Thailand, similar to many other countries in Southeast Asia, has undergone rapid socio-economic development accompanied by increased urbanisation and Westernisation during the past couple of decades(Reference Kosulwat1, Reference Cohen2). This development with advances in technology has resulted in marked changes in lifestyle, diet and physical activity(Reference Kosulwat1, Reference Jitnarin, Kosulwat and Rojroongwasinkul3, Reference Jones4). Unfortunately, along with the economic development, overnutrition has been on the rise, particularly in urban areas(Reference Kosulwat1, Reference Oner, Vatansever and Sari5–Reference Sakamoto, Wansorn and Tontisirin7). Similar changing patterns for health and nutritional status have been observed in rural areas in recent years(Reference Kosulwat1, Reference Hirata, Kuropakornpong and Funahara8). More alarmingly, the rapid rise in childhood obesity and the incidence of diet-related chronic diseases such as non-insulin-dependent diabetes are being reported at progressively younger ages(Reference de Onis and Blössner9–Reference Hossain, Kawar and Nahas11). Concurrently, undernutrition and, more specifically, stunting and subclinical micronutrient deficiencies continue to be challenges, despite the implementation of successful community-based nutrition programmes during the 1980 s in children aged < 5 years, school-aged children and pregnant/lactating mothers(Reference Hunt12, Reference Behrman, Alderman and Hoddinott13). It is known that undernutrition can contribute to compromised physical and cognitive abilities in children. It is speculated that with changing family lifestyles, food accessibility and social environment, infant and young child feeding practices, dietary patterns and physical activities will also change dynamically(Reference Kosulwat1, Reference Jitnarin, Kosulwat and Rojroongwasinkul3, Reference Hirata, Kuropakornpong and Funahara8, Reference de Onis and Blössner9, Reference Hunt12, Reference Doak, Adair and Monteiro14). The recent National Nutrition Survey in Thailand has demonstrated that in children aged 1·0–5·9 years the prevalence of underweight and stunting is higher in rural children than in urban children (underweight 5·3 v. 3·5 %; stunting 6·6 v. 5·8 %). In children aged 6·0–12·9 years, the prevalence of obesity is higher in urban children (11·8 %) than in rural children (7·3 %)(Reference Aekplakorn15).
Micronutrient deficiencies, either alone or coexisting, have been reported among Thai school children(Reference Thurlow, Winichagoon and Pongcharoen16). Fe deficiency (ID) has long been identified as a public health problem, but recently it has been shown that vitamin D deficiency is an emerging concern for Asian populations. According to the Ministry of Public Health, though the prevalence of anaemia in Thai pregnant women decreased from 27·3 % in 1988 to 10·6 % in 2005, anaemia remains a health burden for lactating women and children(17). Limited data have shown that the prevalence of anaemia in children aged < 5 years is about 25 % and between 32 and 62 % in young infants(Reference Winichagoon18). Similar data on vitamin D status of Thai children are still scarce. Global estimates, however, suggest that 1 billion people worldwide have vitamin D deficiency (VDD) or insufficiency(Reference Holick19). Children and young adults are potentially at a high risk for developing VDD. Nutritional rickets exists as a public health problem with significant morbidity in many Asian countries(Reference Thacher, Fischer and Strand20).
‘Double burden of malnutrition’ affects Thailand and other countries in the development transition(Reference Florentino21). However, there are no large-scale surveys that have addressed the present nutrition situation comprehensively. Hence, the objective of the present study was to evaluate the nutritional status, anthropometry and blood parameters of children aged 0·5–12·9 years in both rural and urban Thailand.
Subjects and methods
The Nutrition Survey of Thai children was a part of the South East Asian Nutrition Survey (SEANUTS), which was a multi-centre study carried out in 16 744 children aged 0·5–12·9 years in four countries (Thailand, Indonesia, Malaysia and Vietnam). The study was registered in the Netherlands Trial Registry as NTR2462. It was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures were approved by the committee on human rights related to research involving human subjects of the Faculty of Medicine Ramathibodi Hospital, Mahidol University (MURA 2010/467), Thailand. Written informed consent was obtained from the parents or caretakers of all participants.
