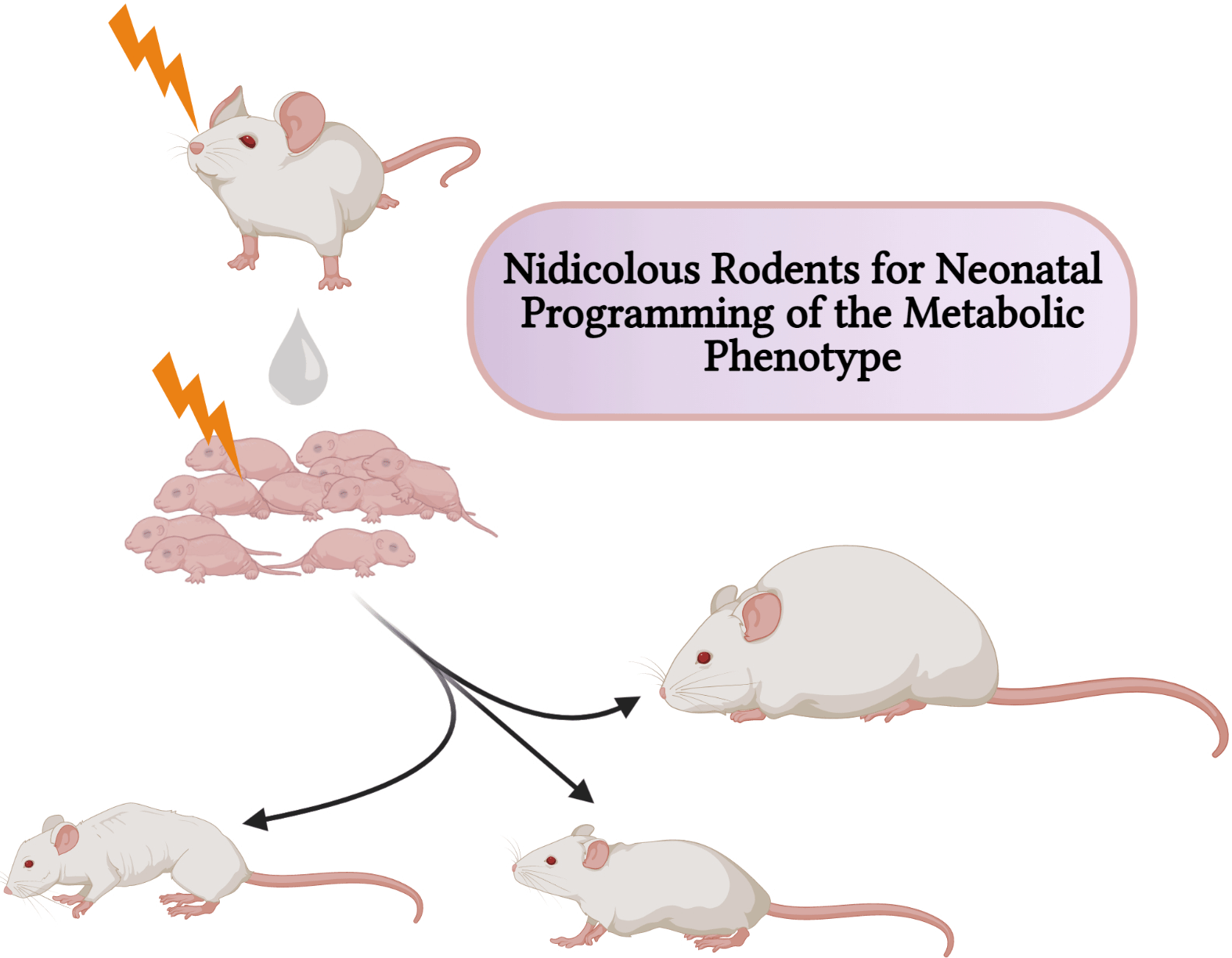
Obesity and non-communicable diseases like type 2 diabetes mellitus constitute global health concerns due to their high morbidity and mortality(Reference Saklayen1). These and other metabolic diseases arise due to one or more abnormalities in the components of cellular metabolism. Studies have implicated not only genetic variations like polymorphisms and mutations of certain genes but also prenatal and postnatal environmental factors that influence the expression of the genes responsible for the metabolism of carbohydrates and lipids(Reference Hernández-Granados, Ramírez-Emiliano, Franco-Robles and Ibeh2).
The term ‘developmental programming’ describes adaptive phenotypic changes that occur in later life following environmental exposure to certain factors, termed ‘stressors’ or ‘cues’, at critical periods of early life. The intrauterine and neonatal life represent critical and sensitive periods of programming for cellular metabolism. In some cases, the subsequent reprogramming of neonatal life outweighs the programmed developments of intrauterine life(Reference George, Draycott and Muir3,Reference Vithayathil, Gugusheff and Ong4) . Despite the need for more scientific investigations on developmental programming of neonatal life, several factors, such as ethical considerations, genetic heterogeneity, differential disease outcomes, intensive care treatment and methodological constraints, limit direct investigations on the human neonate. Consequently, studies have predominantly adopted the rodent model to study neonatal exposure and adult health(Reference Puimana and Stolla5). These animal models are useful for the study of malleable environmental factors such as diets, acting predominantly through epigenetic routes(Reference Cheng, Zheng and Almeida6).
A number of reviews identify the general use of adult animal models programmed for specific pathologies such as metabolic syndrome(Reference Panchal and Brown7,Reference Wong, Chin and Suhaimi8) , diabetes and obesity(Reference Hernández-Granados, Ramírez-Emiliano, Franco-Robles and Ibeh2,Reference Alquier and Poitout9) . Some others focus on the use of single faceted nutritional models to neonatal programming(Reference Puimana and Stolla5,Reference Flamm10–Reference McClain, Keller and Casciano12) . However, the predominant use of rodents (rats and mice) for neonatal programming lacks full evaluation. Also, emphasis has been raised for the documentation of newer models and clarification of the considerations and caveats for their selection(Reference Hernández-Granados, Ramírez-Emiliano, Franco-Robles and Ibeh2,Reference Wong, Chin and Suhaimi8,Reference Alquier and Poitout9) . Herein, we employed a pioneer attempt to consolidate the altricial models of neonatal programming and document their suitability in the prevention and treatment of metabolic disorders. Furthermore, we present some guidelines and confounding points for model selection and animal experimentation.
Neonatal programming
Previously, the ‘Foetal Origins of Adult Disease’ hypothesis was used to define the relationship between gestational exposure to stressors and the emergence of adult metabolic disorders(Reference Barker and Osmond13–Reference Hales and Barker15). This phenomenon was termed ‘fetal programming’ due to its recognition of the fetal life as a period of plasticity. Subsequently, it was established that exposure to stressors in the postnatal (suckling) period also programme the outcome of adult health(Reference Koletzko16). This recognition of ‘neonatal programming’ led to a shift in the hypothesis to ‘the Developmental Origins of Adult Health and Disease’. The overall phenomenon thus became ‘Developmental programming’(Reference Armitage, Taylor and Poston17,Reference Mandy and Nyirenda18) .
While the stressors that trigger developmental programming could be either exogenous, endogenous or both(Reference Reichetzeder, Dwi Putra and Li19), they are not always detrimental, and the outcomes of developmental programming could be inherited intergenerationally or transgenerationally(Reference Xu, Wang and Chen20). For instance, Benyshek et al. (Reference Benyshek, Johnston and Martin21) demonstrated transgenerationally altered glucose metabolism of F3 descendants of rats (F1) undernourished during lactation. Furthermore, exposure to stressors could either be gestational, triggering fetal programming, or may occur in the early postnatal period, causing alterations in adult metabolism due to neonatal programming(Reference Aiken, Tarry-Adkins and Ozanne22).
Neonatal programming occurs during the suckling period. This period for humans, if allowed, can naturally extend from the neonatal phase of development (birth to 1 month) into infancy (1 month to 2 years)(Reference Kramer and Kakuma23). Comparatively, the suckling period for laboratory altricial rodents (rats and mice) extends to 3 weeks (day 21) after birth and weaning is marked by separation of pups from the dams. However, this may also extend naturally past the 21 d period. The suckling period for rodents comprises of a neonatal phase (birth to 1 week) and an infancy phase (week 1 end to week 3 end) (Reference Barrow, Barbellion and Stadler24,Reference Picut, Dixon and Simons25) .
Mechanistic bases for the metabolic programming in early life
With respect to Developmental Origins of Adult Health and Disease, metabolic alterations rely on the mechanistic ‘retention’ of the impact made by an exposure at early life. So far, such mechanisms are accounted for by some morphological memories and epigenetics(Reference Fall and Kumaran26). As outlined later on, morphological changes (particularly of the gut) are peculiar to the suckling period. These include tissue remodelling, altered cell numbers, cell distribution or cell selection which may alter in response to nutrition and environment. Accumulating evidence also shows that epigenetics is a primary mechanism by which developmental programming influences and prepares an organism for adult life(Reference Goyal, Limesand and Goyal27). This includes changes in the DNA methylome, histone modifications and regulatory noncoding RNA, all affecting gene expression. Notably, the DNA methylome is particularly re-established in early life. During fertilisation and implantation, huge portions of the mammalian DNA methylation are lost, but later regained during the developmental periods (intra-uterine life, suckling period and puberty)(Reference Fall and Kumaran26). This re-methylation can be modified by nutrition or environment, and a number of reports have demonstrated the role of DNA methylome to neonatal programming. For instance, Plagemann et al. (Reference Plagemann, Roepke and Harder28) illustrated that over-nourished rat pups possessed a dose-dependently increased methylation of their hypothalamic insulin receptor promoter sites, predisposing them to diabesity in later life. We believe, at least anecdotally, that the nutritional influence on epigenetics is partly a function of the one-carbon cycle, which generates the methyl groups needed for DNA methylation. This is because nutrients, such as vitamin B12, folic acid, choline and betaine present in milk, are relevant for the maintenance of this cycle(Reference Lillycrop and Burdge29). Further investigations are required to fully understand these mechanisms and possibly uncover new ones.
