Intestinal tract is an important organ not only responsible for the digestion and absorption of nutrients, but also constitutes a physical and immunological protective barrier to the body health(Reference Hinsberger and Sandhu1,Reference Allaire, Crowley and Law2) . It is well known that gastrointestinal mucosa is the largest immune system in the body(Reference Chase3). Gut-associated lymphoid tissue, immune cells and cytokines are all important components of the mucosal immune system, which resist the invasion of ingested infectious agents and maintain intestinal homeostasis(Reference Lillehoj and Trout4,Reference Okumura and Takeda5) .
The impact of diet on animal health has long been a topic of research. Some dietary nutritional components, including proteins, fatty acids and carbohydrates, etc., have been fully studied regarding their effects on immune status of some domestic animals(Reference Tang, Sun and Yao6–Reference Al-Khalaifah, Al-Nasser and Givens8). Starch is a main source of carbohydrates in animal diets, and it is generally classified into rapidly digestible starch (digested within 20 min), slowly digestible starch (digested between 20 min and 120 min) and resistant starch (RS, undigested after 120 min) based on the rate of starch hydrolysis in vitro (Reference Englyst, Kingman and Cummings9). RS is defined as the fraction of starch that is resistant to digestion by host amylases in the upper digestive tract and transits intact to the large bowel, where it is fermented by gut microbiota(Reference Englyst, Kingman and Hudson10). One of the most important chemical features of RS is that high amylose molecules content, which contributes to the indigestibility(Reference Trinh11). The fermentation of RS in hindgut will alter microbiota composition and enhance the production of SCFA, which have multiple beneficial effects on health(Reference Maier, Lucio and Lee12,Reference Topping and Clifton13) . Thus, the nutritional effects of RS have been notably recognised.
Several studies have provided conclusive evidence of RS in regulating both mucosal and systemic immunity. 5 % potato RS can alter cecal immunological tolerance in pigs to enhance mucosal immunity by increasing the regulatory T cells number and IL-6 mRNA expression in the caecum(Reference Trachsel, Briggs and Gabler7). Corn RS can also improve the colonic immunity of pigs by increasing the abundance of anti-inflammatory cytokine IL-10(Reference Fan, Archbold and Lackeyram14). In addition, a metabolomics study has shown that rice RS promoted systemic immunity by decreasing serum TNF-α levels compared with the rice digestible starch(Reference Wang and Pang15). For poultry, our previous studies have found that feeding broilers with higher concentrations of RS can alter the microbial composition and diversity and modulated the metabolic pathways of microbial metabolism in caecum, but retard the growth performance(Reference Zhang, Liu and Li16,Reference Liu, Zhang and Li17) . Whether these changes will further alter the immune status remains unclear. Based on the results of our previous studies, we hypothesised that RS could elevate the immune function of broiler chickens. Therefore, the objective of this study was to assess the effects of graded levels corn RS on mucosal immune and systemic immunological characteristics in broilers.
Experimental methods
Ethics approval
All experimental protocols and procedures involving birds were conducted in accordance with the guidelines established by the Institutional Animal Care and Use. This study was approved by the Animal Care and Use Committee of Nanjing Agricultural University (permit number: GB14925, NJAU-CAST-2011-093).
Animals, experimental design and sample collection
In all, 320 newly hatched male broilers (Arbor Acres) were purchased from a commercial hatchery (Hewei Agricultural Development Co. Ltd). Chicks were weighed and randomly assigned to five treatment groups, each group had eight replicates (one replicate/cage) with eight broilers per replicate. The five dietary treatment groups are as follows: (1) NC, a basic normal corn–soyabean diet; (2) CS, a basic diet supplementation with 20 % corn starch; (3) 4 % RS, (4) 8 % RS and (5) 12 % RS, the diet supplementation with 40 g/kg, 80 g/kg and 120 g/kg RS (Hi-Maize® 260, type II RS, 60 % purity; Ingredion Inc.), respectively. The ingredients and nutrient levels of all diets were formulated to meet the NRC (1994) nutrient requirements of broiler(18) chickens (Table 1). Birds were allowed free access to feed and water in a temperature-controlled room at Nanjing Kangxin Poultry Industry. The room temperature was kept at 33°C for the first week, and then was reduced by 3°C each week until the final temperature was around 26°C. Birds were exposed to light for 23 h/d throughout the whole experimental period. The feeding trial lasted 42 d, the number of dead birds in each cage and the corresponding weight were recorded during the trial.
