Obesity is defined as abnormal or excessive body fat, and it is a major public health problem. In recent times, the prevalence of obesity has increased dramatically and it continues to increase in middle-aged and older adults worldwide, with a doubling in prevalence since 1980(1,2) . With age, obesity prevalence also increases, so in an ageing population this obesity epidemic represents a mounting financial concern in regard to healthcare resources(Reference Torjesen3–Reference Zamboni, Mazzali and Fantin6). Obesity is a well-established risk factor for CVD and mortality in adult populations(Reference Yusuf, Hawken and Ounpuu7–Reference Wormser, Kaptoge and Di Angelantonio10); typically ‘U’ or ‘J’ shaped curves are seen between obesity (measured by BMI) and CVD or mortality, with increased risks in underweight and overweight middle-aged adults. However, there is controversy surrounding the effects of overweight and obesity on CVD and mortality in older people. Some studies even suggest that overweight and obesity, measured by BMI, are apparently associated with a lower mortality risk(Reference Zamboni, Mazzali and Zoico11) (known as the obesity paradox). A previous large meta-analysis, including thirty-two studies, of almost 200 000 individuals aged 65 years and older, showed a U-shaped relationship between BMI and mortality, with the lowest risk in those with a BMI between 24 and 30 kg/m2, and mortality risk only began to increase when BMI exceeded 33 kg/m2(Reference Winter, MacInnis and Wattanapenpaiboon12). This obesity paradox may be partly explained by the fact that BMI is an imprecise measure of body fat which does not distinguish between fat and lean body mass, with have opposing effects on mortality risk; fat mass is positively associated and lean mass is negatively associated with mortality risk(Reference Allison, Zhu and Plankey13).
As people age, some important changes to body composition occur, which includes a relative increase in visceral abdominal fat and a gradual loss of muscle mass(Reference Enzi, Gasparo and Raimondo Biondetti14–Reference Ponti, Santoro and Mercatelli16). Increased visceral fat is a risk factor for developing metabolic disorders, such as hypertension, dyslipidaemia and insulin resistance, and also CVD. The progressive loss of muscle strength and mass which happens with age is known as sarcopenia(Reference Morley, Baumgartner and Roubenoff17). Many factors contribute to the development of sarcopenia but its aetiology is not completely understood. A number of pathological mechanisms have been suggested to underlie age-related muscle loss including neuronal and hormonal changes, being underweight, under-nutrition including low protein intake, physical inactivity and inflammation(Reference Zamboni, Mazzali and Fantin6,Reference Visvanathan and Chapman18–Reference Petermann-Rocha, Chen and Gray22) . Sarcopenia is of major concern in ageing populations as it is associated with metabolic impairment, cardiovascular risk factors and physical disability(Reference Abellan van Kan23–Reference Choi25). Although sarcopenia has been less extensively studied in relation to incident CVD morbidity and mortality, there is increasing evidence that muscle mass or muscle strength components of sarcopenia are associated with increased risk of CVD(Reference Tyrovolas, Panagiotakos and Georgousopoulou26–Reference Spahillari, Mukamal and DeFilippi30). It is well documented that sarcopenia is a significant predictor of death in older adults, including those who are community-dwelling, care home residents or hospitalised patients(Reference Liu, Hao and Hai31–Reference Vetrano, Landi and Volpato33). A recent meta-analysis of six prospective cohort studies examined the association between sarcopenia and mortality, including 7367 community-dwelling older adults(Reference Liu, Hao and Hai31). The pooled estimate showed that sarcopenic individuals had a 60 % increase in the risk of mortality (hazard ratio (HR) 1·60, 95 % CI 1·24, 2·06) compared with those without sarcopenia. Sarcopenia is also a significant predictor of all-cause mortality among older nursing home residents, with a meta-analysis of six studies showing an 86 % increase in risk (pooled HR 1·86, 95 % CI 1·42, 2·45)(Reference Zhang, Wang and Dou32).
Despite it being the case that body composition can greatly change with age, the fact that visceral fat tends to increase and muscle mass tends to decrease means that there may be no significant change to an individual’s overall body weight or BMI(Reference Zamboni, Mazzali and Fantin6,Reference Zamboni, Mazzali and Zoico11) . Body composition changes with age can see the co-occurrence of sarcopenia with increases in fat mass. Sarcopenic obesity has recently emerged as a new category of body composition(Reference Zamboni, Mazzali and Fantin6) (Fig. 1). The body composition of older adults can therefore be categorised as: normal, sarcopenic, obese or sarcopenic obese. Visceral fat and muscle mass are connected pathogenically and share common pathways including decline in physical activity, low energy expenditure, increase in insulin resistance and inflammation(Reference Zamboni, Mazzali and Fantin6,Reference Kalinkovich and Livshits34–Reference Livshits and Kalinkovich37) . The co-existence of both sarcopenia and obesity in older adults may therefore interact and increase their effects on risk of CVD and mortality, which may result in older adults with a sarcopenic obese body composition having the worse disease and mortality outcomes(Reference Zamboni, Mazzali and Fantin6,Reference Kohara38–Reference Stenholm, Harris and Rantanen40) .