Epigenetic changes alongside morphological memories (such as cellular distribution/tissue remodelling) may influence metabolic activity, the development of hormonal systems and trigger programming to a degree dependent on timing, type, magnitude and duration of stressor exposure, from conception to the first 1000 d of human life(Reference Mandy and Nyirenda18,Reference Fall and Kumaran26) . This period of life stretches from the beginning of gestation to the end of the early postnatal life. Notably, prematurity presents a classic case of exposure to adversity during these 1000 d, both gestational and postnatal(Reference Reichetzeder, Dwi Putra and Li19).
Neonatal programming usually primes the subject to cope with future adversity such as a scarcity of nutrients. This ‘predictive adaptive response’ is an anticipatory process involving developmental adjustments after early-life exposure to stressors(Reference Wells30). It occurs to match and adapt an organism’s phenotype against later life exposure(Reference Bateson, Gluckman and Hanson31). However, stress results when the predictive adaptive response is mismatched with the future condition resulting in cardiovascular and metabolic disorders(Reference Goyal, Limesand and Goyal27). In line with this concept of mismatch, studies relating the neonatal environment to developmental conditions reveal that offspring suckled by diabetic, obese or malnourished mothers are predisposed to metabolic disorders in later life(Reference Fante, Simino and Reginato32–Reference Wattez, Delmont and Bouvet36). Even so, the collective mechanisms by which such responses develop are yet to be fully elucidated. Notwithstanding, this critical neonatal period is a potent window for developing some targeted metabolic studies and possible preventive measures(Reference Lewis, Galbally and Gannon37).
Altricial rodents
The nidicolous or altricial rat and mouse are subsets of the largest mammalian order, Rodentia, whose blind pups display absolute dependency on the dams, including a need for stimulation to micturate and defecate(Reference Otto, Franklin, Clifford, Fox, Anderson and Otto38,Reference Aalinkeel, Srinivasan and Song39) . For reasons we shall discuss, these altricial mammals present a stream of species with conditions that translate to the human state and may prove useful in investigating and understanding the fundamental mechanisms involved in neonatal programming. Research with these animal models targets some environmental and/or even genetic events that may shape the metabolic development of a suckling pup, at a period of developmental plasticity. Furthermore, beside the traditional approaches to disease treatment using these animal models(Reference Liu, Srinivasan and Mahmood40,Reference Schroeder, Shbiro and Moran41) , some novel attempts at disease prevention have been established. For instance, neonatal intake of Hibiscus Sabdariffa was shown to protect against fructose-induced dyslipidaemia(Reference Ibrahim, Chivandi and Mojiminiyi42), while neonatal intake of oleanolic acid was reported to repress the development of fructose-induced non-alcoholic fatty liver disease in adult rats(Reference Nyakudya, Mukwevho and Nkomozepi43).
Why rats and mice represent good animal models for investigating the neonatal development of metabolic disorders?
Altricial rodents are the most widely used laboratory animals due to their relatively short life span, little space for maintenance, fecundity, short gestation period and well-defined genetic background, completely sequenced genome of mice (2002: Mouse Genome Sequencing Consortium) and rats (2004: Rat Genome Consortium) (Reference Otto, Franklin, Clifford, Fox, Anderson and Otto38,Reference Gibbs, Weinstock and Metzker44,Reference Waterston, Lindblad-Toh and Birney45) . Models of these rodents are useful in evaluating maternal interventions that exert influence on the metabolic development of the neonate. Their known genomic status provides foundation for genetically modified forms that can be purposely bred to investigate specific disorders(Reference Vandamme46).
During the neonatal period, the maternal milk, via nutrients and bioactive factors, has the major influence on pup development. Though the pups are blind at birth, they are attracted to the mother’s nipples by certain odoriferous cues that coat them(Reference Aïn, Belin and Schaal47). Milk-laden factors, antigens, immunoglobulins, bacteria and macromolecules are absorbed across a highly permeable enteric epithelium. For rats and mice, this permeability to macromolecules completely halts after weaning, a process called ‘gut closure’ which limits the absorption some dietary inclusions(Reference Teichberg, Wapnir and Moyse48,Reference Xu, Collins and Ghishan49) . Like humans, suckling rodents have a hardwired age-related increase in receptor number, sensitivity and signal transduction than weaned rodents. This comes with associated consequences. For instance, the increased receptors and sensitivity for enterotoxins of Escherichia coli and cholera in the suckling period increase neonatal susceptibility to diarrhoea in man and rat(Reference Cohen, Guarino and Shukla50–Reference Swenson, Mann and Jump53). A similar increase with development is noted for certain transporters such as sodium-glucose transporter 1, apical sodium-dependent bile acid transporter, water and Na transport channels in neonatal man, rats and mice(Reference Xu, Collins and Ghishan49). Also, a precociously increased expression of other transporters like GLuT-2 and GLuT-5 can occur through transcriptomic influence in response to early dietary exposure such as a high fructose intake(Reference Xu, Collins and Ghishan49). This has been adopted for the neonatal induction and accelerated development of adult metabolic disorders in these rodents(Reference Ibrahim, Wright and Chivandi54,Reference Toop, Muhlhausler and O’Dea55) .
Furthermore, altricial rodents are characterised by low forms of developmental maturity surrounding aspects of gastrointestinal nutrition and evacuation, thermoregulation, locomotion with most ontogeny finishing after birth. The periodical similarity of rodent-human ontogeny of several organs or systems makes suckling rodents suitable targets for neonatal research directed at specific metabolic conditions. Underneath this descriptive similarity of the suckling period of rodents to humans are certain changes that mirror the different phases of human development. For instance, some underdevelopments of the brain and gut show similarity to human premature babies and third trimester of human gestation(Reference Puimana and Stolla5). The enteric maturation occurring within the suckling period of altricial rodents relates to in utero intestinal development for humans. The time lapse is perhaps due to the shorter gestational period for rodents as compared with the longer gestational periods in man(Reference Xu, Collins and Ghishan49). More so, the developmental increase in disaccharidases (sucrase, isomaltase) for humans occurs from the 12th week of gestation while that for rats and mice is increased at weaning(Reference Antonowicz and Lebenthal56–Reference Ménard and Pothier58).
Like humans, suckling rodents express critical development in white adipose tissue(Reference Butruille, Marousez and Pourpe59); immune system and adaptation(Reference Pérez-Cano, Franch and Castellote11); establishment of neural networks (hypothalamus) and astrocyte development(Reference Canal, Mohammed and Rodriguez60) and permanent metabolic programming of appetite and growth dynamics. All these become useful targets and biomarkers for neonatal programming which are leveraged upon by researchers to test hypotheses and bridge scientific gaps. Specifically, these similarities found in suckling altricial rodents have created opportunities to assess food composition/quality and natural products (for prophylaxis)(Reference Gumede, Lembede and Nkomozepi61,Reference Le Dréan, Pocheron and Billard62) ; understand gut microbes and mucosal homoeostasis(Reference Steegenga, Mischke and Lute63); conduct immune programming (immuno-nutrition) and genetic studies (advantaged in toe clipping)(Reference Pérez-Cano, Franch and Castellote11,Reference Paluch, Lieggi and Dumont64) among others.
Despite some marked differences from humans and a major setback in repeated blood sampling due to low blood volume, altricial rodents retain benefits to their undersize and underdevelopment. Rodent pups bear underdeveloped guts and brains that mature during lactation, before weaning and prove useful in the shared similarity with that of a premature human infant(Reference Puimana and Stolla5). Though adult rodents can be surgically manipulated, the diminutive pup size, particularly of mice, presents a key obstacle for studies beginning at postnatal day 1. Regardless of this challenge, scientists the world over have developed and are still brainstorming upon newer techniques that rightly fit for neonatal programming. This has come with some potent breakthroughs(Reference Wattez, Delmont and Bouvet36,Reference Beierle, Chen and Hartwich65–Reference Willingham, McNulty and Anderson71) .
Notably, no animal species possess complete similarities to human, along enzymatic form and activity. However, some enzyme isoforms acting upon specific metabolites show similarity in humans and rodents. For instance, the human cytochrome P450, subfamily 1A enzyme (CYP1A), has shown similarity with mice, while human cytochrome P450, subfamily 3A enzyme (CYP3A) exists similarly in both mice and male rats(Reference Marcella, Groothuis and Kanter72). On the other hand, certain specie-specific differences also exist with some other enzyme isoforms, their organ specificity, expression and/or catalytic action. Marcella et al. (Reference Marcella, Groothuis and Kanter72) gave a detailed comparison of enzymes critical to oxidative metabolism in mice, rats and humans. This knowledge is essential for extrapolation of data to humans particularly in studies for preclinical drug development, metabolism and/or drug interactions. Additionally, altricial rodents share similarities with humans in many genes and biochemical pathways(Reference Puimana and Stolla5). Consequently, experimental methods into the metabolic programming of neonatal rodents are useful in establishing developmental mechanisms and assessing natural products that potentially prevent deficits of early life from triggering the metabolic adversities of later life.
Models of altricial rodents for neonatal programming
Animal models are useful representations of humans that allow for extensive hypotheses testing and interventions. The animal models for neonatal programming study direct or indirect postnatal exposure to stressors in the suckling stage of life. These animal models have specific focus on either postnatal exposure and neonatal programming or on the combinational effects of a longitudinal exposure from the pre-conceptional or in utero period to the postnatal period(Reference Vithayathil, Gugusheff and Ong4,Reference Toop, Muhlhausler and O’Dea55) . In this review, we categorised the models into five, based on the approaches used to trigger neonatal programming (see Tables 1–5 for summary of some articles). These models include altered litter size models, models of altered lactational conditions of the dams, models of altered condition of the pups (diet, photic experience, smoking and maternal separation), foster models and genetic models. These models alongside control groups are useful, singly or in conjunction, to study the development of metabolic phenotype. Using these models, structural changes in the brain, GIT, skeletal muscle, liver and plasma, alongside epigenetic events have been identified in the development of metabolic disruptions(Reference Canal, Mohammed and Rodriguez60,Reference Steegenga, Mischke and Lute63,Reference Liu, Mahmood and Srinivasan73–Reference Yuan, Tsujimoto and Hashimoto76) .