Table 1. Ingredient composition and nutrient contents of experimental diets, on an as-fed basis

NC, normal corn–soyabean; CS, corn starch; RS, resistant starch.
* NC, a basic normal corn–soyabean diet; CS, the diet contains 20 % corn starch; 4 % RS, 8 % RS and 12 % RS, the diets contains 4 %, 8 % and 12 % corn RS by replacing corn starch of CS diet with 6.67 %, 13.3 %, and 20 % Hi-Maize® 260 (type II RS, 60 % purity), respectively.
† Premix provided per kilogram of diet: trans-retinyl acetate, 30 mg; cholecalciferol, 0.075 mg; DL-α-tocopherol acetate, 30 mg; menadione, 1.3 mg; thiamine, 2.2 mg; riboflavin, 8.0 mg; nicotinamide, 40 mg; choline, 400 mg; pantothenic acid (D-Ca pantothenate), 15 mg; pyridoxine HCl, 4 mg; biotin, 0.04 mg; folic acid, 1 mg; cobalamin, 0.013 mg; Fe, 80 mg; Cu, 8.0 mg; Mn, 110 mg; Zn, 60 mg; I, 1.1 mg; se, 0.3 mg.
At 21 and 42 d of age, one bird from each experimental unit was selected for sample collection, the cage was used as the experimental unit. The body weight of the selected birds is close to the average weight of the birds in the experimental unit. About 10 ml blood samples from the jugular vein were collected and centrifuged to separate plasma. Then these birds were electrically stunned (50 V, alternating current; 400 Hz for 5 s each one) and killed via exsanguination. After the birds were dissected, the thymic lobes, spleen and bursa were removed and weighed. About 1 cm of the middle portion of jejunum segments were excised, washed in saline solution (0·75 % NaCl) and fixed in buffered formalin. The jejunal mucosa was gently scraped, frozen in liquid N2 and stored at –80°C for further analysis.
Lymphoid organ index
The thymus, spleen and bursa index were determined as described by Li et al. (2018)(Reference Li, Ren and Zhu19). Before the birds were slaughtered, the live weight of the birds was recorded. And the thymus, spleen and bursa of the slaughtered birds were removed and weighed separately and recorded. Then the equation for calculating the lymphoid organ index is as follows: lymphoid organ index (g/kg·body weight) = the weight of immune organ (g)/body weight (kg).
Intraepithelial lymphocyte counts in jejunum
Intestinal samples were dehydrated in a graded ethanol series, clarified with fresh xylene and then embedded into paraffins. About 5 μm cross-sections were cut for hematoxylin–eosin staining. All sections were observed under a light microscope at 400× amplification (Scope A1, Carl Zeiss Co. Ltd.), and the microscopic images were analysed by Image-Pro Plus 6.0 software (Media Cybernetics). The density of intraepithelial lymphocytes (IEL) was defined as the intraepithelial lymphocyte count/100 enterocytes (n/100 enterocytes). Data were presented as the mean of eight villis from one intestinal cross-section per chicken was used.
IgA-producing cells immunohistochemistry
The histological sections (5 μm) were prepared using the same protocol as described above. Immunostaining was performed by incubating tissue sections with mouse anti-chicken monoclonal antibody (Cat no. 8330-01; Southernbiotech), followed by the use of a diaminobenzidine staining kit (Cat no. K5007; Angle Gene Bioengineering). After immunohistochemical staining, the number of IgA-producing cells in the intestinal lamina propria was counted using a light microscope (Axio Scope A1, Carl Zeiss) at 400× amplification. Four different fields per section in each chicken were analysed. The results were expressed as the number of IgA positive cells/mm2.
Analysis of NO, IL-2, IL-4 and IFN-γ concentration, NOS and iNOS activities in plasma
The concentration of nitric oxide (NO) (catalogue no. A013-2-1), IL-2 (catalogue no. H003), IL-4 (catalogue no. H005) and interferon (IFN)-γ (catalogue no. H025) and the activities of total nitric oxide synthase (TNOS) and inducible nitric oxide synthase (iNOS) (catalogue no. A014-1-2) in plasma were determined with the corresponding commercial diagnostic kits (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s instructions.