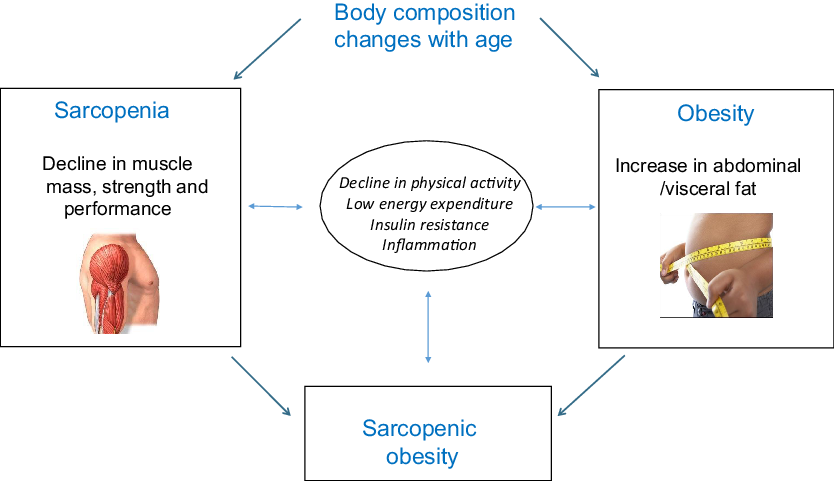
Fig. 1. Body composition changes with age and the interplay between sarcopenia and obesity. Adapted from Wannamethee & Atkins(Reference Wannamethee and Atkins35).
Sarcopenic obesity
The term ‘sarcopenic obesity’ was first coined by Baumgartner(Reference Baumgartner41) and combines the body composition categories of both sarcopenia and obesity. BMI (weight divided by height squared) is the most commonly used measure of adiposity, with obesity defined as greater than or equal to 30 kg/m2(42). However, the validity of BMI in adequately measuring adiposity has been questioned, especially in older age, as BMI does not differentiate between fat mass and lean mass(Reference Allison, Zhu and Plankey13). Alternative obesity definitions have therefore focused on the distribution of body fat, with central or visceral obesity being commonly measured(Reference Prentice and Jebb15). Fat mass can be assessed using bioimpedance analysis (BIA) or dual-energy X-ray absorptiometry (DXA)(Reference Ponti, Santoro and Mercatelli16). Computerised tomography or MRI can also be used to evaluate adipose tissue or its quality(Reference Pescatori, Savarino and Mauri43) (Table 1). Measures of central obesity have also been shown to be stronger predictors of CVD and mortality than BMI in older adults(Reference de Koning, Merchant and Pogue44,Reference de Hollander, Bemelmans and Boshuizen45) . Two such anthropometric measures of central adiposity are waist:hip ratio (≥0·90 cm for men; ≥0·85 cm for women) and waist circumference (>102 cm for men; >88 cm for women), as defined by the WHO(46).
Sarcopenia has been defined using many different measurement methods (Table 1), and the diagnostic criteria are not uniform(Reference Xie, Xiao and Fan47). Sarcopenia was originally defined by Baumgartner et al.(Reference Baumgartner, Koehler and Gallagher19) as appendicular skeletal muscle mass two sd below the sex-specific reference for a young healthy person, assessed using DXA and height adjusted. Janssen et al.(Reference Janssen, Heymsfield and Ross48) developed an alternative sarcopenia definition of skeletal muscle mass measured by BIA. DXA and BIA are more commonly used to assess muscle mass in research settings, but some clinical setting may also use computerised tomography or MRI scans. In 2010, The European Working Group on Sarcopenia in Older People (EWGSOP) published a sarcopenia definition to assist in case finding in older adults(Reference Cruz-Jentoft, Baeyens and Bauer49). This definition included the presence of both low muscle function (low strength and/or low physical performance) and low muscle mass. The EWGSOP sarcopenia definition recommended measuring physical performance using gait speed and measuring muscle strength using handgrip strength(Reference Cruz-Jentoft, Baeyens and Bauer49). In 2014, an alternative and more specific sarcopenia definition was recommended by the Foundation for the National Institutes of Health Sarcopenia Project; appendicular lean mass cut-points adjusted for BMI (<0·789 for men; <0·512 for women) and for grip strength cut-points (<26 kg for men; <16 kg for women)(Reference Studenski, Peters and Alley50). Recently, there has been much increased acknowledgement of the importance of sarcopenia in older adults, and in 2016 sarcopenia was officially recognised as a disease and assigned an International Classification of Disease-10 code(Reference Anker, Morley and Haehling51). In 2019, the EWGSOP published an updated sarcopenia definition and cut-points (the EWGSOP-2) which focused on low muscle strength as a key sarcopenia characteristic, used detection of low muscle quantity and quality to confirm the diagnosis and used poor physical performance to indicate severe sarcopenia(Reference Cruz-Jentoft, Bahat and Bauer52). Comparison of the old and new EWGSOP definitions of sarcopenia in the UK Biobank cohort study suggests that the new EWGSOP-2 definition recognises fewer people as sarcopenic (0·36 %) compared with the old definition (8·14 %)(Reference Petermann-Rocha, Chen and Gray53).