Table 1. Summary of some publications using the altered litter size model for neonatal programming
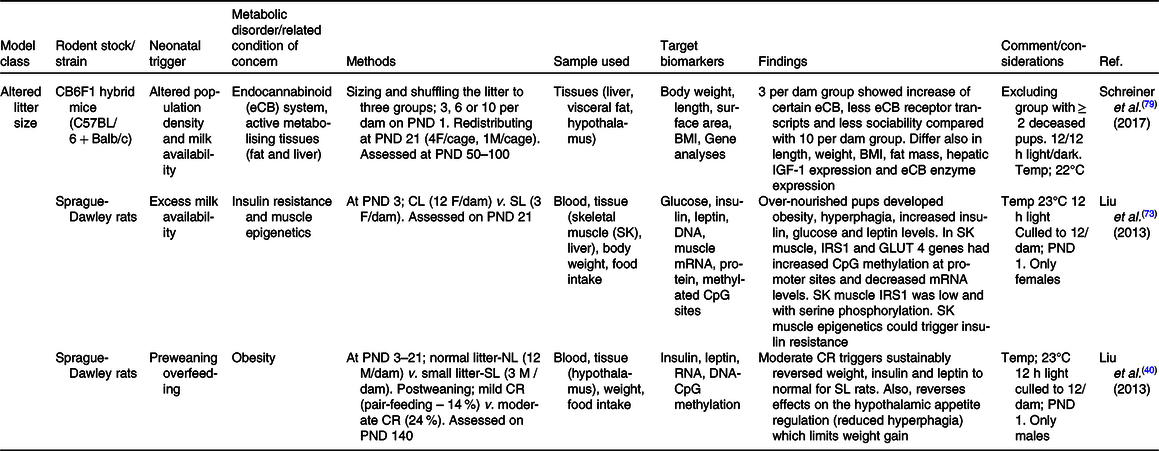
CL, control litter; CR, energetic restriction; GLUT, glucose transporter; ipGTT, intraperitoneal glucose tolerance test; ipITT, intraperitoneal insulin tolerance test; IRS, insulin receptor substrate; NL, normal litter; PND, postnatal day; SL, small litter.
Table 2. Summary of some publications using the model of altered lactational condition of the dam for neonatal programming
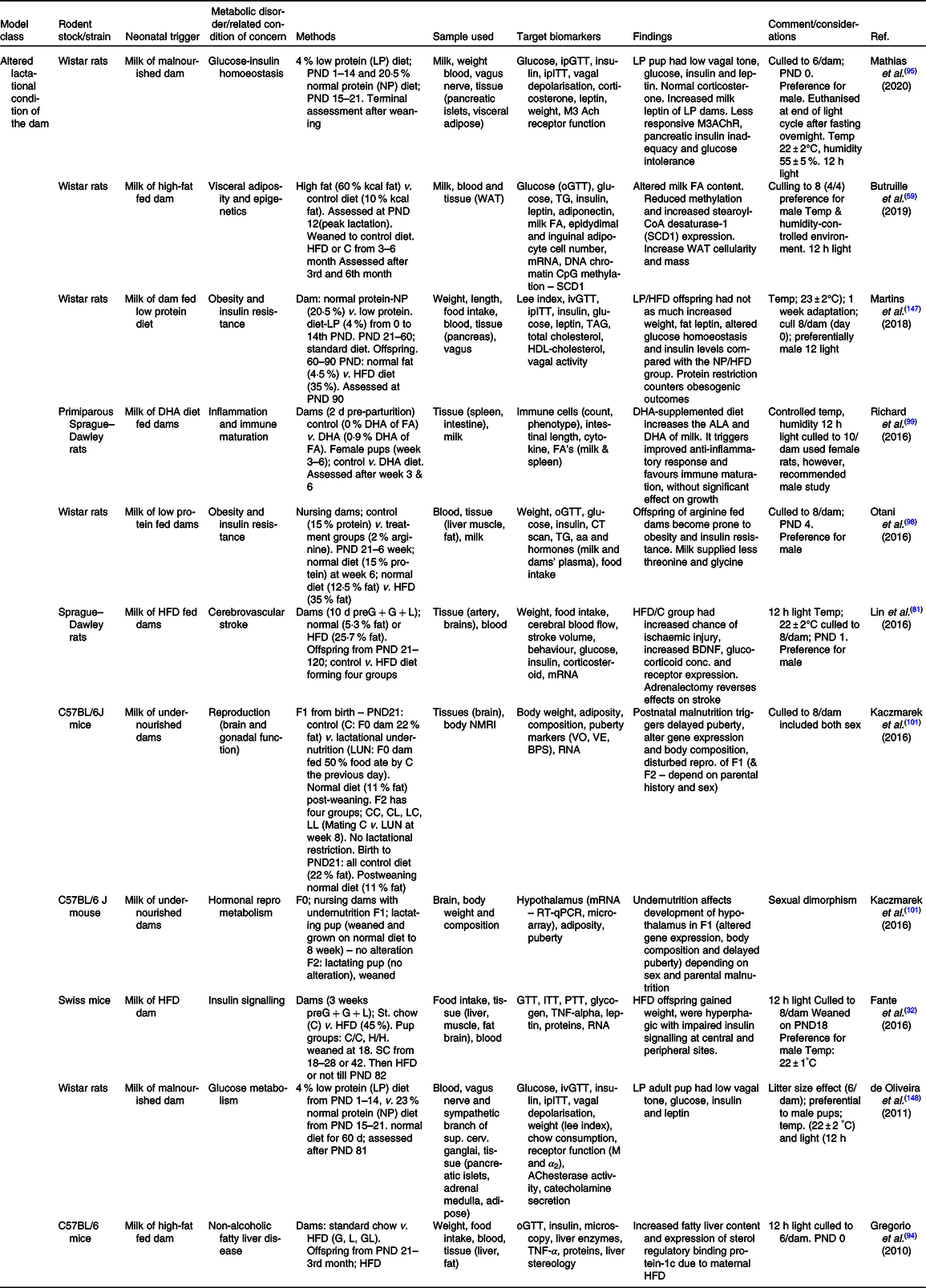
ACh, acetylCholine; ALA, α-linolenic acid; BDNF, brain-derived neurotrophic factor; BPS, balano preputial separation; C/C, control-control diet; C, control; C57BL/6, C fifty-seven black six mice; F0/1/2, filial generation 0/1/2; FA, fatty acid; G, gestation period; GL, gestation and lactation period; H/H, HFD to HFD dam; HFD, high-fat diet; ipITT, intraperitoneal insulin tolerance test; ivGTT, intra-venous glucose tolerance test; L, lactation period; LUN, lactation undernutrition condition; M, muscarinic; NMRI, nuclear magnetic resonance imaging; o/ip-GTT, oral/intraperitoneal-GTT; PND, postnatal day; PTT, pyruvate tolerance test; SCD1, stearoyl-CoA desaturase-1; VE, age of vaginal oestrous; VO, age of vaginal opening; WAT, white adipose tissue.
Table 3. Summary of some publications using the model of altered lactational condition of the pup for neonatal programming
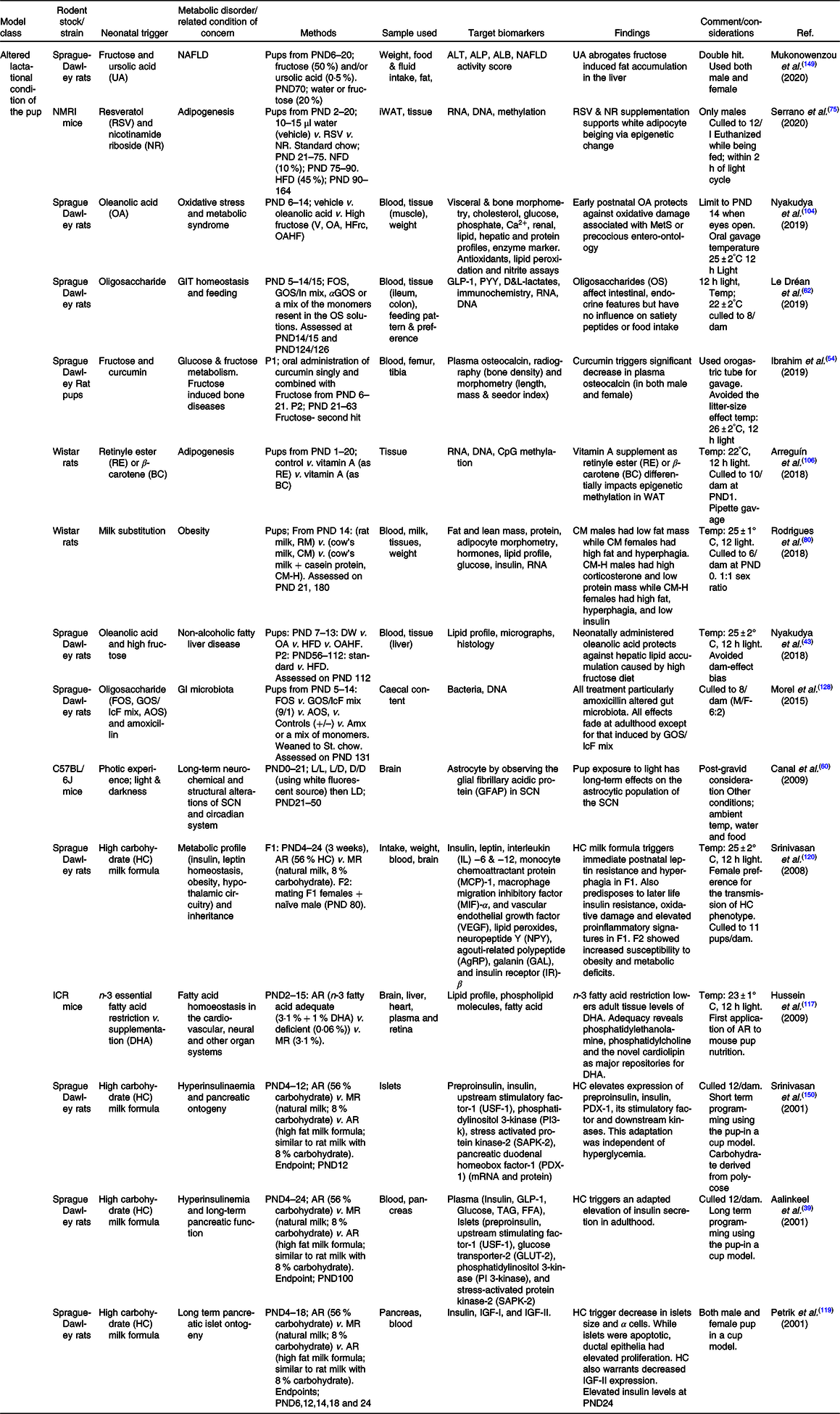
Abbreviations: ALB = albumin; ALP = alkaline phosphatase; ALT = alanine aminotransferase; Amx = amoxicillin; AOS = pectin-derived acidic oligosaccharides; AR = artificial rearing; D/D = dark/dark; DHA = docosahexanoic acid; DW = Distilled water; F0/1/2 = filial generation 0/1/2; FOS = fructo-oligosaccharides; GLP-1 = glucagon like peptide; GLP-1 = glucagon like peptide; GOS/In = beta-galacto-oligosaccharides/inulin mix; GOS/lcF = galacto-oligosaccharides/long-chain fructan mix; HFD = high fat diet; HFrc = high fructose; IGF = insulin growth factor; iWAT = inguinal white adipose tissue; L/D = light/dark; L/L = light/light; MR = maternal rearing; NAFLD = non-alcoholic fatty liver disease; NFD = normal fat diet; NR = nicotinamide riboside; OA = oleanolic acid; OA = oleanolic acid; OAHF = oleanolic acid and high fructose; PND = postnatal day; PYY = peptide YY (tyrosine tyrosine); RSV = resveratrol; SCN = suprachiasmatic nucleus; V = vehicle; αGOS = alpha-galacto-oligosaccharides.