For the determination of NO concentration, add 160 μl of double-distilled water, sodium nitrite standard solution (20 μmol/l) and plasma into 96-well plate, then add 80 μl of chromogen reagent and left for 15 min, then the optical density (OD) at 550 nm was determined. The NO concentrations were calculated according to OD values.
For the determination of TNOS and iNOS activities, 30 μl plasma was added to the assay tube, followed by 100 μl of double-distilled water, 200 μl of reagent I, 10 μl of reagent II and 100 μl of reagent III, respectively, followed by a water bath at 37°C for 15 min. Then 100 μl of reagent IV and 2000 μl of reagent V were added. The blank assay tube was replaced with 30 μl of double-distilled water instead of plasma, and the rest of the reagents added were the same as the assay tube. Zeroing with distilled water, the OD value was measured at 530 nm. TNOS and iNOS activities were calculated according to OD values.
For the determination of IL-2, IL-4 and IFN-γ concentration, take 50 μl of the standard solutions and plasma samples into the microtiter plate, then add 50 μl of biotin antigen and react at 37°C for 30 min. Wash the sample wells and standard wells, then add 50°C avidin–horseradish peroxidase (HRP) and react at 37°C for 30 min. Wash the sample wells and standard wells again and add 50 μl of chromogen A and chromogen B, respectively. React for 10 min protected from light, and finally add 50 μl of termination solution. The OD value was measured at 450 nm. Calculate the regression equation of the standard curve based on the standard concentrations and OD values, and then use the regression equation and the sample OD values to calculate the sample concentrations.
RNA extraction and real-time PCR analysis
Total RNA was extracted from the jejunal mucosa samples using Trizol reagent (Takara Biotechnology Co. Ltd.). The purity and quantity of the RNA were measured with a Nanodrop ND-1000 spectrophotometer (Thermo Scientific). Total RNA was RT to cDNA using a PrimeScript RT Master Mix kit (Takara Biotechnology). The RT reactions were incubated for 15 min at 37°C, followed by 5 s at 85°C. The RT products (cDNA) were stored at −20°C.
Real-time quantitative PCR (RT-qPCR) was carried out in optical 96-well plates on an QuanStudio6 RT-qPCR detection system (Applied Biosystems) using SYBR Premix Ex Taq kits (Takara Biotechnology). The amplification was performed in a total volume of 20 μl, containing 10 μl of SYBR Premix Ex Taq, 0·4 μl of each primer (10 μM), 0·4 μl of ROX Reference Dye II, 2 μl of cDNA and 6·8 μl of sterilised doubled-distilled water. The following cycling conditions were used: 95°C for 30 s, followed by 40 cycles of denaturation at 95°C for 5 s and anneal at 60°C for 30 s and the collection of the fluorescence signal at 60°C. The pairs of specific primers for target genes were presented in Table 2. The expression of target gene relative to 18S rRNA were calculated using the 2−ΔΔCT method(Reference Livak and Schmittgen20).
Table 2. Primer sequences for real-time quantitative PCR analysis

Statistical analysis
The sample size was calculated using data (plasma IgG concentration) from a previous study. The study compared the effects of a prebiotic xylo-oligosaccharide and flavomycin on immune function of broilers and found that the plasma IgG concentration was 2·03, 2·19 and 2·45 mg/ml, and sd was 0·16, 0·13 and 0·21, respectively(Reference Yuan, Li and Huo21). Accordingly, using the PASS 15.0 software, it was determined that a sample size of n=6 would allow the power of 80 % at the level of significance of 5 %. By considering a dropout, the required sample size was n=8. The mortality data were analysed using Kruskal–Wallis of nonparametric test using SPSS software (Version 20.0, SPSS Inc.). Other data were analysed using one-way ANOVA and checked for normal distribution and homogeneity of variance using the Shapiro–Wilk tests. The effect of graded levels dietary RS was determined by orthogonal polynomial contrasts. The model included linear and quadratic contrasts for effects of supplemental RS. P < 0·05 was considered statistically significant. The results are presented as means with their standard errors (se). The cage (replicate) was the sampling unit and the experimental unit. A total of eight replicate were used per treatment (n=8).