The operational definition of sarcopenic obesity is still under discussion(Reference Zamboni, Rubele and Rossi36) and hence there is no universally accepted classification(Reference Waters and Baumgartner24,Reference Stenholm, Harris and Rantanen40,Reference Cruz-Jentoft, Bahat and Bauer52) . There is a marked heterogeneity in definitions and approaches to diagnose sarcopenic obesity(Reference Donini, Busetto and Bauer54). The threshold values used in previous literature to define both sarcopenia and obesity have varied significantly depending on population, age, sex and ethnicity(Reference Lee, Shook and Drenowatz55). It is therefore a challenge to compare the results of findings between studies using different sarcopenic obesity definitions. Prevalence estimates for sarcopenic obesity have therefore also differed greatly, from 0 to 25 % in older adults across different studies, with an average prevalence of 5–10 %(Reference Lee, Shook and Drenowatz55). In a review of eight sarcopenic obesity definitions, Batsis et al.(Reference Batsis, Barre and Mackenzie56) estimated that prevalence can vary up to 26-fold depending on definition used. Such a high degree of variability is suggestive of a need to establish a consensus definition that can be reliably applied across different clinical and research settings(Reference Zamboni, Rubele and Rossi36).
Sarcopenic obesity and cardiovascular risk factors
There has been a rapid growth in the literature in the last couple of decades which has examined the associations between sarcopenic obesity and cardiovascular risk factors(Reference Wannamethee and Atkins35,Reference Atkins and Walrand39,Reference Gusmão-Sena, Curvello-Silva and Barreto-Medeiros57) . Table 2 summarises relevant studies, discussed below, that investigate the associations of sarcopenic obesity and cardiovascular risk factors in older people. In Korean older adults, several cross-sectional studies have shown that sarcopenic obese individuals have the highest cardiovascular risk; sarcopenic obesity (classified by skeletal muscle mass assessed by DXA and obesity assessed by either DXA, BMI or waist circumference) was associated with up to an 8-fold increase in the risk of the metabolic syndrome, an increased risk of hypertension, insulin resistance, dyslipidaemia, higher fasting glucose levels and a lower cardiorespiratory fitness, compared with the non-sarcopenic, non-obese group(Reference Baek, Nam and Han58–Reference Kim, Yang and Yoo66). A study in Taiwanese older adults (defined by BIA-measured muscle mass and BMI) is also comparable in that the sarcopenia obese group also had the highest risk of the metabolic syndrome, with a 12 times increased risk, compared with the non-sarcopenic, non-obese group(Reference Lu, Yang and Chang67). A large cross-sectional study of over 14 000 adults in the National Health and Nutrition Examination Survey showed that sarcopenic obese individuals (defined by BIA-measured muscle mass and BMI) also have the highest risk of dysglycaemia and insulin resistance(Reference Srikanthan, Hevener and Karlamangla68).
Table 1. Methods for measuring sarcopenia and obesity

MAMC, midarm muscle circumference; WC, waist circumference; WHR, waist:hip ratio.