Table 4. Summary of some publications using cross-foster or genetic models for neonatal programming
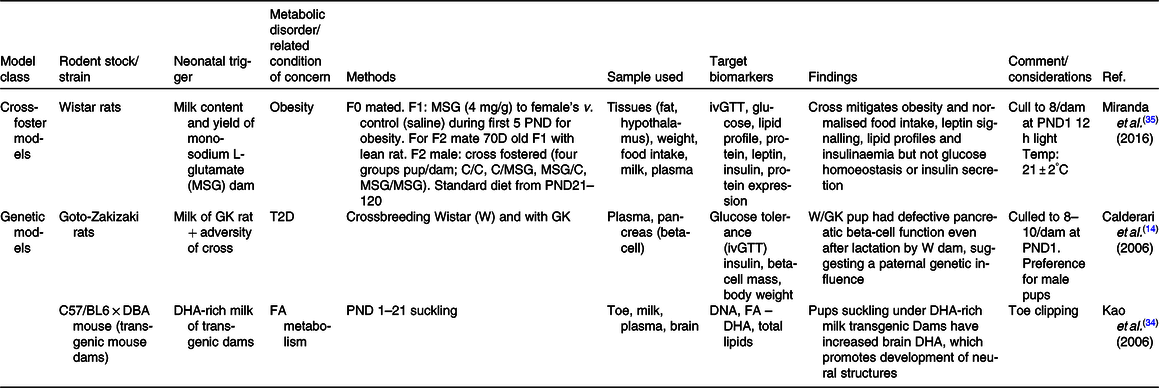
FA, fatty acid; ivGTT, intra-venous GTT; PND, postnatal day; T2D, type II diabetes.
Table 5. Summary of some publications using combined models for neonatal programming
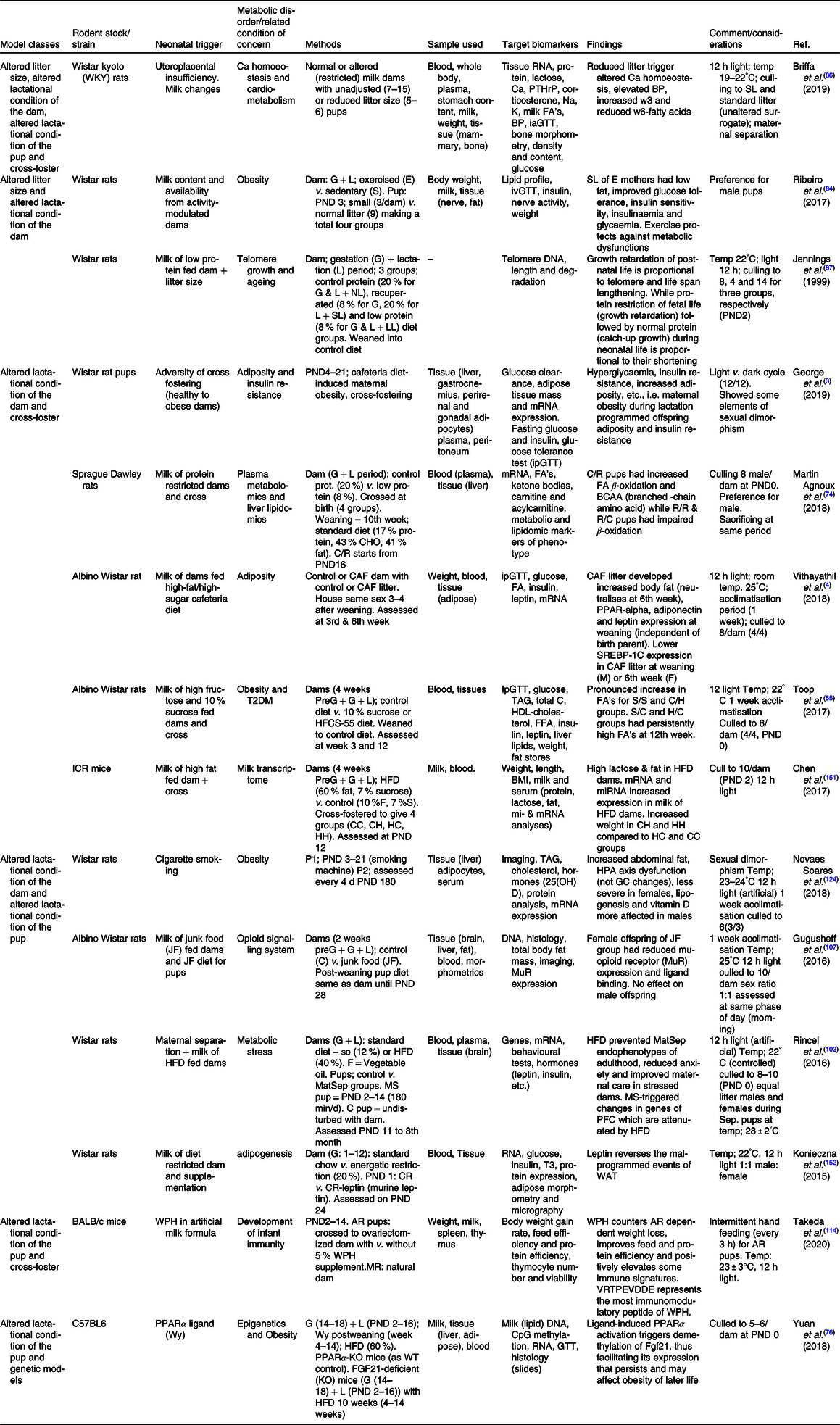
AR, artificially reared; BP, blood pressure; C/H, control-HFCS diet; C/R, control dam-low protein dam; CH, control to HFD dam; CHO, carbohydrate; CR, energetic restriction; FGF21, fibroblast growth factor-21 gene; G, gestation period; GC, glucocorticoid; H/C, HFCS-control diet; HC, HFD to control dam; HFCS, high fructose corn syrup; HFD, high-fat diet; HH, HFD to HFD dam; HPA, hypothalamo–pituitary–adrenal axis; ia/ip/oGTT, intra-arterial/intraperitoneal/oral-GTT; L, lactation period; LL, large litter; MatSep, maternal separation; MR, maternally reared; NL, normal litter; P1, phase one; P2, phase two; PFC, pre-frontal cortex; PND, postnatal day; PPAR, peroxisome proliferator activator receptor; PPARα-KO, peroxisome proliferator activator receptor alfa – knock out; PTHrP, parathyroid hormone-related protein; R/C , low protein dam – control dam; R, protein-restricted dam; S/C, sucrose-control diet; S/S, sucrose–sucrose diet; SL, small litter; SL, small litter; SREBP-1c, sterol regulatory element binding protein-1c; T3, triiodothyronine; WAT , white adipose tissue; WPH, whey protein hydrolysate; WT, wild type.
Altered litter size model
The suckling period is a time of intense dependency of the offspring on the mother for caregiving. As this secondary mother–offspring interaction occurs at a periodical hallmark of development for vital organs, studying variations programmable at different stages in this period is commonplace. Also, modelling in this form is a potent tool for comparing and deepening knowledge about rodents and humans. The maternal, paternal, littermate, environmental and predatory events are associations with varying contributions to neonatal programming(Reference Lucion and Bortolini77). Studying one factor effectively involves altering the variable while normalising the others.
For long, nursing of fewer human offspring is believed to facilitate weight gain and predispose to other disorders of adult life(Reference Widdowson and McCance78). Consequently, altering the litter has been used singly or in combination with other models(Reference Schreiner, Ackermann and Michalik79), to study neonatal programming. Following its pioneer usage by Widdowson and McCance(Reference Widdowson and McCance78), modelling alterations in litter size of maternally reared pups (littermate) are currently employed in investigations of under- (large litter) and over-nutrition (small litter) on the programming of new born animals (see Table 1 ). Techniques such as this use carefully planned mating strategies. For instance, in the pioneer study, female rats were placed in groups of six across five cages and one male was introduced per cage. This ensured that at least two litters were born on same day. Once pregnant, the rats were moved to separate cages where they delivered. Same-day-old pups were immediately mixed and redistributed into small and large litters for maternal rearing. It should be noted that while some studies count the day of birth as postnatal day (PND) 1(Reference Rodrigues, Moura and Bernardino80), others mark the day of parturition as PND 0(Reference Lin, Shao and Zhou81). Though the use of PND 0 predominates (see Table 1), whatever protocol is adopted should be indicated.