Results
Growth performance and mortality
In our previous study(Reference Zhang, Liu and Li16), the birds fed RS diets had lower (P < 0·05) average daily gain, average daily feed intake and gain to feed ratio (G/F) than those receiving the control diet. As shown in Fig. 1, feeding RS diets did not have a significant effect (P > 0·05) on mortality in the five treatment groups.

Fig. 1. Mortality of broilers fed diets supplemented with graded levels of corn resistant starch (RS). NC, a basic normal corn–soyabean diet; CS, a corn–soyabean–based diet supplementation with 20 % corn starch (CS); 4 % RS, 8 % RS, and 12 % RS, the corn–soyabean–based diets supplementation with 4, 8, and 12 % corn RS, respectively.
Lymphoid organ development
Birds fed RS-treated diets linearly increased (P < 0·05) the relative weights of spleen, thymus and bursa at 21 d of age (Table 3). Meanwhile, the relative weight of spleen in 8 % RS group and the relative weight of bursa in groups of 4 % RS, 8 % RS and 12 % RS were higher (P < 0·05) than those of NC group at 21 d of age. The relative weight of spleen in groups of CS, 4 % RS, 8 % RS and 12 % RS were higher (P < 0·05) than those of NC group at 42 d of age.
Table 3. Immune organ index of broilers fed diets supplemented with graded levels of corn resistant starch (RS)
(Mean values with their standard errors of the mean, n=8)

NC, normal corn–soyabean; CS, corn starch; RS, resistant starch; BW, body weight.
* NC, a basic normal corn–soyabean diet; CS, a corn–soyabean–based diet supplementation with 20 % CS; 4 % RS, 8 % RS, and 12 % RS, the corn–soyabean–based diets supplementation with 4, 8, and 12 % corn RS, respectively.
† Orthogonal polynomial contrast was used to determine linear and quadratic effects of increasing concentrations of resistant starch in CS and RS diets.
a,b,cMeans in a row without a common superscript letter significantly differ (P < 0·05).
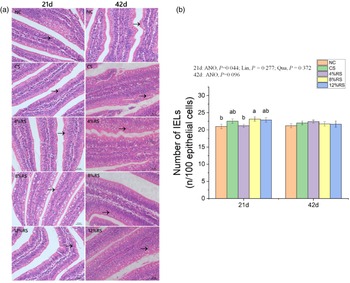
The number of intraepithelial lymphocytes in jejunum
Birds from 8 % RS group exhibited higher (P < 0·05) number of jejunal IEL compared with those in NC and 4 % RS groups at 21 d of age (Fig. 2). There is no observable difference (P > 0·05) in jejunal IEL number of birds among all groups at 42 d of age.

Fig. 2. (a) Morphological observation of intraepithelial lymphocytes in jejunum of broilers based on hematoxylin–eosin staining. (b) Intraepithelial lymphocytes density in jejunum of broilers fed diets supplemented with graded levels of corn resistant starch. The results are represented as the mean value ± se (n=8). Means without a common letter significantly differ (P < 0·05). NC, a basic normal corn–soyabean diet; CS, a corn–soyabean–based diet supplementation with 20 % corn starch (CS); 4 % RS, 8 % RS, and 12 % RS, the corn–soyabean–based diets supplementation with 4, 8, and 12 % corn resistant starch (RS), respectively.
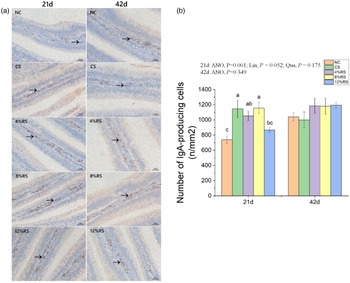
The density of IgA-producing cells in jejunum
In comparison with the NC group, higher (P < 0·05) density of jejunal IgA-producing cells were presented in birds treated with CS, 4 % RS and 8 % RS diets at 21 d of age (Fig. 3). No significant difference (P > 0·05) in density of IgA-producing cells among all groups at 42 d of age.

Fig. 3. (a) The immunohistochemical assessment of IgA-producing cells in jejunum. (b) Number of IgA-producing cells in jejunum of broilers fed diets supplemented with graded levels of corn resistant starch (RS). The results are represented as the mean value ± se (n=8). Means without a common letter significantly differ (P < 0·05). NC, a basic normal corn–soyabean diet; CS, a corn–soyabean–based diet supplementation with 20 % corn starch (CS); 4 % RS, 8 % RS, and 12 % RS, the corn–soyabean–based diets supplementation with 4, 8, and 12 % corn RS, respectively.