Study population
The cross-sectional survey was conducted in 3119 children aged 0·5–12·9 years from January to August 2011. A multistage cluster sampling method was used to select participants from four regions, i.e. central, north, northeast and south regions of Thailand (seventy-six provinces), including Bangkok. In the first stage of sample selection, random sampling was done to select the representative province for each region, one province from the central, north and south regions and two provinces from the northeast region as the largest part of the country. Bangkok, the capital city, was chosen as one study site due to its difference in characteristics compared with other provinces in each region that might influence the lifestyle and was considered as urban. In the second stage, each province (except Bangkok) was divided into urban (municipal area) and rural (non-municipal area) areas, and then villages in each area were randomly selected. Within each village, a random sample of households was selected, and only one child per household was recruited. The number of children in each region was estimated based on the proportion of children in the age group 0–14 years in the population of each region as well as the ratio of the population in urban and rural areas. The sample was then weighted to reflect the distribution of sex, age, region and area of residence in the general population within Thailand (population data from 2010 from the Department of Provincial Administration, Ministry of Internal Affairs, compiled by the National Statistical Office, Thailand, unpublished results). The comparison of different parameters was made by residential area and three age group categories (0·5–2·9, 3·0–5·9 and 6·0–12·9 years) to represent the life pattern changes in infants and children. These age categories are commonly used in Thailand.
Data collection
Trained staff carried out all the measurements, including structured questionnaire administration, anthropometric measurements and blood withdrawal. Information on the study population and on the sociodemographic characteristics of their parents or caretakers was obtained by interviewing the parents or caretakers using questionnaires.
Anthropometric measurements
Body weight and height were measured in all the children. Weight was measured with a Seca digital weighing scale model 882 (GmbH&Co. KG) to the nearest 0·1 kg. Height was measured in the standing position for children aged >2 years with a microtoise (Stanley-Mabo Limited) to the nearest 0·1 cm. Length in children aged < 2 years was measured in the supine position with a wooden measuring board to the nearest 0·1 cm. The instruments were calibrated daily. BMI was calculated as body weight divided by height squared (kg/m2).
The standard deviation scores (z-scores) of weight, height/length and BMI were derived using the age- and sex-specific WHO growth references for 0–5 years(22) and for 5–19 years(Reference de Onis, Onyango and Borghi23). Anthropometric status was assessed using the following indicators: weight-for-age z-scores < − 2 sd for underweight; height-for-age z-scores < − 2 sd for stunting; BMI-for-age z-scores < − 2 sd for thinness, ≥ 1 to ≤ 2 sd for overweight and >2 sd for obesity.
Skinfold thickness was measured at four sites (tricipital, bicipital, subscapular and suprailiac) to the nearest 0·2 mm using a Holtain caliper (Holtain Limited)(Reference Lohman, Roche and Martorell24). Measurements were taken in triplicate, and the average skinfold thickness value was used in the statistical analyses. Mid-upper arm circumference was measured on the left arm, at the midpoint between the acromion process and the olecranon process of the ulna. Waist circumference was measured midway between the lower rib margin and the iliac crest using a Seca 201 (Seca Corp. Chino, CA, USA) measuring tape to the nearest 0·1 cm(25).