The mothers’ milk contains essential micronutrients and components which are substrates for metabolism and basis for development. The female rats and mice have six and five pairs of mammary glands, respectively(Reference Brower and Skinner82). Adjustments in the litter size affect milk availability. A reduction in litter size below normal creates an increased availability of milk for the pups(Reference Boersma, Bale and Casanello83). Such offspring subjected to increased feeding by the ‘litter size effect’ have been reported to come up with weight gain and developmental changes through adult life. Small litter offspring of both rats and mice commonly develop overweight as the resulting phenotype and as such are used for investigating obesity(Reference Liu, Srinivasan and Mahmood40,Reference Ribeiro, Tófolo and Martins84) . Notably, suckling generates a directly proportional surge in oxytocin, and this endogenous oxytocin increases milk supply via the milk let-down reflex(Reference Agnish and Keller85). Given the maximum nipple occupation for mice and rats are five and six, respectively, only small litters below these numbers are useful for initiating litter size effects. Also, caution must be exercised in the use of exogenous oxytocin to stimulate milk release as this affects the study of litter size. Aside obesity, small litters have also been used in the study of Ca metabolism, the homoeostatic endocannabinoid system(Reference Schreiner, Ackermann and Michalik79,Reference Briffa, O’Dowd and Romano86) .
In contrast to the small litter, increased litter size results in an initial rise in milk volume until a limit is reached (approximately fourteen pups), beyond which milk deprivation occurs. Large litter models of numbers beyond this limit may be used to investigate growth retardation and telomere shortening(Reference Jennings, Ozanne and Dorling87). The length of chromosomal telomeres is significant indicators of early life exposure to multiple forms of stress, and they have been reported to shorten in disease states associated with stress exposure such as obesity, diabetes mellitus, CVD and more(Reference Ridout, Ridout, Goonan and Fink88). Furthermore, large litters have also been used in the study of obesity and insulin resistance(Reference Liu, Srinivasan and Mahmood40,Reference Liu, Mahmood and Srinivasan73) . Large litters are associated with lesser nursing time and selective killing of runts between postnatal days 3 and 7(Reference Gandelman and Simon89,Reference Grota and Ader90) . Ranges for small, normal and large litter studies have been described(Reference Patel and Srinivasan91) (see Fig. 1). With large litter sizing, groups with death count of two or more pups are excluded to avoid auxological development, which may present similar outcomes as small litters(Reference Schreiner, Ackermann and Michalik79). In addition, the type of diet, the class and animal species are equally important in investigating for emergence of metabolic disorders resulting from feeding(Reference Ehrampoush, Homayounfar and Davoodi92).
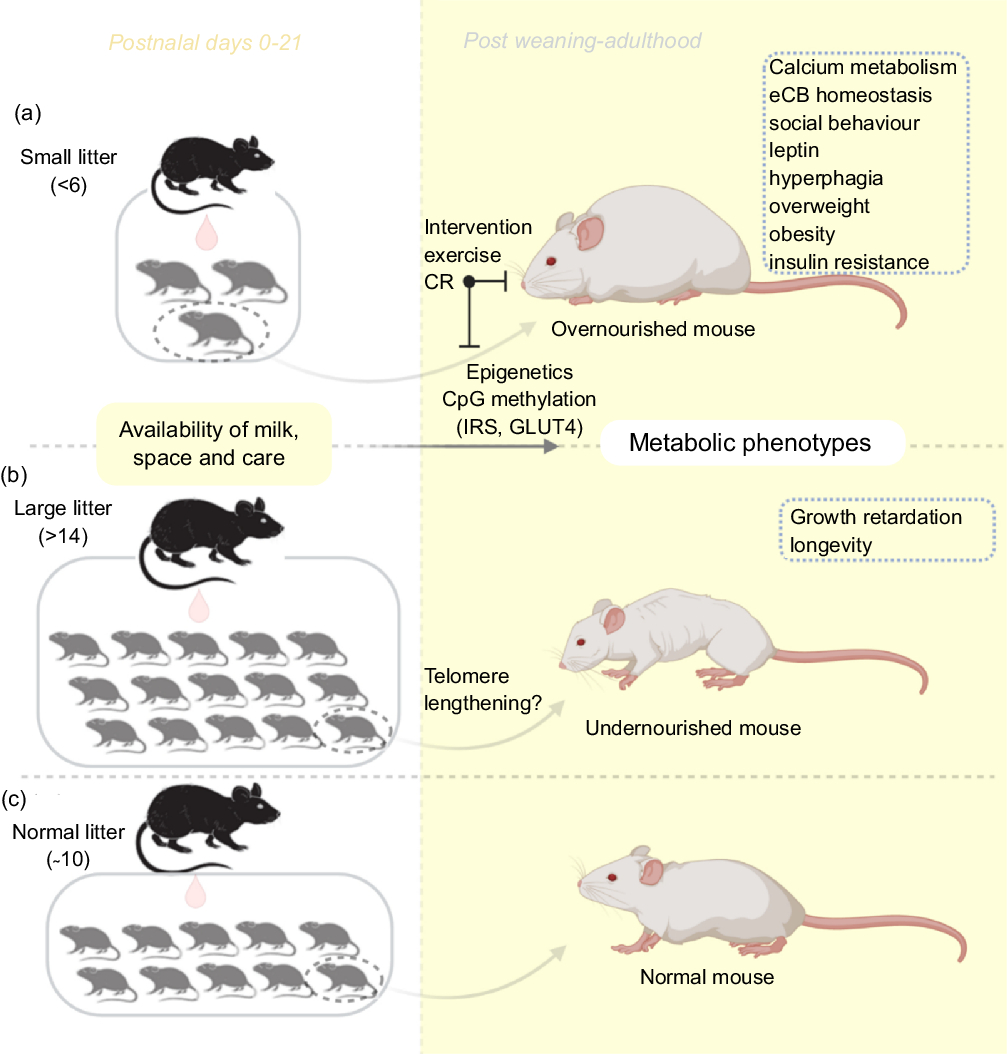
Fig. 1. An illustration of the litter size model useful for metabolic programming at early postnatal life. Adjustments in litter size (a) small or (b) large; determine milk availability and trigger the development of metabolic phenotypes with age. Using this model, several behaviours and phenotypes have been studied (trace boxes). CR, energetic restriction; eCB, endocannabinoid; GLUT4, glucose transporter 4; IRS, insulin receptor substrate.
Overall, the litter size correlates with variable diets in little or excess. A small litter may initiate overnutrition while a large litter may initiate undernutrition. Consequently, investigations that adopt other neonatal models for programming take measures to avoid the ‘litter size effect’ by adjusting/controlling litter size to an average number (commonly 8–12) per dam, a term known as ‘culling’ or ‘to standardise’. This helps ensure that any observed phenotypic change is due to the experimental variable and not masked by the litter size. Additionally, culling has been adopted in balancing and avoiding biased sexual ratios(Reference Agnish and Keller85,Reference Suvorov and Vandenberg93) . However, variability in culling protocols may result in variability in results and as such care must be taken when choosing a protocol. The optimum number of rodents to cull ranges from 6 to 10 pups(Reference Agnish and Keller85,Reference Gregorio, Souza-Mello and Carvalho94) ; usually on the first(Reference Lin, Shao and Zhou81), second(Reference Jennings, Ozanne and Dorling87), third(Reference Liu, Srinivasan and Mahmood40), fourth, fifth or sixth PND(Reference Agnish and Keller85).
Model of altered lactational condition of the dam
Most investigations in the lactational period of the nursing dam involve nutritional perturbations. Additional studies examine some other perturbations like maternal disorders, surgical alterations and even exercise on neonatal development(Reference Ribeiro, Tófolo and Martins84,Reference Briffa, O’Dowd and Romano86,Reference Mathias, Miranda and Barella95) . Pups suckled by diabetic and obese mothers are also common (see Table 2). Using this approach, the nursing mother is influenced, and the response is sought in the milk fed offspring. For instance, the maternal postnatal undernutrition during suckling has been studied and is implicated in the programming of metabolic diseases via changes in milk composition(Reference Wattez, Delmont and Bouvet36,Reference Martin Agnoux, Antignac and Boquien96) . Also, nutritional protocols such as the maternal low-protein diets have been developed(Reference Ma, Ozanne and Guest97) (see Fig. 2). These exposure of dams to several cues during lactation represents the commonest of models for usage in neonatal programming and like other models can be adopted singly or in combination(Reference Otani, Mori and Koyama98). With this model, it is common to quantitatively and qualitatively assess milk production. Consequently, several milk measurement strategies have been developed and adopted to determine the immediate effects of maternal disruptions to milk yield and content in mice and rats(Reference Wattez, Delmont and Bouvet36,Reference DePeters and Hovey66,Reference Gómez-Gallego, Ilo and Jaakkola67,Reference Paul, Hallam and Reimer70,Reference Willingham, McNulty and Anderson71) . Some investigators also adopt milk collection from the pup stomach(Reference Richard, Lewis and Goruk99). In addition, variabilities in milk volume have been documented, with decreased amounts being collected from inbred and primiparous compared with outbred and multiparous dams(Reference Gómez-Gallego, Ilo and Jaakkola67). Consequently, we recommend that comparative group studies should use dams of same stock/strain and parity. Furthermore, aside the weight-suckle-weight method of quantifying milk output, a water turnover method has been established(Reference Sevrin, Alexandre-Gouabau and Darmaun100).