Plasma NO concentration, TNOS and iNOS activities
Birds fed RS diets linearly increased (P < 0·05) the NO concentration of plasma at 21 d of age and the TNOS and iNOS activities of plasma at 21 and 42 d of age (Table 4). Meanwhile, the plasma NO concentration in birds from the 4 % RS, 8 % RS and 12 % RS groups were higher (P < 0·05) than that in the NC group at 21 d of age. The activities of plasma TNOS and iNOS in broilers from 12 % RS group were higher (P < 0·05) than those in the NC group at 42 d of age.
Table 4. Plasma NO concentration, TNOS and iNOS activities of broilers fed diets supplemented with graded levels of corn resistant starch
(Mean values with their standard errors of the mean, n=8)

NC, normal corn–soyabean; CS, corn starch; RS, resistant starch.
* NC, a basic normal corn–soyabean diet; CS, a corn–soyabean–based diet supplementation with 20 % CS; 4 % RS, 8 % RS, and 12 % RS, the corn–soyabean–based diets supplementation with 4, 8, and 12 % corn RS, respectively.
† Orthogonal polynomial contrast was used to determine linear and quadratic effects of increasing concentrations of resistant starch in CS and RS diets.
a,bMeans in a row without a common superscript letter significantly differ (P < 0·05).
The concentrations of IFN-γ, IL-2 and IL-4 in plasma
Dietary RS supplementation linearly increased (P < 0·05) plasma IL-4 content of bird at 21 d of age and plasma IL-2 content at 42 d of age (Table 5). Moreover, compared with the NC group, birds fed the RS supplementation diets showed higher (P < 0·05) plasma IFN-γ content and birds consumed the 8 % RS diets also exhibited higher (P < 0·05) plasma IL-4 content at 21 d of age (P < 0·05).
Table 5. Plasma interferon (IFN)-γ, IL-2 and IL-4 concentrations of broilers fed diets supplemented with graded levels of corn resistant starch
(Mean values with their standard errors of the mean, n=8)

NC, normal corn–soyabean; CS, corn starch; RS, resistant starch; IFN, interferon.
* NC, a basic normal corn–soyabean diet; CS, a corn–soyabean–based diet supplementation with 20 % CS; 4 % RS, 8 % RS, and 12 % RS, the corn–soyabean–based diets supplementation with 4, 8, and 12 % corn RS, respectively.
† Orthogonal polynomial contrast was used to determine linear and quadratic effects of increasing concentrations of resistant starch in CS and RS diets.
a,b,cMeans in a row without a common superscript letter significantly differ (P < 0·05).
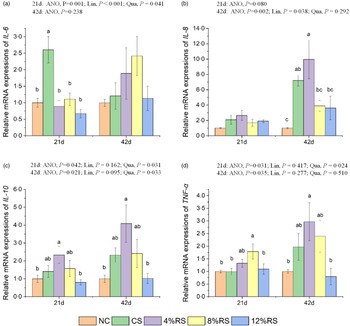
The mRNA expressions of cytokines in jejunal mucosa
The broiler jejunal mRNA expressions of the IL-6 at 21 d and IL-8 at 42 d in RS-treated groups were linearly down-regulated (P < 0·05), while the mRNA expressions of IL-6, IL-10 and TNF-α at 21 d as well as IL-10 at 42 d in RS-treated groups were quadratically up-regulated (P < 0·05). In comparison with the NC group, the broiler jejumal IL-10 mRNA expression in 4 % RS group and the TNF-α mRNA expression in 8 % RS group were higher (P < 0·05) at 21 d of age (Fig. 4). Meanwhile, the mRNA expressions of IL-8 and IL-10 in 4 % RS group were also higher (P < 0·05) than those in the control group at 42 d of age.

Fig. 4. Relative mRNA expressions of (a) IL-6, (b) IL-8, (c) IL-10 and (d) TNF-α in jejunum of broilers fed diets supplemented with graded levels of corn resistant starch (RS). The results are represented as the mean value ± se (n=8). Means without a common letter significantly differ (P < 0·05). NC, a basic normal corn–soyabean diet; CS, a corn–soyabean–based diet supplementation with 20 % corn starch (CS); 4 % RS, 8 % RS, and 12 % RS, the corn–soyabean–based diets supplementation with 4, 8, and 12 % corn RS, respectively.