Biochemical assessment
Of the total subjects, 20 % of the infants and children were sampled for blood collection. In children aged < 3 years, 10 μl of blood were collected through finger prick in a microcuvette to the brim. Hb concentration was measured using the HemoCue instrument (HemoCueHb201+, HemoCue Diagnostics B.V. Eindhovern, The Netherlands). In children aged >3 years, 5 ml of fasting venous blood were collected. Blood was collected into two tubes, 2 ml in each tube containing EDTA for the determination of complete blood count. Hb concentration was measured using a colorimetric method, and another 3 ml of whole blood were subsequently separated from the serum for the determination of retinol, soluble transferrin receptor (s-TfR) and total 25-hydroxyvitamin D (25(OH)D) concentrations. s-TfR concentration was determined using an enzyme immunoassay(26) (Ramco Lab, Inc.). Serum retinol concentration was determined using a HPLC technique, and retinyl acetate was used as an internal standard. Serum 25(OH)D concentration was determined using a chemiluminescence immunoassay (LIAISON®, Diasorin, Inc.). The intra-assay and inter-assay CV were 1·0 and 2·1 % for Hb, 6·2 and 7·1 % for s-TfR, 1·3 and 4·7 % for serum retinol and 3·9 and 5·5 % for serum 25(OH)D. The cut-off points to define anaemia in children aged < 5, 5–11 and 12–14 years were Hb concentrations < 110 g/l, < 115 g/l and < 120 g/l, respectively(26). A cut-off value for s-TfR concentration >8·3 mg/l(Reference Phiri, Calis and Siyasiya27) was used to define ID. ID anaemia (IDA) was defined as the combination of anaemia and ID. The cut-off value of serum retinol concentration < 0·7 μmol/l was used to define vitamin A deficiency (VAD)(Reference de Pee and Dary28). The prevalence of VDD was based on the criterion that serum 25(OH)D concentration was < 50 nmol/l(Reference Mithal, Wahl and Bonjour29, Reference Greer30).
Dietary intake
Dietary intake data were collected using a 1 d 24 h recall. The computer-assisted recall was completed by the mothers or caretakers for younger children (0·5–9·9 years), and children aged ≥ 10 years attended the recall interview by themselves. Portion sizes were estimated using measuring cups and spoons. Pictorial food models of commonly eaten foods were also used to closely estimate the amount consumed. In addition, cooked rice (source of staple food) as normally consumed was weighed during the interview. Food recall data were converted into nutrient intakes using the INMUCAL-N V. 2.0 computer software (Institute of Nutrition, Mahidol University).
As only one single 24 h recall had been collected, the distribution of observed intakes had to be adjusted to partially remove the day-to-day variability in intakes (within-person variation)(Reference Barr, Murphy and Poos31), for example, by adjusting the nutrient intake distribution using external variance estimates derived from Thai children of the same age and sex from the Thai National Food Consumption Survey conducted in 2003–4(Reference Jitnarin, Kosulwat and Rojroongwasinkul32). The Personal Computer Version of Software for Intake Distribution Estimation (PC-SIDE) (version 1.0, 2003; available from the Department of Statistics, Iowa State University) produces an empirical estimate of the usual nutrient intake of each age subgroup(Reference Dodd33, Reference Dodd34).
Power analysis
The power analysis for estimating the number of participants to be included in the present survey was based on two age groups (0·5–5·9 and 6·0–12·9 years) and the potential occurrence (prevalence) of anaemia (estimation: 22 %).
The formula(35) used was as follows:
in which n is the total number of participants in each group, Z is the confidence level (α: 0·05 and Z: 1·96), p is the prevalence (%) of anaemia, DEFF is the estimated design effect (ratio of the actual variance within the sampling method used to that of simple random sampling, in the present study estimated as 2·0) and tolerable error is the level of specificity, being 3 % for anaemia. The outcome of the calculation revealed the total number of children to be included as approximately 1545 per age group, applying a response rate of 95 %. Therefore, in the present study, a total of 3100 was used.
With respect to blood collection, a randomised (per region and province for both urban and rural areas) subsample of approximately 20 % of the total number of children was selected. As stated above, in children aged < 3 years, blood was collected through a simple finger prick, whereas in older children venous blood was sampled. This 20 % subsample was based on the same formula used to quantify the total number of children, using the same information on anaemia, applying a prevalence of 22 % and accepting a response rate of 95 % and a tolerable error of 4·5. The latter was done to compensate for a potential power-limiting effect, not only due to physical variations but also due to methodological errors.
Representation of the collected data
Since the total number of children per province and per country was only available for 2010, the growth rate information in 2011 per sex per year was used to calculate actual numbers in the present study. Furthermore, the total number of children per age year in Thailand is different, and weighted factors were applied to obtain this distribution. Moreover, the number of children living in rural areas is approximately twice as that of children living in urban areas, which reflects in the proportion of sampled children between both the strata in the present study. Hence, data collection in the present study was carefully considered based on various factors involved and should, therefore, represent the country information.