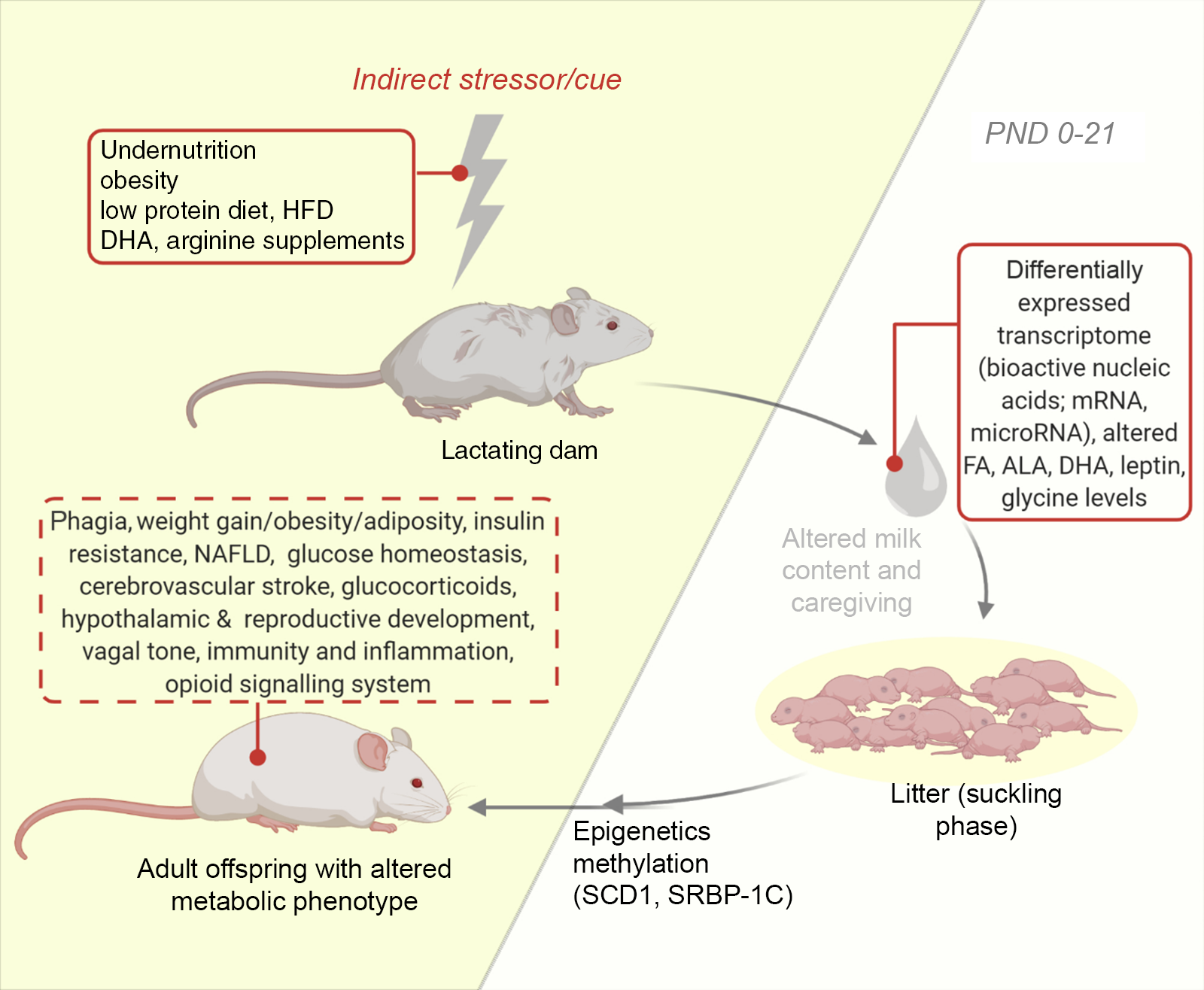
Fig. 2. An illustration of the model of altered lactational condition of the dam. Development of the offspring metabolic health following lactation is influenced by maternal exposure and health during lactation via notable changes in milk content (untraced boxes). Using this model, several behaviours and metabolic phenotypes have been studied (traced box). ALA, α-linolenic acid; DHA, docosahexaenoic acid; FA, fatty acid; HFD, high-fat diet; NAFLD, non-alcoholic fatty liver disease; PND, postnatal day; SCD1, stearoyl-CoA desaturase-1; SREBP-1C, sterol regulatory element-binding protein-1c.
Models of altered lactational condition of the pup
Modifications in pup diet and other direct neonatal experiences can programme the development of adult metabolic phenotypes. Consequently, investigators commonly adopt the model of altered lactational condition of the pup to test for metabolic phenotypes(Reference Ribeiro, Tófolo and Martins84,Reference Otani, Mori and Koyama98,Reference Kaczmarek, Mendoza and Kozak101,Reference Rincel, Lépinay and Delage102) and examine prophylactic agents which may protect from adversities of later life(Reference Ibrahim, Chivandi and Mojiminiyi42,Reference Gumede, Lembede and Nkomozepi61,Reference Serrano, Asnani-Kishnani and Couturier75,Reference Muhammad, Lembede and Erlwanger103,Reference Nyakudya, Isaiah and Ayeleso104) . Here we highlight models that employ diet, maternal separation, photic experience or cigarette smoking in the investigation of neonatal programming (see Table 3).
Dietary inclusions
Altered diet models provide means for assessing the outcomes of several administrations or intakes – nutrients, toxins, food composition, energy – on premature gut development and metabolism. Though the mouse and rat widely differ from humans in bioavailability of nutrients, the differences on how they metabolise, utilise and dispose of nutrients are well documented and suited for research(Reference Neal-Kluever, Fisher and Grylack105). Consequently, the neonatal mice and rats have been adopted for varying dietary protocols. Additionally, due in part to their lack of emesis and increased intestinal permeability of pups as compared with adults(Reference Xu, Collins and Ghishan49), we are able to examine the influence of several dietary inclusions on neonatal development and adult metabolic health(Reference Puimana and Stolla5).
Dietary inclusion as a model of altered lactational condition of the pup may involve either maternally or artificially reared pups. The dietary inclusions may be supplementations to maternal suckling or synthesised substitutes for artificial rearing. Unlike the altered litter size models, a precise control of neonatal nutrition in terms of composition or amount can be achieved by artificial rearing. Also, aside gavage techniques, commonly adopted for supplementing littermate (maternally reared, MR) pups, intragastric feeding tubes are employed when administering rodent milk substitutes (RMS) to artificially reared pups. Feeding procedures include the ‘pup in a cup’ technique as established by Hall(Reference Hall68) for artificially reared pups and a hand-feeding technique first described by Hoshiba(Reference Hoshiba69) for MR pups. Using comparable RMS formula to natural milk composition, artificially reared pups have been proven to exhibit non-distinct growth and development from MR pups(Reference Beierle, Chen and Hartwich65,Reference Hall68) . Some investigators of neonatal programming limit dietary exposure to the PND 14 based on the fact that pups gain sight and begin to forage/nibble upon ambient substances aside the dams’ milk, i.e. pelleting(Reference Le Dréan, Pocheron and Billard62,Reference Rincel, Lépinay and Delage102,Reference Nyakudya, Isaiah and Ayeleso104) . However, others extend the period of exposure to PND 21 (weaning) as all the pups are still exposed to the same confounding variables(Reference Ibrahim, Wright and Chivandi54,Reference Serrano, Asnani-Kishnani and Couturier75,Reference Rodrigues, Moura and Bernardino80,Reference Arreguín, Ribot and Mušinović106) . Some others even extend further (PND 28) (Reference Gugusheff, Bae and Rao107).
Rodent milk substitute
Unlike maternal rearing, the artificial rearing of pups requires alternatives to the natural milk. The common alternative regimes use synthetic modifications that are comparable with the natural rodent milk composition and are termed RMS. RMS are chemically derived from diary or non-diary formula under aseptic conditions for the artificial rearing of rat(Reference Kanno, Koyanagi and Katoku108–Reference Patel, Vadlamudi and Johanning110) and mice pups(Reference Yajima, Kanno and Yajima111). So far, RMS include several diet models: high-carbohydrate diet model, low-protein diet(Reference Ma, Ozanne and Guest97) and high-fat diary diet model(Reference Ehrampoush, Homayounfar and Davoodi92). Some of these diets, like the high-fat diary diet, have proven useful in investigating ameliorants of metabolic phenotypes(Reference Serrano, Asnani-Kishnani and Couturier75), as well as protective strategies(Reference Rincel, Lépinay and Delage102). Notably, modifications to enrich or deplete specific composition(s) may be made to a RMS in order to study specific interventions(Reference Motoki, Naomoto and Hoshiba112).
The pup in a cup; a technique for artificial rearing
The rat ‘pup’ in a ‘cup’ technique developed in 1975 by Hall(Reference Hall68) involves the transfer of few day old rat pups into several styrofoam cups floating on a thermo-regulated water bath(Reference Puimana and Stolla5). A miniaturised intragastric feeding cannula and an incubator housing arrangement are the two major components of this technique(Reference Hall68) (see Fig. 3). The introduction of intragastric feeding cannula (a polyethylene tubing) for mouse by Beierle et al. (Reference Beierle, Chen and Hartwich65) opened doors to the permissive usage of nutritional manipulations with a mouse pup in a cup. Thus, pup in a cup method is currently useful for both rats and mice and has proven helpful with rearing transgenic rodents(Reference Puimana and Stolla5). The insertion of intragastric cannulas employ a surgical technique for mice(Reference Beierle, Chen and Hartwich65) and nonsurgical technique for rats(Reference Hall68). A useful comparison of these techniques is found in the paper by Patel et al. (Reference Patel, Vadlamudi and Johanning110). However, such neonatal handling methods reportedly produce reproductive deficits(Reference Boersma, Bale and Casanello83). Further descriptions of artificial rearing and the pup in a cup, and their usefulness to nutritional research, has also been provided by Patel et al. (Reference Patel, Vadlamudi and Johanning110) and Patel and Hiremagalur(Reference Patel and Hiremagalur113). Using artificial rearing, several research works have examined the immediate metabolic outcomes of neonatal nutrition in mice – body weight, immunomodulation(Reference Takeda, Harauma and Okamoto114), – and rats – hyperinsulinaemia and glucose metabolism(Reference Haney, Estrin and Caliendo115,Reference Aalinkeel, Srinivasan and Kalhan116) . Also, certain long-term metabolic consequences of specific neonatal nutrition have been established for mice – cardiovascular(Reference Hussein, Fedorova and Moriguchi117) – and rats – hyperinsulinaemia and adult-onset obesity(Reference Aalinkeel, Srinivasan and Song39,Reference Vadlamudi, Hiremagalur and Tao118) , glucose and insulin homoeostasis(Reference Petrik, Srinivasan and Aalinkeel119) and leptin homoeostasis(Reference Srinivasan, Dodds and Ghanim120).