Discussion
The immune-promoting effects of RS on humans and mammals have been widely recognised, but little is known about avian species. In the present study, we have confirmed our hypothesis that RS could elevate the immune function of broiler chickens. We showed that dietary supplementation with RS can improve mucosal immune and systemic immune function, which evidenced by the increased density of immune cells IEL and IgA-producing cells, and the relative weight of spleen and bursa, as well as the increased concentrations of plasma NO and cytokines. These results can provide a theoretical basis for the rational use of RS in poultry diets
Our previous study has demonstrated that dietary supplementation with RS significantly reduced average daily gain, average daily feed intake and G/F(Reference Zhang, Liu and Li16), but it had no effect on mortality in this study. These results indicated that RS may have a beneficial effect on the immune function of broilers. A possible mechanism for the reduction of average daily feed intake and average daily gain by RS is that the RS supplementation can reduce the proportion of rapidly digestible starch in diets, which would affect the energy metabolism of broilers. And RS has also been proved to promote the secretion of glucagon-like peptide-1 (GLP-1) and peptide tyrosine tyrosine (PYY), which have an appetite suppressing effect and may contribute to the reduction of average daily feed intake and average daily gain(Reference Zhou, Martin and Tulley22,Reference De Silva and Bloom23) . However, RS can be used by hindgut microorganisms to ferment and produce the metabolite, SCFA, which is known to regulate the production, transport and function of innate and adaptive immune cells(Reference Gonçalves, Araújo and Di Santo24). And we have reported that RS does increase the production of SCFA in broiler caecum(Reference Zhang, Liu and Li25). This leads us to believe that RS may have the effect of improving immune characteristics of broilers.
The body immune system includes three major components, the immune organs, immune cells and immunologically active substances(Reference Yu, Ma and Gong26). In poultry, thymus and bursa of Fabricius are central lymphoid organs, and spleen is the peripheral lymphoid organ(Reference Oláh, Nagy, Vervelde, Schat, Kaspers and Kaiser27). These immunological organs are responsible for the immune cells production, maturation, storage and release, and their weight is often as a measure of the body’s immune capacity(Reference Oláh, Nagy, Vervelde, Schat, Kaspers and Kaiser27,Reference Kwak, Austic and Dietert28) . The thymus is where the lymphocyte progenitor cells develop into T cell subpopulations(Reference Kaspers, Göbel and Ratcliffe29). The bursa of Fabricius is a central lymphoid organ unique to poultry, also the place where B lymphocytes occur, differentiate and mature(Reference Kaspers, Göbel and Ratcliffe29,Reference Ratcliffe, Härtle, Schat, Kaspers and Kaiser30) . In this experiment, birds fed RS significantly elevated the relative weight of bursa, and also had an increasing trend for the relative weight thymus at 21 d of age. These might be an initial positive signal for the immunity improvement of the chicks. But for the 42-d-old birds, there was no difference in the relative weight of thymus and bursa in all treatment groups. One possible reason may be thymus and bursa are essential in neonatal life(Reference Cheville31,Reference Cooper, Raymond and Peterson32) . Then thymus will start to degenerate after growing up(Reference Bredenkamp, Nowell and Blackburn33). Meanwhile, we also found that birds fed RS diet increased the relative weight of the spleen. For poultry, the spleen is a more important peripheral immune organ than that in mammals because of the poorer development of lymphatic vessels and nodes(Reference Oláh, Nagy, Vervelde, Schat, Kaspers and Kaiser27). In some pathological conditions, avian immune organs may degenerate and decrease in weight(Reference Quinteiro-Filho, Ribeiro and Ferraz-de-Paula34,Reference Sun, Cui and Liu35) . Whereas prebiotic supplementation has the effect of increasing the weight of the lymphoid organs and improve other immunological indicators. For example, dietary supplementation with oligochitosan can increase the relative weight of bursa and thymus, and there was also an increasing trend in the relative weight of spleen. Meanwhile, serum concentrations of IgG, IgA and IgM were also increased(Reference Huang, Deng and Yang36). Similar results were obtained for RS in our study. These results suggest that RS can promote the development of lymphoid organs and may futher modulate the immune function of broilers.