Table 2. Summary of studies examining the association between sarcopenic obesity and cardiovascular risk factors in older people*
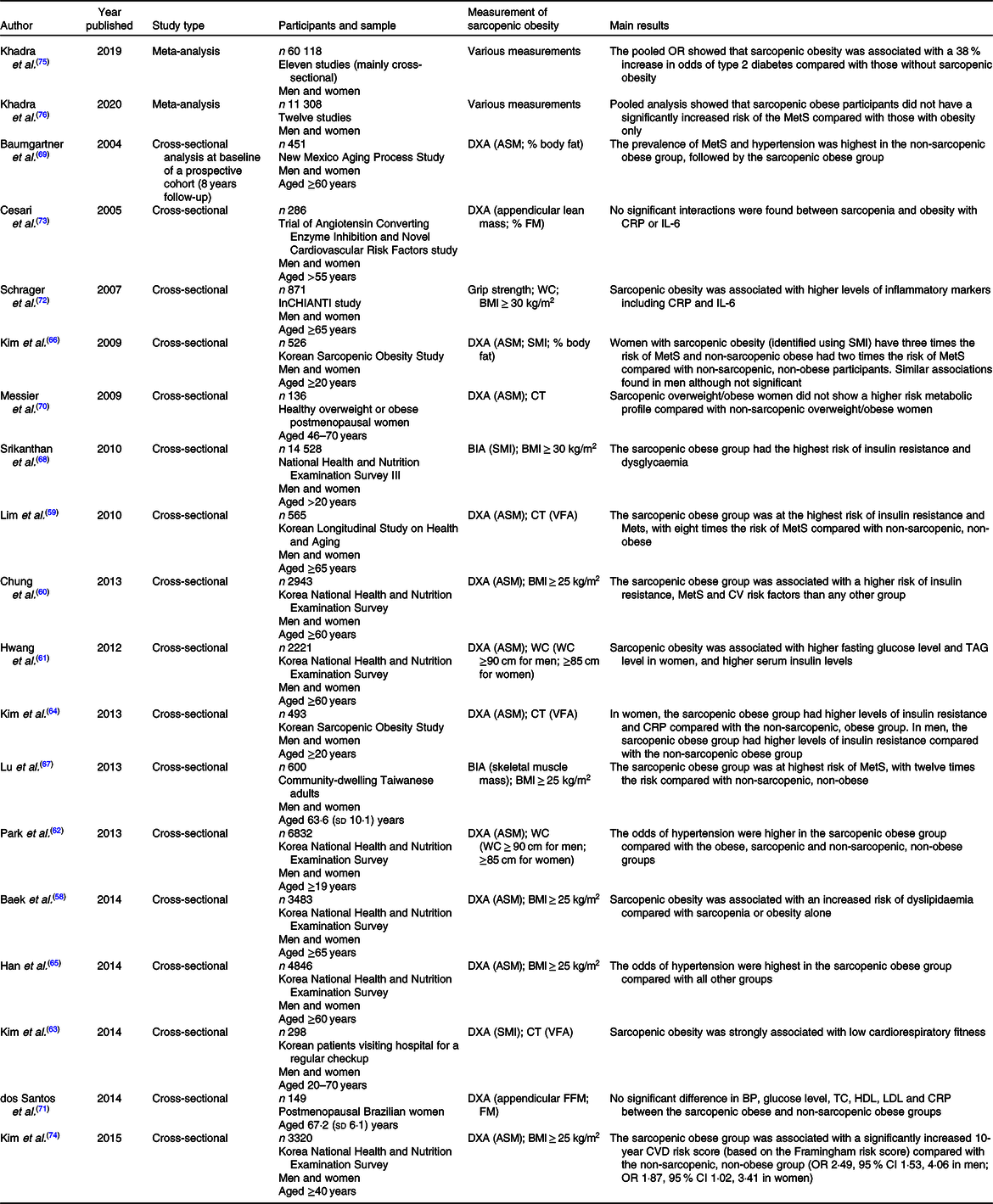
ASM, appendicular skeletal muscle mass; BIA, bioelectrical impedance analysis; BP, blood pressure; CRP, C-reactive protein; CT, computerised tomography; DXA, dual-energy X-ray absorptiometry; FFM, fat-free mass; FM, fat mass; MetS, metabolic syndrome; SMI, skeletal muscle mass index; TC, total cholesterol; VFA, visceral fat area; WC, waist circumference.
* Table adapted from Atkins & Wannamethee(Reference Atkins and Wannamethee82). Studies arranged by type (meta-analysis, cross-sectional) and by year.
Not all studies, however, show that sarcopenic obese older adults have the highest cardiovascular risks, with some studies suggesting that obese older adults have higher levels of cardiovascular risk factors. The New Mexico Aging Process Study examined older adults aged 60 years and over, and found that the prevalence of hypertension and the metabolic syndrome was highest in the non-sarcopenic obese group, followed by the sarcopenic obese group (assessed using DXA measurements)(Reference Baumgartner, Wayne and Waters69). Studies in older, postmenopausal women have also shown that sarcopenic obese individuals did not have a worse metabolic profile compared with non-sarcopenic obese individuals(Reference Messier, Karelis and Lavoie70) and that glucose level, lipid profile and blood pressure were not significantly higher in sarcopenic obese compared with non-sarcopenic, non-obese people(Reference dos Santos, Gadelha and Safons71).
In spite of inflammation being on a common pathway for both sarcopenia and obesity, differing results have been found regarding the association between sarcopenic obesity and inflammatory or haemostatic markers. The ‘Invecchiare in Chianti’ (InCHIANTI) study of older adults, aged 65 years and older, showed that sarcopenic obesity (defined using grip strength and waist circumference measurements) was associated with higher levels of inflammatory markers including IL-6 and C-reactive protein in cross-sectional analysis(Reference Schrager, Metter and Simonsick72). Similarly, a study found that in older Korean women, the sarcopenia obese group had the highest C-reactive protein levels(Reference Kim, Park and Lim64). However, baseline analysis of the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study found that although obesity and sarcopenia were both significantly associated with higher C-reactive protein and IL-6 levels, the relationship of inflammation with sarcopenia was not independent of the relationship with obesity(Reference Cesari, Kritchevsky and Baumgartner73). A study of postmenopausal women also found that C-reactive protein levels were not significantly different between the sarcopenic obese and non-sarcopenic, non-obese groups(Reference dos Santos, Gadelha and Safons71).