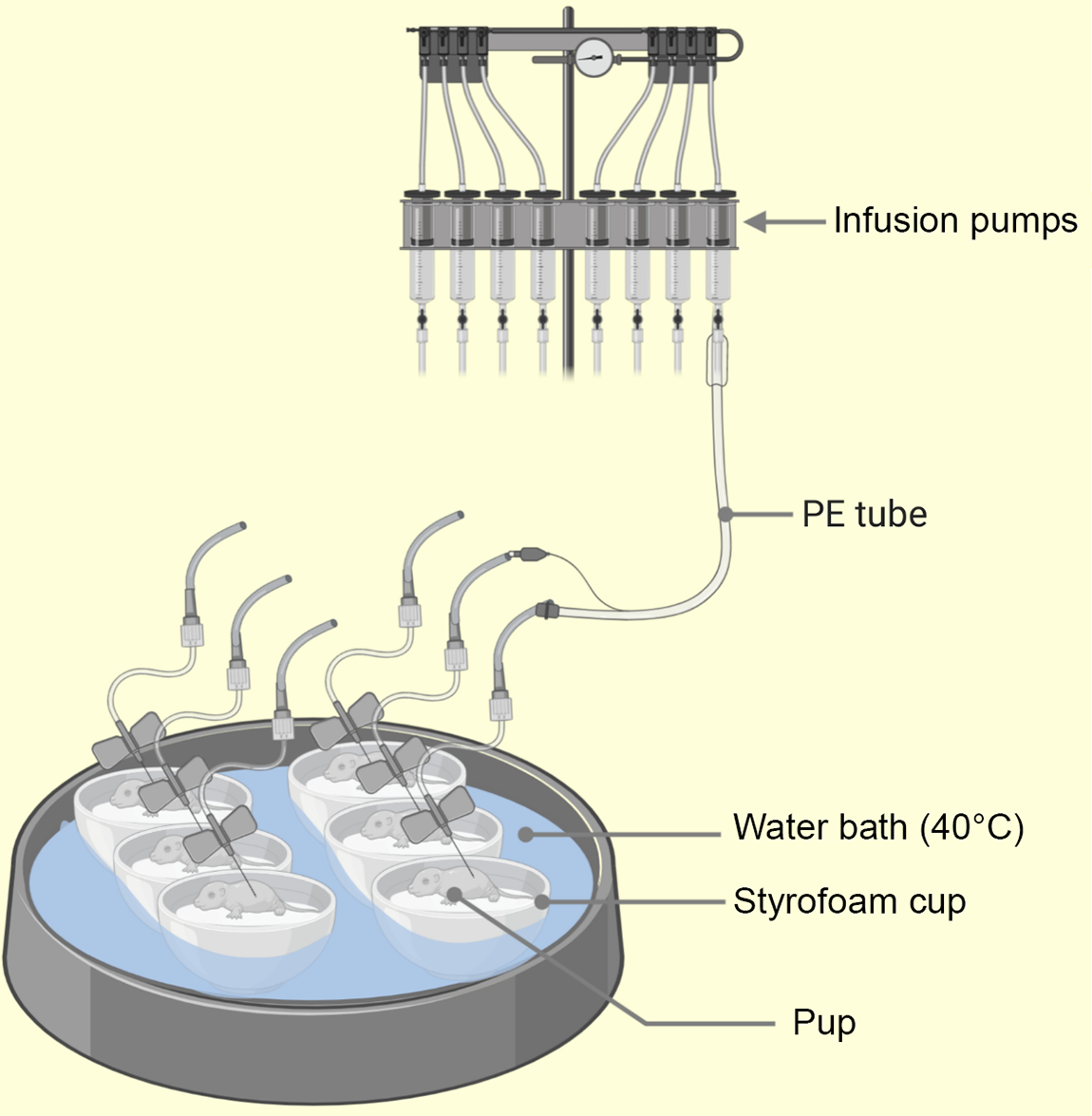
Fig. 3. Illustration of the pup-in-a-cup model used for breeding artificially reared (AR) pups. The pups in warm moist incubators (floating Styrofoam cups insulate the pups from direct contact with heat source, i.e. water bath) water at 40°C from 2 to 13 postnatal day (PND) to keep pup axillary temp at 32–36°C. The intragastric cannula (PE leads) emerge from the lids of the cups (not shown) and pass across to syringe mounted infusion pumps(Reference Hall68). PE, polyethylene.
Hand-feeding technique for maternal rearing
Rodents are omnivores and several commercial diets are available for their intake(Reference Duke Boynton, Dunbar and Koewler121). Oral gavage represents the classical involuntary and most common way of administering liquid compounds to conscious pups. Of the different types of gavage needles (curved or straight, flexible or rigid), we recommend the use of the curved flexible kind due to lower risks of oesophageal damage and since they cannot be chewed by the pups (see Fig. 4). Also in use is a hand-feeding technique first described by Hoshiba(Reference Hoshiba69). This uses a surrogate nipple for artificially reared mouse pups, which despite tediousness and time devotion, enables post-gravid study of pups and prevents physical injuries. Several nipple sizes have been described for mice and rats(Reference Hoshiba69). With this method, mouse pups receive calibrated amounts of nutrients with relatively reduced chances of physical trauma as inherent in the native gavage method of larger rats(Reference Halpern, Khailova and Molla-Hosseini122).
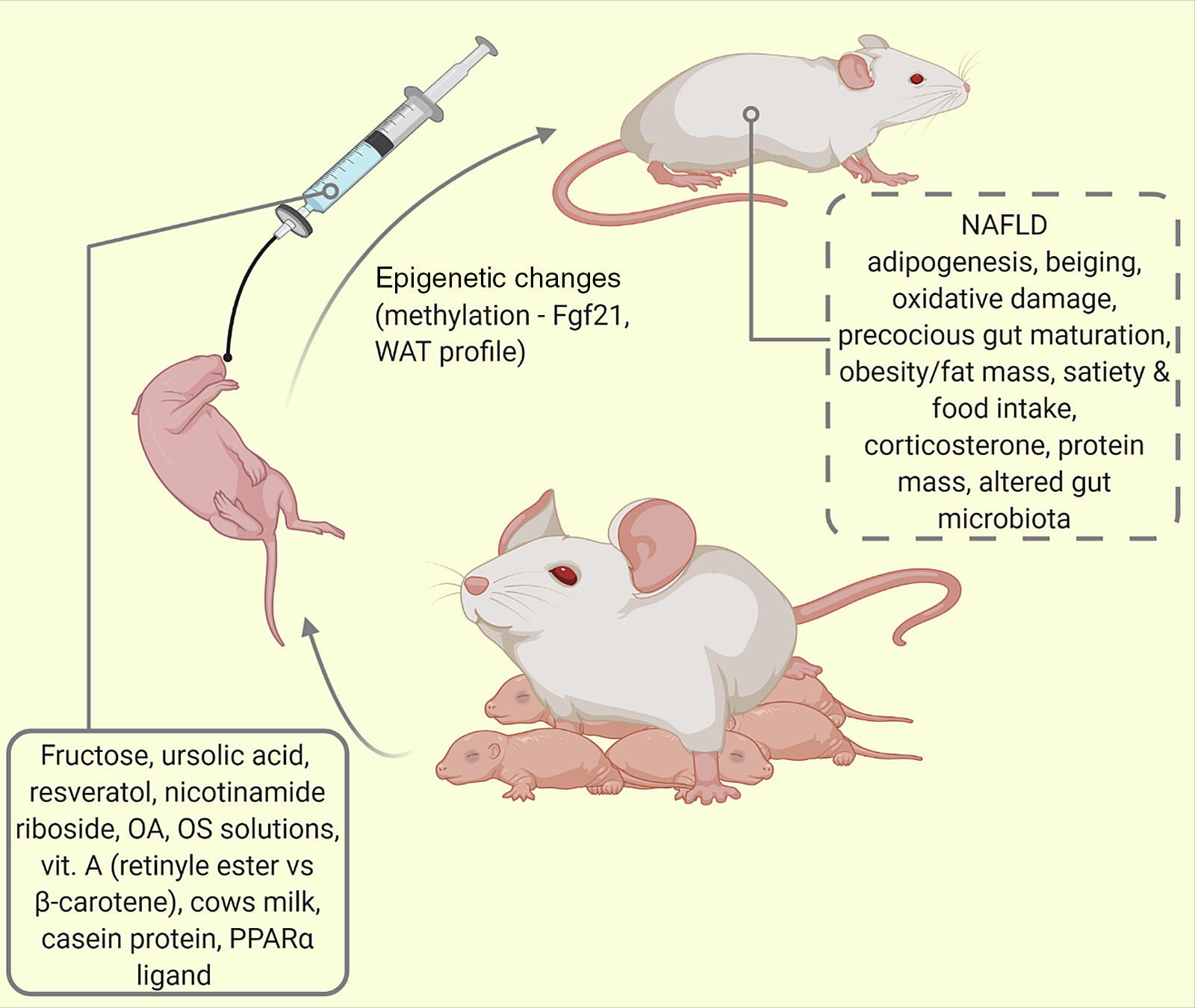
Fig. 4. Oral supplementation/intermittent feeding of maternally reared (MR) pups. Using this model of altered lactational condition of the pup, several behaviours and phenotypes have been studied (traced box). Fgf21 = fibroblast growth factor-21; NAFLD, non-alcoholic fatty liver disease; OA, oleanolic acid; OS, oligosaccharide; PPAR-α, peroxisome proliferator activated protein -alpha; WAT, white adipose tissue.
Maternal separation
Some studies have adopted maternal separation (MatSep) as a model for simulating neglect and predisposing to metabolic disorders(Reference Rincel, Lépinay and Delage102). The suckling pups are typically separated from the dam for 2–8 h daily across several days(Reference Boersma, Bale and Casanello83). A similar procedure to MatSep called ‘neonatal handling’ involves lesser timed separations of pup or litter from the dam and has been described elsewhere(Reference Raineki, Lucion and Weinberg123). Furthermore, with MatSep, the outcomes appear to be more prominent with inbred rat strains than outbred stocks. Lastly, when conducting MatSep, it is important to consider the age at initiation, short (<24 h) or long term.