Statistical analyses
All statistical analyses were performed on weighted data. ANCOVA was used to compare the mean differences in anthropometric, nutrient intake and biochemical data between the residential areas adjusted by age. Furthermore, differences in the proportion of anthropometric and biochemical status indices by residential area were examined using the χ2 statistic. The analyses were carried out using the STATA/IC 12.1 for Windows (StataCorp LP). Statistical tests were considered significant at P< 0·05.
Results
Subject characteristics
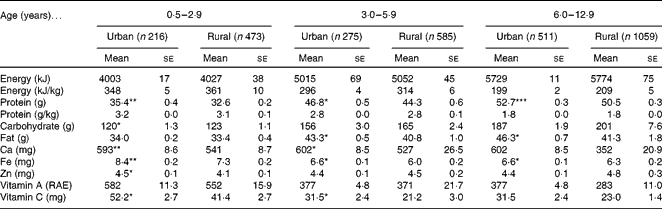
A total of 3119 children aged 0·5–12·9 years participated in the present cross-sectional study. Table 1 describes the anthropometric characteristics of children by age group and residential area. The household income of the parents in urban areas was three times higher than that of those in rural areas (34 908+5367 v. 11 027+2168 baht, 1US$ = 30 baht). Compared with only 6 % of the rural mothers, one-third of the urban mothers received their bachelor degree or a higher degree. The remaining rural mothers received a secondary school or lower degree (data not shown).
Table 1 Anthropometric characteristics of children, by age group and area of residence (Mean values with their standard errors)

Mean values were significantly different from those of the rural areas: * P< 0·05; ** P< 0·01; *** P< 0·001 (ANCOVA, corrected for age).
† Sum of four skinfolds: sum of bicipital, tricipital, subscapular and suprailiac skinfolds.
Among all the participants, at the age of 6·0–12·9 years, urban children had greater weight, height, BMI, arm and waist circumferences and sum of four skinfolds than the rural children. In the younger age group, only height was significantly different between urban and rural children, but from age 3 years onwards. Weight and arm and waist circumferences were greater in urban children.
Nutritional status
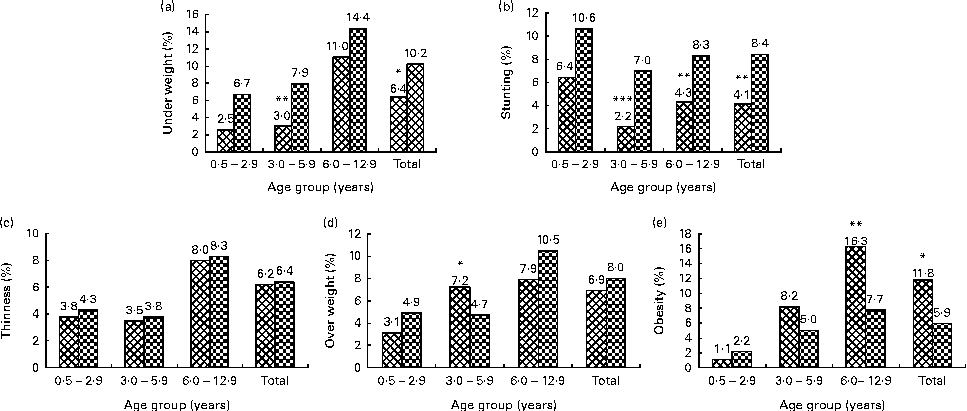
Figure 1 shows the prevalence of underweight, stunting, thinness, overweight and obesity. Underweight and stunting were more prevalent in rural children than in their urban counterparts, although the differences were not always significant in the separate age groups. The prevalence of thinness and overweight did not show a clear difference between urban and rural areas. In contrast, obesity was more prevalent in urban children, especially in the older age groups.