The 5th Korean National Health and Nutrition Examination Survey (n 3320; ≥40 years) assessed the association between sarcopenic obesity and a number of cardiovascular risk factors, encapsulated within the Framingham risk score(Reference Kim, Cho and Park74). The Framingham risk score was calculated based on age, sex, total cholesterol, HDL-cholesterol, systolic blood pressure, smoking and diabetes. The sarcopenic obese group was associated with a significantly increased 10-year CVD risk (OR 2·49, 95 % CI 1·53, 4·06 in men; OR 1·87, 95 % CI 1·02, 3·41 in women) compared with the non-sarcopenic, non-obese group. However, sarcopenic non-obese and non-sarcopenic obese participants were not associated with an increased 10-year CVD risk.
More recently, a meta-analysis of studies examining the associations between sarcopenia obesity and type 2 diabetes has been performed, with eleven studies including a total of 60 118 adults who were overweight or obese(Reference Khadra, Itani and Tannir75). Sarcopenic obesity was significantly associated with a 38 % increase in risk of type 2 diabetes compared with those without sarcopenic obesity (OR 1·38, 95 % CI 1·27, 1·50), but results should be interpreted with caution as the majority of studies were cross-sectional. A meta-analysis has also been carried out to synthesise the results of studies assessing the associations between sarcopenia obesity and the metabolic syndrome, including twelve studies with a total of 11 308 overweight or obese adults(Reference Khadra, Itani and Chebaro76). Pooled analysis showed that sarcopenic obese participants did not have a significantly increased risk of the metabolic syndrome (risk ratio 1·08, 95 % CI 0·99, 1·17) compared with those with obesity only.
Sarcopenic obesity and CVD
In spite of the growth of literature on the associations between sarcopenic obesity and cardiovascular risk factors in recent years, to date only a limited number of studies have assessed the associations between sarcopenic obesity and CVD risk in older adults(Reference Wannamethee and Atkins35,Reference Atkins and Walrand39,Reference Gusmão-Sena, Curvello-Silva and Barreto-Medeiros57,Reference Prado, Wells and Smith77) . Table 3 summarises relevant studies, discussed below, that investigate the associations of sarcopenic obesity and the risk of CVD in older people. Cross-sectional studies have yielded inconsistent results. A cross-sectional analysis in the New Mexico Aging Process Study compared CVD prevalence across body composition groups in older adults, aged 60 years and older(Reference Baumgartner, Wayne and Waters69). The prevalence of CVD was not higher in sarcopenic obese individuals (11·5 %), defined with appendicular skeletal muscle and percentage body fat from DXA, compared with non-sarcopenic, non-obese individuals (13·7 %). Conversely, a cross-sectional study of older adults aged 65 years and older, in the Korea National Health and Nutrition Examination Survey, found that the sarcopenic obese group (based on appendicular skeletal muscle from DXA and BMI ≥ 25 kg/m2) showed a slightly higher but non-significant prevalence of CVD (12·3 %) compared with non-sarcopenic obese (10·0 %)(Reference Chin, Rhee and Chon78).
Table 3. Summary of studies examining the association between sarcopenic obesity and risk of CVD in older people*
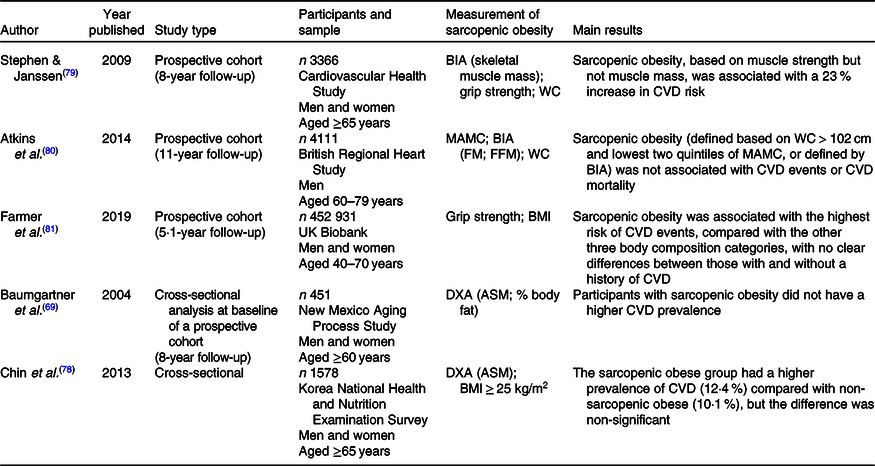
ASM, appendicular skeletal muscle mass; BIA, bioelectrical impedance analysis; DXA, dual-energy X-ray absorptiometry; FFM, fat free mass; FM, fat mass; MAMC, midarm muscle circumference; WC, waist circumference.
* Table adapted from Atkins(Reference Atkins and Walrand39). Studies arranged by type (prospective cohort, cross-sectional) and by year.
