative stress induced by Cd and its impact on birth measures. They concluded that the extent of benefit afforded by Se is not governed only by its concentration but also by the different chemical forms of Se that interact with various proteins. Chen et al. ( Reference Chen, Myers and Wei 35 ) attempted to clarify concentrations for Cd, Hg, Pb and Se in mothers and newborns and their placental transfer. Finding a high degree of maternal–fetal Se transfer, they concluded that there was free trans-placental passage of Se from mother to fetus. However, our results do not support that conclusion; the different pattern of transfer of SeP and SeAlb implies that different mechanisms are involved in the transfer of these different Se species from mother to fetus.
It appears likely that receptor-mediated uptake exists in humans, as previously found in pregnant mice( Reference Burk, Olson and Hill 5 ). Burk et al.( Reference Burk, Olson and Hill 5 ) identified two mechanisms of Se transfer; from early to mid-gestation, plasma GPx-3 and SeP were transported via the uterine fluid, probably by pinocytosis, whereas in the latter half of gestation, placental transfer of maternal SeP occurred through the apoE receptor 2. In fact, the higher levels of SeAlb in cord serum compared with the levels in maternal serum suggest the existence of a further maternal–fetal Se transfer mechanism( Reference Burk, Olson and Hill 5 , Reference Anan, Ogra and Somekawa 37 ). Studies in mice and rats suggest that this other pathway involves the transfer of Se as SeMet-containing proteins or SeMet itself (via methionine transporters) to the fetus( Reference Burk, Olson and Hill 5 , Reference Anan, Ogra and Somekawa 37 ). This mechanism depends on the amount of SeMet in the diet of the mother, appears to be unregulated and is highly dependent on the mother’s Se status; thus, it will be less effective under conditions of Se deficiency( Reference Burk, Olson and Hill 5 ). The similar concentrations for SeMetab and their strong correlation in maternal and cord sera can be explained by a simple diffusion mechanism of trans-placental passage that does not occur for other Se species.
GPx-3 activity was measured to see whether there was a correlation between GPx-3 concentrations and GPx-3 activity. As expected, the GPx-3 activity in maternal serum significantly correlated with maternal total Se and GPx-3 concentrations, but GPx-3 activity in cord serum only correlated with gestational age and birth weight. These results are in agreement with previous findings( Reference Darlow, Inder and Graham 38 ); it seems that GPx-3 activity in mothers at delivery is related to maternal Se status, whereas the GPx-3 activity in cord serum depends on gestational age( Reference Makhoul, Sammour and Diamond 22 ).
Finally, our study compared different methods of determining SeP: 2D/SE-AF-HPLC and ELISA. The growing replacement of traditional immunoassay techniques by liquid chromatography tandem MS has highlighted the advantages and limitations of each system as well as the disparity in results by the two methods( Reference Taylor, Keevil and Huhtaniemi 39 ). These two methods have been previously used and published( Reference García-Sevillano, García-Barrera and Gómez-Ariza 17 , Reference Yang, Hwang and Choi 40 ). In our sample, the concentration of SeP in maternal serum measured by both methods gave a similar result, but this was not the case in cord serum where SeP concentration measured by ELISA was significantly lower than that measured by HPLC, and did not correlate with any other parameters. Although these results may suggest that ELISA is not a reliable method of quantifying SeP in cord serum, other explanations are possible. SeP has three transcripts in humans, although all variants begin with the same non-coding exon( Reference Ballihaut, Kilpatrick and Kilpatrick 41 ). The most abundant transcript in all tissues was SePa. However, all variants (SePa, b and c) showed tissue and developmental stage-specific expression patterns in neonatal and adult tissues( Reference Ballihaut, Kilpatrick and Kilpatrick 41 ). These transcripts can synthesise different isoforms, varying in size and Se content( Reference Ballihaut, Kilpatrick and Kilpatrick 41 ), as previously proposed( Reference Akesson, Bellew and Burk 42 ). Moreover, the silencing of each transcript results in a differential synthesis of SeP isoforms( Reference Ballihaut, Kilpatrick and Kilpatrick 41 ). In this respect, the antibodies used in ELISA kits could have a different affinity for the SeP isoforms present in serum. This could affect the binding of the antibody to the SeP protein in the ELISA and therefore give different results than techniques that do not use antibodies. A further determination of SeP levels by the use of kits from other suppliers could be considered but, unfortunately, we have no remaining cord blood samples. Alternatively, this discordance in the results of SeP concentration between 2D/SE-AF-HPLC and ELISA may suggest that, in the newborn, the predominant SeP isoform is different from that found in the mothers and, perhaps, with different functions( Reference Ballihaut, Kilpatrick and Kilpatrick 41 ). It is also likely that full-length SeP has a higher Se content than the shorter isoforms( Reference Burk and Hill 15 ). It has been suggested that while the liver exports full-length SeP, other tissues secrete mostly shorter SeP isoforms, possibly for local use as a redox enzyme or as a signalling molecule( Reference Burk and Hill 15 ). Our finding supports the hypothesis that circulating truncated SeP isoforms might not reach the concentrations necessary for successful identification by ELISA( Reference Hybsier, Schulz and Wu 43 ).
The current study has some limitations. First, the absence of a FFQ did not allow us to assess Se intake. Furthermore, the small proportion (25 %) of women taking a supplement containing Se and the lack of compliance data may have prevented us from finding significantly higher markers of status in that group.
Our study has also certain strengths; our design made possible the collection of data and samples from healthy pregnant women and their newborns at the time of birth. The multiple biomarkers studied from the same patients and the availability of a robust and reliable method to simultaneously determine different Se species gave us an insight into the relationships between these Se species in mothers and newborns.
Conclusion
There is a different pattern of Se species found in maternal and cord sera at the time of birth, which suggests the existence of different mechanisms of trans-placental passage of Se species from mother to fetus, which are species dependent.
The lack of concordance in the SeP concentration measured by 2D/SE-AF-HPLC and ELISA suggests the possibility that the predominant SeP isoform in the newborn may be different from that found in the mother; this possibility needs to be followed up in a later study.
Acknowledgements
The authors sincerely thank all the mothers and their babies for voluntarily participating in the study and the staff of the Maternity ward for their involvement and support.
This study was undertaken with the aid of a grant from the Consejería de Salud of the Junta de Andalucía, Spain (PI-0373/2012) and by projects CTM2012-38720-C03-01 from the Spanish Ministry of Economy and Competitiveness (MINECO) and P12-FQM-0442 from the Regional Ministry of Economy, Innovation, Science and Employment (Andalusian Government, Spain). B. C.-L. thanks the Spanish Ministry of Education, Culture and Sport for a doctoral grant.
C. S. and I. V. designed the study, recruited participants, collected the samples and wrote the manuscript. E. G.-F. performed the biochemical tests and the statistical analysis. T. G.-B. and J. L. G.-A. designed the analytical experiments, the instrumental couplings and supervised the results. C. S. and B. C.-L. performed the analytical experiments and the tandem MS analysis. M. P. R. contributed intellectually to the study and to the writing of the manuscript.
The authors declare that there are no conflicts of interest.