Fig. 1 Prevalence (%) of anthropometric indices, by age group and area of residence. Values were significantly different between the urban (![]() ) and rural (
) and rural (![]() ) areas: * P< 0·05; ** P< 0·01; *** P< 0·001 (χ2 test). Classification based on the WHO: (a) underweight, weight-for-age z-scores < − 2 sd; (b) stunting, height-for-age z-scores < − 2 sd; (c) thinness, BMI-for-age z-scores < − 2 sd; (d) overweight, BMI-for-age z-scores >2 sd to ≤ 3 sd in children aged ≤ 5 years and BMI-for-age z-scores >1 sd to ≤ 2 sd in children aged >5 years; (e) obesity, BMI-for-age z-scores >3 sd in children aged ≤ 5 years and BMI-for-age z-scores >2 sd in children aged >5 years.
) areas: * P< 0·05; ** P< 0·01; *** P< 0·001 (χ2 test). Classification based on the WHO: (a) underweight, weight-for-age z-scores < − 2 sd; (b) stunting, height-for-age z-scores < − 2 sd; (c) thinness, BMI-for-age z-scores < − 2 sd; (d) overweight, BMI-for-age z-scores >2 sd to ≤ 3 sd in children aged ≤ 5 years and BMI-for-age z-scores >1 sd to ≤ 2 sd in children aged >5 years; (e) obesity, BMI-for-age z-scores >3 sd in children aged ≤ 5 years and BMI-for-age z-scores >2 sd in children aged >5 years.
Dietary intake
The mean daily intakes of macronutrients and micronutrients are given in Table 2. Generally, intakes were not much different between urban and rural children, but for some nutrients in some age groups, differences reached a significance level, with mostly urban intakes being higher than the rural intakes.
Table 2 Daily nutrient intakes of children, by age group and area of residence (Mean values with their standard errors)

Mean values were significantly different from those of the rural areas: * P< 0·05; ** P< 0·01; *** P< 0·001 (ANCOVA, corrected for age); RAE, Retinoic acid equivalents.
Prevalence of iron, vitamin A and vitamin D deficiencies
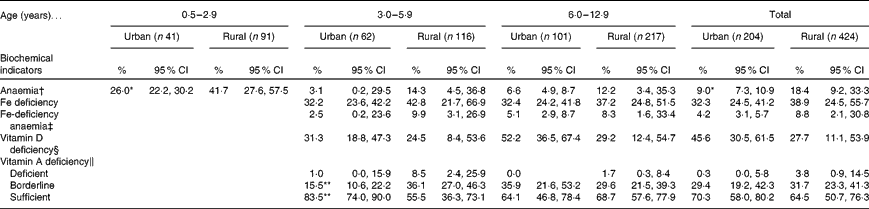
The prevalence of micronutrient deficiencies is summarised in Table 3. Using Hb as an indicator, a significantly higher prevalence (P< 0·05) of anaemia in infants and young children was found in rural areas than in urban areas. Almost 40 % of the children had Fe deficiency, on using s-TfR as an indicator, with no difference being observed between the residential areas. The prevalence of IDA was about 10 %, with no difference being observed between the rural and urban areas. The prevalence of VAD was low, with no difference being observed between the residential areas. The prevalence of VDD was more pronounced in urban children than in rural children; however, no significant difference was observed. About half of the urban children aged 6·0–12·9 years had VDD, whereas one-third of the rural children were reported to have it in the present study.
Table 3 Prevalence of nutritional status from biochemical indicators in children, by age group and area of residence (Percentages and 95 % confidence intervals)

Values were significantly different from those of the rural areas: * P< 0·05 and ** P< 0·01 (χ2 test).
† Hb concentrations: < 110 g/l in children aged < 5 years, < 115 g/l in children aged 5–11 years and < 120 g/l in children aged 12–13 years.
‡ Serum transferrin receptor concentration >8·3 mg/l and Hb concentration < cut-off.
§ 25-Hydroxyvitamin D concentration < 50 nmol/l.
∥ Based on serum retinol concentration: deficient, < 0·7 μmol/l; borderline, 0·7–1·05 μmol/l; sufficient, >1·05 μmol/l.