There are a small number of prospective studies that have examined the association between sarcopenic obesity and CVD risk over time. Stephen & Janssen(Reference Stephen and Janssen79) analysed data from a prospective study of 3366 community-dwelling older adults (aged 65 years and over) followed over 8 years, in the Cardiovascular Health Study. The risk of CVD events was not significantly raised in the sarcopenic obese group (defined using waist circumference and BIA-measured muscle mass) compared with the normal body composition group. However, it seems that muscle strength could be better measure of sarcopenia than muscle mass in predicting CVD risk, as within the same study population, when sarcopenic obesity was defined by grip strength and waist circumference, there was a 23 % increase in CVD risk in the sarcopenic obese group, compared with the non-sarcopenic, non-obese group(Reference Stephen and Janssen79). The sarcopenic obese group also had the highest risk of CVD compared with the sarcopenic group and the obese group(Reference Stephen and Janssen79). A prospective study of 4111 men from the British Regional Heart Study, aged 60–79 years and followed-up for 11 years, showed no association between sarcopenic obesity (defined by midarm muscle circumference and waist circumference) and CVD events or CVD mortality(Reference Atkins, Whincup and Morris80). However, this study did not examine muscle strength measures when defining sarcopenic obesity. Recently, Farmer et al.(Reference Farmer, Mathur and Schmidt81) explored the association between sarcopenic obesity and CVD in the largest prospective study of its kind to date in UK Biobank (452 931 community volunteers, aged 40–70 years, followed up for 5·1 years). Sarcopenic obesity (defined by hand grip strength and BMI) was associated with the highest risk of CVD events, compared with the other three body composition categories, with no clear differences between those with and without a history of CVD. However, there is no clear evidence of an interaction between obesity and sarcopenia for CVD events. Overall, it seems that research to date from these cross-sectional and prospective studies shows heterogeneity in associations between sarcopenic obesity and risk of CVD; the literature is not consistent as to whether the sarcopenic obese group has the highest risk of CVD, but these inconsistencies are perhaps due to the different sarcopenic obesity definitions used between studies.
Sarcopenic obesity and mortality
Recently, there has been a growing number of prospective studies which have examined the risk of all-cause mortality in sarcopenic obese older adults(Reference Atkins and Walrand39,Reference Atkins and Wannamethee82,Reference Tian and Xu83) . Table 4 summarises relevant studies, discussed below, that investigate the associations of sarcopenic obesity and mortality risk in older people. In a community-based study of 4107 men, aged 60–79 years (the British Regional Heart Study) the risk of all-cause mortality was increased in sarcopenic obese individuals; those with a high waist circumference (>102 cm) and in the lowest quartile of midarm muscle circumference had a 55 % increase in mortality risk compared with non-sarcopenic, non-obese individuals over a follow-up period of 6 years(Reference Wannamethee, Shaper and Lennon84). This same population was re-examined more recently after 11 years of follow-up(Reference Atkins, Whincup and Morris80). The risk of mortality was increased in the sarcopenic group (41 % increase in risk) and the obese group (21 % increase), with the highest risk in sarcopenic obese men (72 % increase) compared with the non-sarcopenic, non-obese group, after adjustment for lifestyle and cardiovascular risk factors(Reference Atkins, Whincup and Morris80).
Table 4. Summary of studies examining the association between sarcopenic obesity and risk of mortality in older people*