The intestinal mucosa separates internal from external environment, preventing the invasion of potentially harmful substances. The IEL constitute a population of cells dwelling interspersed in intestinal epithelial cells and represent a unique immunological compartment in the intestines(Reference Hoytema van Konijnenburg, Reis and Pedicord37). We thus measured the lymphocyte density in jejunal epithelium. Our results showed that 8 % RS treatment significantly improved the jejunal IEL density of birds at 21 d of age compared with birds in the NC and 4 % RS groups, suggesting that RS can promote the development of IEL in the early stage of broilers. An increased number of intestinal immune cells implies the improvement of mucosal immune function. Secreted sIgA promote the clearance of antigens and pathogenic microorganisms from the intestinal lumen by blocking their access to epithelial receptors, entrapping them in mucus(Reference Pabst38). In the current study, the density of jejunal IgA-producing cells were higher in groups of 4 % RS and 8 % RS than those in the NC group at 21 d of age. The trend of the results of the effect of RS on IgA-producing cells is consistent with that of RS on IEL, indicating that RS enhances mucosal immunity in broiler chickens by increasing the density of immune cells.
Cytokines is mainly secreted by T cells and can be used as a measure of immune response ability of the body(Reference Raphael, Nalawade and Eagar39). The helper T cells of the body are divided into Th1 and Th2 subgroups. Th1 subgroup mainly mediates cellular immunity and inflammatory response and secretes cytokines such as IL-2 and IFN-γ, which are important for antiviral and antibacterial immunities. Th2 subgroup mainly mediates humoral immunity and secretes IL-4 etc., which is responsible for the immunity of extracellular pathogens(Reference Swain40). All RS diet treatments increased plasma IFN-γ concentration, and 8 % RS treatment also increased plasma IL-4 concentration of broilers at 21 d of age compared with the NC, CS and 4 % RS groups. These results demonstrated that both cellular and humoral immunity were elevated when broilers were fed RS diets. In addition, 4 % RS up-regulated the mRNA expression pro-inflammatory cytokines IL-8 and TNF-α in jejunum mucosa. Meanwhile, the mRNA expression of anti-inflammatory cytokines IL-10 was also up-regulated in 4 % RS group compared with the NC and 12 % RS treatments. The up-regulation of both pro-inflammatory and anti-inflammatory cytokines in the 4 % RS group might create a new state of immunological balance.
NO is a kind of small molecular substance synthesised by NOS with extensive functions and unique properties in biology. NO not only acts as the nervous system signaling molecule, but also plays an important role in the immune defense(Reference Coleman41). When the organism is induced by cytokines or infected by bacteria, viruses and parasites, the iNOS existing in macrophages will catalyse the release of NO(Reference Kleinert, Pautz and Linker42). In this study, dietary RS supplementation increased the plasma NO concentration of birds at 21 d of age, and bird in 12 % RS group showed the highest plasma TNOS and iNOS activities at 42 d of age. There is an increasing evidence that NO is essential for the regulation of the inflammatory response(Reference Sharma, Al-Omran and Parvathy43). Therefore, the increased plasma NO concentration and iNOS activity may have the ability to relieve the underlying inflammation in the chicken body.
In summary, the present study investigated the effect of dietary corn RS on broiler immunological characteristics. Our results revealed that dietary corn RS supplementation can modulate mucosal and systemic immune function in broiler chickens by increasing jejunal immune cells IEL and IgA-producing cells density and cytokines mRNA expression, as well as elevating the relative weight of lymphoid organs and plasma NO concentration during the starter growth period. These alterations may enrich the nutritional theory of starch research in broiler chickens.
Acknowledgements
We thank the members of Quality and Safety Control, Joint International Research Laboratory of Animal Health and Food Safety for the animal feeding and sample collection.
This study was financially supported by the National Key Research and Development Programme of China (2017YFD0500505) and the Earmarked Fund for Jiangsu Agricultural Industry Technology System (JATS[2021]459).
The authors’ contributions were as follows: Y.-Y. Z. and F. G. designed the research; Y.-Y. Z., Y.-S. L., T. X., J.-L. L., Y. J., L. Z. and F. G. performed the research; Y.-Y. Z. analysed data and wrote the paper; all authors read, edited and approved the final manuscript.
The authors declare that there are no conflicts of interest.