Discussion
The Thai SEANUTS is a cross-sectional survey that collected data from Thai children aged 0·5–12·9 years in both urban and rural areas. The present study concentrated on the nutritional status, nutrient intake and biochemical indicators such as vitamin A, vitamin D and Fe status. The same information is available from other SEANUTS countries in the region, i.e. Malaysia, Indonesia and Vietnam.
Nutritional status
Overweight and obesity are problems that are on the rise in Thailand. The Thai National Health Examination Survey has reported an increasing prevalence of childhood obesity from 5·8 % in 1997 to 7·9 % in 2001 for children aged 2–5 years and from 5·8 to 6·7 % for children aged 6·0–12·9 years(Reference Aekplakorn and Mo-suwan36). Moreover, in 2009, the prevalence increased further in the two age groups to 8·5 and 8·7 %, respectively(Reference Aekplakorn15), with an increase especially in the urban areas.
The results from the present study reveal a further increase in obesity up to 16·3 % in urban children aged 6·0–12·9 years. Though the prevalence of obesity was higher in urban children aged 6·0–12·9 years, interestingly that of overweight was higher in rural school children.
The prevalence of stunting in rural children was twice as high as that in urban children, with the highest prevalence being observed in the age group 0·5–2·9 years. Underweight tended to be higher in rural children with a relatively high prevalence of 2·5–14 % depending on the age group and area of residence. On the other hand, thinness (BMI-for-age z-scores < − 2 sd) was not different between urban and rural areas and was at a level of 4 % (youngest age group) to 8 % (oldest age group).
Underweight and thinness are most prominent in populations in Southeast Asia and Africa. In school-aged children in Latin America, the prevalence of underweight and thinness is generally lower and less than 10 %. Overnutrition is primarily prevalent in Latin American countries, where about 20–35 % of the school-aged children are overweight and 10–20 % are obese. In Africa, Asia and the Eastern Mediterranean, the prevalence of overweight and obesity together is generally below 15 %. Overnutrition is mainly detected in urban populations from all the reviewed regions(Reference Best, Neufingerl and van Geel37). Both undernutrition and overnutrition during the school age years have detrimental impacts on the development and health of children. Stunting is associated with long-term consequences, including impaired intellectual achievement and school performance(Reference Frongillo38, 39) and small body size in adulthood(39). The underlying causes of undernutrition might be the poor child feeding practice and low food intake in both urban and rural areas(40).
Thinness defined as BMI-for-age z-scores < − 2 sd has been adopted to indicate recent nutritional deprivation. This indicator demonstrated insufficient dietary intake that inevitably results in adverse health outcomes. The immediate health consequences of being excessively thin in the school-aged children include delayed pubertal maturation and reduced muscular strength and work capacity(25). In the other extreme nutritional problems, overweight and obesity also have a negative impact on health, which includes metabolic syndrome indicators as well as psychological disturbance(Reference Cook, Weitzman and Auinger41). The alarming consequence of obesity, among other things, is a greater risk factor for the onset of type 2 diabetes in children(42). Both overnutrition and undernutrition are important public health problems to deal with in transition countries. Proper strategies to alleviate the immediate and the long-term health consequences are crucial and needed.
Macronutrient and micronutrient intakes
The results of nutrient intakes in the present study were adjusted to get the usual intake distribution. The possibility of using an appropriate external estimate of within-person variability to analyse a subgroup of interest in relation to the Dietary Reference Intake (DRI) has been suggested(Reference Barr, Murphy and Poos31, Reference Murphy and Poos43–Reference Murphy45). As the present study has no replicate day of recall, we can estimate within-person variation from our previous datasets(Reference Jitnarin, Kosulwat and Rojroongwasinkul32) using PC-SIDE (Department of Statistics, Iowa State University)(Reference Dodd33, Reference Dodd34). When the intake distribution is adjusted using an external variance estimate from a different population, the prevalence estimate is much less biased. This adjustment provides a more realistic estimation of the prevalence in the population at risk of inadequate nutrient intakes than when the distribution is unadjusted(Reference Jahns, Arab and Carriquiry46). Although the method for adjusting distributions can effectively account for some of the variations in dietary intake data, others such as over- and under-reporting of certain foods or the inaccuracies present in the food composition database cannot be addressed satisfactorily in this way(Reference Carriquiry47).