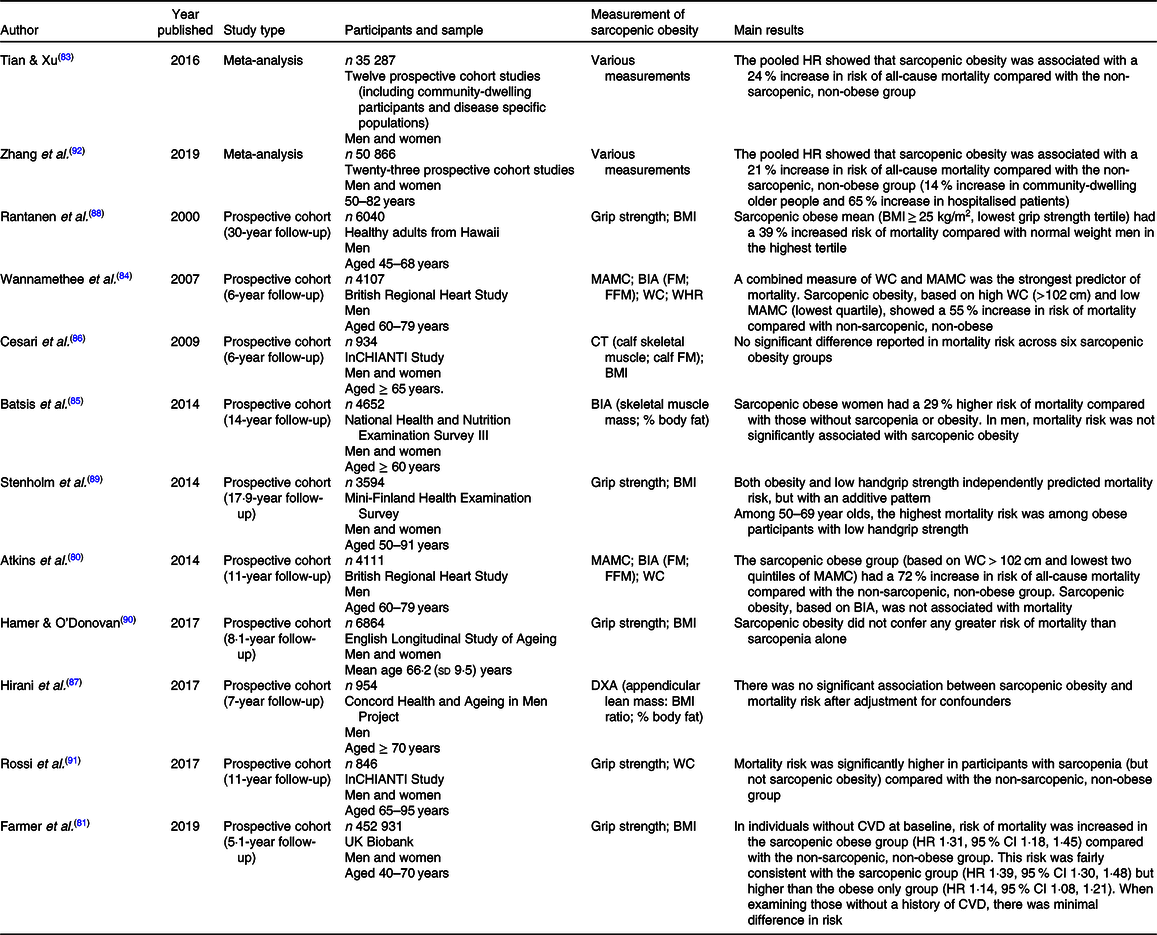
BIA, bioelectrical impedance analysis; CT, computerised tomography; DXA, dual-energy X-ray absorptiometry; FFM, fat free mass; FM, fat mass; HR, hazard ratio; MAMC, midarm muscle circumference; WC, waist circumference; WHR, waist:hip ratio.
* Table adapted from Atkins(Reference Atkins and Walrand39). Studies arranged by type (meta-analysis, prospective cohort) and by year.
The National Health and Nutrition Examination Survey has also assessed the association between sarcopenic obesity and mortality in more than 4000 participants, aged 60 years or above, followed-up for 14 years(Reference Batsis, Mackenzie and Barre85). Sarcopenic obesity was classified according to BIA-measured skeletal muscle mass and body fat, and sarcopenic obese women had a significantly increased mortality risk of 29 % compared with those with no sarcopenia or obesity, after adjustment for age, sex, ethnicity and cardiovascular risk factors(Reference Batsis, Mackenzie and Barre85). However, there was no significant increase in mortality risk in sarcopenic obese men in this cohort(Reference Batsis, Mackenzie and Barre85). The InCHIANTI study assessed risk of mortality in 934 males and females aged 65 years and above, across six different sarcopenic obesity combinations, combining sarcopenia (measured with calf skeletal muscle) or no sarcopenic with three adiposity categories (obese, overweight or normal body weight measured by BMI)(Reference Cesari, Pahor and Lauretani86). Over a follow-up period of 6 years, no significant difference in risk of mortality was observed across the six sarcopenic obesity groups. Similarly, a recent small study in 954 men (aged 70 years and above, followed-up for over 7 years) from the Concord Health and Ageing in Men Project, categorised sarcopenic obesity based on DXA-measured lean mass and body fat, also found no significant association between sarcopenic obesity and mortality risk after adjustment for confounders(Reference Hirani, Naganathan and Blyth87).
A number of prospective cohort studies examining mortality risk have defined sarcopenic obesity using a measure of muscle strength instead of muscle mass, with grip strength being a common measurement method. In over 6000 healthy adult men aged 45–68 years living in Hawaii, with follow-up of more than 30 years, mortality risk was significantly increased by 39 % in sarcopenic obesity men (BMI ≥ 25 kg/m2; lowest tertile of grip strength)(Reference Rantanen, Harris and Leveille88). The Mini-Finland Health Examination Survey, including 3594 participants followed over 17 years, categorised sarcopenic obesity using BMI and grip strength (BMI ≥ 30 kg/m2; lowest tertile of grip strength), found that sarcopenia and obesity each independently predicted mortality, but with an additive rather than a multiplicative effect(Reference Stenholm, Mehta and Elo89). A prospective study of 6864 older adults in the English Longitudinal Study of Ageing, which categorised sarcopenic obesity using BMI and grip strength (BMI ≥ 30 kg/m2; lowest tertile of grip strength) found that sarcopenic obese individuals did not have a significantly greater risk of mortality than those with just sarcopenia, but the risk was higher than in those with just obesity(Reference Hamer and O’Donovan90). Another prospective study, categorising sarcopenic obesity using tertiles of waist circumference and tertiles of grip strength (n 846 older adults from InCHIANTI), found that after adjustment for confounders, only the sarcopenic group (and not the obese group or the sarcopenic obese group) had a significantly increased mortality risk(Reference Rossi, Bianchi and Volpato91). Although the above studies have all used tertiles of grip strength, this will have resulted in different grip strength cut-offs in different settings. This could confound results and makes valid comparison of findings problematic.