In the present study, the energy intake of the majority of children was low compared with the estimated average recommendation (data not shown). This finding seems to be contradictory with the existing relatively high prevalence of overweight and obesity. However, bias towards underestimation of energy intake has been reported previously(Reference Black, Goldberg and Jebb48). Since an overnutrition problem is the outcome of a long-term positive energy balance, other lifestyle factors such as physical activity of children must also be considered.
The present study indicates that more than 50 % of Thai children have a low intake of Ca, Fe, Zn, vitamin A and vitamin C. This value was similar to the results from previous reports(Reference Aekplakorn and Satheannoppakao49). In the infant group, the results might be due to low micronutrient content in complementary foods(40). In pre-school and school children, low intakes might be due to the poor quality of foods. Childhood is the period of life when dietary habits are being formed and may persist in adult life. Food consumption patterns in childhood tend to be associated with subsequent risks of developing chronic diseases in adult life(Reference Kalkwarf, Khoury and Lanphear50, Reference Nicklas51). In other words, establishing a healthy diet in early childhood may be one way of contributing to the prevention of future morbidity and mortality.
Micronutrient deficiencies
Anaemia results from a wide variety of causes, and the most significant nutritional factor of anaemia is ID. The global prevalence of anaemia was 47·4 % in pre-school children and 25·4 % in school-aged children as reported by the WHO in 2008(52), and hence IDA is one of the major public health problems worldwide, particularly in developing countries. The present results indicate an overall prevalence of anaemia between 9·0 and 18·4 % among Thai infants and children using Hb as a proxy indicator. When using s-TfR as an indicator, the prevalence of ID was remarkably higher than that of anaemia: 32·3–38·9 % for all areas, implying that ID is an important nutritional problem in Thai children. Another study has reported a prevalence of IDA in school children as low as 4 % using low Hb and serum ferritin cut-offs(Reference Aekplakorn15). The high prevalence of IDA requires policy action at the community level to alleviate the problem. The use of s-TfR index has the advantage that it can differentiate ID from anaemia resulting from other chronic deficiencies(Reference Baille, Morrison and Fergus53, Reference Cook54), and levels of s-TfR are thought to be directly proportional to a functional Fe deficit after Fe status is depleted(Reference Russia, Flowers and Madan55). More rural children than urban children were classified as having IDA by combined low Hb concentration and high level of s-TfR. Multiple factors can cause IDA such as poor food intake with low dietary Fe bioavailability, increased Fe requirement and/or parasitic infestation.
It has been reported that in Asia IDA affects 40–50 % of pre-school and school children(Reference Khor56). ID or IDA is also consistently associated with impaired cognitive function and lower school performance in school-aged children(Reference Grantham-McGregor and Ani57–Reference Otero, Pliego-Rivero and Porcayo-Mercado60).
The overall prevalence of VAD in Thai children in the present study ranged from 0·3 to 3·8 %. According to WHO definitions, a prevalence of VAD < 10 % is considered a public health problem of a mild degree(61). In contrast, other Southeast Asian countries have reported a higher prevalence of VAD between 9·3 and 44·7 %(Reference Pasricha and Biggs62). Although we found a rather low prevalence of VAD in the present study, one-third of the children were of the suboptimal vitamin A status. Therefore, these children may be at a risk of having a low vitamin A status in the future. Further studies need to determine the habitual food patterns and/or other influencing factors that affect serum retinol concentration among Thai children.