A recent meta-analysis by Tian & Xu in 2016(Reference Tian and Xu83) has amalgamated the results of twelve prospective studies, including 35 287 participants, assessing the association between sarcopenic obesity and risk of mortality. Analysis showed that sarcopenic obesity was associated with a 24 % increase in risk of all-cause mortality compared with those without sarcopenia or obesity (pooled HR 1·24, 95 % CI 1·12, 1·37).
However, this meta-analysis did not perform a subgroup analysis of types of participants and included both community-dwelling individuals and disease-specific populations. Zhang et al.(Reference Zhang, Xie and Dou92) updated this meta-analysis in 2019, to include twenty-three prospective studies, with 50 866 participants aged 50–82 years. Similarly to the previous meta-analysis(Reference Tian and Xu83), a 21 % increase in mortality risk was seen in sarcopenic obese individuals (pooled HR 1·21, 95 % CI 1·10, 1·32) compared with non-sarcopenic, non-obese. This meta-analysis did not present mortality risk in the sarcopenic obese group compared with the sarcopenic only group or the obese only group though. In subgroup analysis, sarcopenic obesity remained a significant predictor of all-cause mortality in community-dwelling older people (HR 1·14, 95 % CI 1·06, 1·23) and especially in hospitalised patients (pooled HR 1·65, 95 % CI 1·17, 2·33)(Reference Zhang, Xie and Dou92). However, the twenty-three studies included a range of countries and various adjustments for confounders, so there was significant heterogeneity across studies. Subgroup analysis by measurement type showed that sarcopenic obesity was associated with all-cause mortality when sarcopenic was measured by skeletal muscle mass (HR 1·12, 95 % CI 1·01, 1·23), muscle strength (HR 1·18, 95 % CI 1·05, 1·33) and skeletal muscle index (HR 1·53, 95 % CI 1·13, 2·07). Additionally, subgroup analysis showed a significant association between sarcopenic obesity and mortality when obesity was measured by waist circumference (HR 1·24, 95 % CI 1·09, 1·40), BMI (HR 1·29, 95 % CI 1·04, 1·59) and visceral fat area (HR 2·54, 95 % CI 1·83, 3·53). This suggests that using SMI and visceral fat to define sarcopenic obesity may be relevant diagnostic criteria to predict mortality risk in older adults.
More recently, Farmer et al.(Reference Farmer, Mathur and Schmidt81) explored the association between sarcopenic obesity and mortality using the UK Biobank, a very large community cohort study of 452 931 volunteers aged 40–70 years, who were followed-up for 5·1 years. Sarcopenic obesity was assessed by grip strength and BMI. In individuals without CVD at baseline, risk of mortality was increased in the sarcopenic obese group (HR 1·31, 95 % CI 1·18, 1·45) compared with the non-sarcopenic, non-obese group. This risk was fairly consistent with the sarcopenic group (HR 1·39, 95 % CI 1·30, 1·48) but higher than the obese only group (HR 1·14, 95 % CI 1·08, 1·21). When examining those without a history of CVD, there was minimal difference in risk.
Conclusion
Sarcopenic obesity is a new category of obesity in older adults, with the co-existence of low muscle mass and strength with high adiposity levels. Studies to date on sarcopenic obesity suggest that this body composition category is associated with higher levels of cardiovascular risk factors and an increased mortality risk in older adults compared with those without sarcopenia or obesity. Efforts to promote healthy ageing should therefore focus on both preventing obesity and maintaining muscle strength and muscle mass. There is some evidence to suggest that the sarcopenic obese group has even higher levels of cardiovascular risk factors and mortality than sarcopenic or obese groups alone. However, the evidence on whether sarcopenic obese have the highest risks of CVD and mortality varies between studies, which is likely to be due to the major differences in sarcopenic obesity classifications used. In recent times, sarcopenia has gained increased recognition as an important condition in older age which has helped to progress and expand the related field of research. However, there is still no universally accepted classification of sarcopenia, or hence therefore of sarcopenic obesity. Therefore, an important need exists to establish a consensus definition of sarcopenic obesity that can be reliably applied across different clinical and research settings.
Acknowledgements
The present review did not receive any financial support.
Both authors contributed to the literature search, manuscript writing and revision of the article.
The authors declare that there are no conflicts of interest.