The present results indicate a high prevalence of VDD in children aged 3·0–12·9 years. Various factors determine vitamin D status, and it is interesting to focus on two primary sources of vitamin D, i.e. sunlight exposure and the child's diet. In addition, the presence of relatively few natural food sources of vitamin D such as whole milk, oily fish and egg yolk might be one associated factor contributing to the low vitamin D status in infants and children. However, because of the limited information on vitamin D content in Thai foods, an accurate estimation of vitamin D intake is difficult. Vitamin D is an essential nutrient as its function is to enhance Ca absorption for optimal bone growth. Since there is no consensus on the standard definition of VDD, the prevalence of VDD varied widely among the regions. In East Asia, the prevalence rate of VDD was found to be 29–71 %, based on different cut-off values. Using the cut-off of serum 25(OH)D concentration < 12·5 nmol/l, high prevalence rates have been reported in China, 29–45 %, and in Mongolia, 32–50 %(Reference Arabi, Rassi and Fuleihan63). Several previous studies have proposed criteria to define the threshold of a low vitamin D status based on plasma 25(OH)D concentrations of less than 50 nmol/l(Reference Lips64), 75 nmol/l(Reference Vieth, Bischoff-Ferrari and Boucher65) and 80 nmol/l(Reference Hollis66), and such serum concentrations have been proved to be associated with adverse health outcomes or clinical manifestations. This association should be warranted and further investigated in Thai infants and children.
Several studies have demonstrated the high prevalence of VDD in European countries (Healthy Lifestyle in Europe by Nutrition in Adolescence study)(Reference González-Gross, Valtueña and Breidenassel67) and in Malaysia(Reference Khor, Chee and Shariff68), using the cut-offs of plasma 25(OH)D concentration < 50 nmol/l ( < 20 ng/ml). Among nine European countries, the prevalence of VDD in boys aged 9–10 years was found to be 42 % when using the same cut-off. Surprisingly, in a tropical country where sunshine is abundant such as in Malaysia, the prevalence of VDD in 401 children aged 7–12 years was even higher (72·3 %). Another report from Australia(Reference Renzaho, Halliday and Nowson69) has demonstrated a higher prevalence in ethnic minority children than in their white counterparts (43·6–48·7 v. 10 %).
A recent study in 150 Thai obese children compared with twenty-nine normal-weight and healthy children has reported a similar prevalence of VDD in both the groups (11·3 v. 10·3 %), suggesting that adiposity is not a determinant of VDD(Reference Poomthavorn, Saowan and Mahachoklertwattana70). The prevalence was relatively low compared with that observed in the present study as the subjects recruited were obese children and the results of their biochemical analysis were compared with those of the normal counterparts. The values that were obtained may not represent the prevalence in Thai children.
In conclusion, a double burden of malnutrition exists in both urban and rural children in Thailand. IDA has been and still is a problem that needs to be addressed. An emerging problem of VDD was observed in the present study. High intakes of dietary protein but low intakes of Ca, Fe, vitamin A and vitamin C were found among Thai children. Proper dietary habits should be formed early in life through the education of mothers and caretakers. This information is useful for policy-makers to endorse more effective strategic plans and actions in the near future.
Acknowledgements
The present study was funded by FrieslandCampina, The Netherlands. The authors thank Associate Professor Pattanee Winichagoon, Institute of Nutrition, Mahidol University, Thailand, for her technical guidance. They also thank Ms Jutatip Wangsai and Ms Chumapa Deesudchit (FrieslandCampina Thailand) for their guidance and coordination among the Thailand research team and other research teams in the SEANUTS project. They are grateful to the participants and their parents for their time and cooperation. Last but not the least, they also thank the enumerators from the Institute of Nutrition, Mahidol University staff and local government staff who helped in logistics arrangement and data collection. N. R. is the principal investigator, designed the study, drafted the paper and carried out the nutrient data analysis. K. K. and U. Y. designed the study and drafted the paper. W. W. carried out the statistical analyses. S. P. prepared the result tables and was involved in the data collection and project coordination. W. T., A. B. and P. K. contributed to the design of the survey and data collection. I. K. was involved in data management, study monitoring and paper drafting. All authors approved the final version of the manuscript. The results of the study will be used by FrieslandCampina, but it had no influence on the outcome of the study. The authors declare that they have no conflicts of interest.
This paper was published as part of a supplement to the British Journal of Nutrition, the publication of which was supported by an unrestricted educational grant from Royal FrieslandCampina. The papers included in this supplement were invited by the Guest Editor and have undergone the standard journal formal review process. They may be cited. The Guest Editor appointed to this supplement is Dr Panam Parikh. The Guest Editor declares no conflict of interest.