Other models of altered pup condition; photic experience and smoking
Though uncommon, pup exposure to light has been investigated and shown to account for phenotypic changes associated with circadian rhythm such as related to astrocytes(Reference Canal, Mohammed and Rodriguez60). The effect of cigarette smoking has also been studied on pup development(Reference Novaes Soares, Silva Tavares Rodrigues and Cherem Peixoto124).
Cross-foster models
Cross-fostering involves the transfer of nursing pups from control dams to either metabolically compromised, stressor exposed or even healthy dams during the period of lactation. This is illustrated in Fig. 5. It is useful in the nutritional, hormonal or behavioural investigation of metabolic phenotypes(Reference Matthews, Samuelsson and Seed125). This model is most advantageous in delineating the outcomes of neonatal/lactational programming from gestational programming(Reference Miranda, da Silva Franco and de Oliveira35).
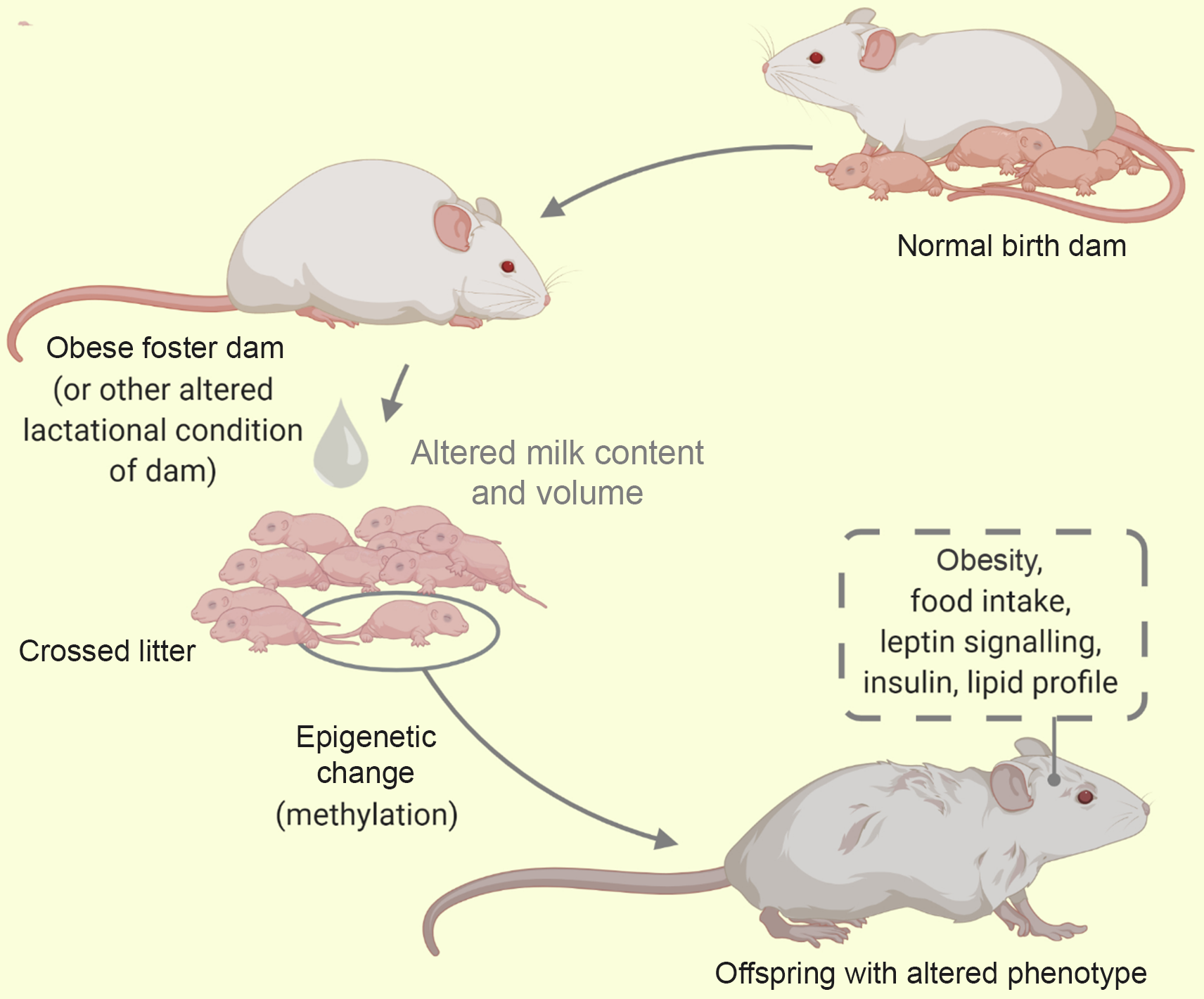
Fig. 5. A cross-foster of litter from a healthy dam to an obese dam. Using the cross-foster model some behaviour and phenotypes have been studied (traced box). Alterations in milk composition influences the offspring metabolic phenotype via epigenetics.
Though sometimes useful as an adversity in itself(Reference Matthews, Samuelsson and Seed125), cross-fostering of offspring from metabolically compromised dams (e.g. obese dams) to normal dams has reportedly mitigated the adverse effects of programming(Reference Miranda, da Silva Franco and de Oliveira35,Reference Romano, Wark and Owens126) . Conversely, cross-fostering of pups from a healthy to a malprogrammed dam (such as protein restricted, diabetic dams) triggers metabolic disruptions of adult life(Reference Martin Agnoux, El Ghaziri and Moyon74,Reference Fahrenkrog, Harder and Stolaczyk127) . When cross fostering, investigators avoid biased maternal care that may result from co-housing of biological and fostered pups. This is by ensuring none of the new born animals remains together with the biological dam(Reference Schreiner, Ackermann and Michalik79). Furthermore, the systematic cross-fostering of pups involves the distribution of pups across dams of the same status and has been adopted in culling pups(Reference Morel, Oozeer and Piloquet128). Following cross-fostering, the pup/dam cages are sometimes maintained for an extended period (e.g. PND 35) to account for natural weaning that may be longer than standard weaning at PND 21(Reference Romano, Wark and Owens126). Additionally, this model has also been adopted alongside other models such as the genetic model(Reference Schroeder, Shbiro and Moran41), models of altered pup condition – MatSep(Reference Huot, Gonzalez and Ladd129) (see Table 4).
Genetic models
More so in mice than rats, rodents are resourceful models in the study of the genetic aetiology of some complex human diseases(Reference Otto, Franklin, Clifford, Fox, Anderson and Otto38). Using potent advances in reproductive and genetic technology, researchers have been able to understand and dissect the molecular basis behind certain metabolic conditions(Reference Agca, Suckow, Hankenson and Wilson130). Genetically modified rodents could be modelled for diseases (resistant or predisposed to the condition) or for identifying/validating new drugs. They include inbred and hybrid (F1 and F2) strains, most of which are products of mutations, transgenic modifications (gain) or gene inactivation (loss of function)(Reference Agca, Suckow, Hankenson and Wilson130,Reference Hedrich and Suckow131) . These models are powerful tools that give room to tease apart the influence of metabolic primers or other selected variables on neonatal development (see Table 4).
Genetic models may serve several roles in neonatal programming. First, as a means of examining the effects of wet nursing in cross-fostering paradigms. Modified rodents are introduced as nursing dams to suckle pups cross-fostered from a control lineage. As like other models, Table 5 shows the combinational utility of this model alongside others. Using such genetic models in combination with cross-fostering, Reifsnyder and co-workers have been able to demonstrate that the maternal environmental influence is due to early obesity-inducing factors present in the milk of obese dams(Reference Reifsnyder, Churchill and Leiter132). In this scenario, the rodents may be engineered to possess a known metabolic disorder. Such studies are usually aimed at elucidating the independent influences the malprogrammed dam has on the suckling pup. In another usage, the offspring are genetically predisposed to or even exempted from a disorder(Reference Yuan, Tsujimoto and Hashimoto76), while testing for the therapeutic impacts that neonatal interventions have on the inheritance and/or development of the adult phenotype, that is, they serve as control subjects in neonatal programming(Reference Yuan, Tsujimoto and Hashimoto76).
Following the development of the first knockout mouse in 1987(Reference Mansour, Thomas and Capecchi133), and the first knockout rat in 2009(Reference Geurts, Cost and Freyvert134), genetic models are gradually permeating investigations in neonatal programming. Interestingly, genetically engineered mice have found use in the development of milk substitutes(Reference Kao, DePeters and Van Eenennaam34) and aid the study of genes in lactational programming(Reference Cowley, Garfield and Madon-Simon135). An example of genetically engineered mice used for neonatal metabolic programming include C57B/6J, New Zealand obese mouse pups (a genetic animal model for obesity and type 2 diabetes)(Reference Reifsnyder, Churchill and Leiter132). Examples of genetically modified rats used for neonatal metabolic programming include ZDF (Zucker rat), GK (Goto-Zakiki rat) – for type 2 diabetes mellitus and Otsuka Long Evans Tokushima Fatty (OLETF) rats (diabetes, obesity and CCK1 receptor)(Reference Schroeder, Shbiro and Moran41).
Considerations when selecting altricial rodents for neonatal programming
Despite the extensive degree of investigations that can be conducted using pups even of inbred status, there remains some potential to derail an experiment, creating bias that hinder the initial focus of experimentation. These biases could arise from methodology chosen and are prevented when pertinent caveats and certain factors such as history, physiology, identification, sample collection, sexual dimorphism and breeding are taken into account. When selecting suitable models that suit research objectives, these principal considerations must be made.
Historical profile
Thorough knowledge of the rodent history is crucial for research. Perhaps, a vital criterion for the examination of an accurate pup phenotype is to obtain a healthy parent. It is important to consider the genetic background of the dam and fetal environment of the pup. Through the right enquiry, the source of the animal, age, stock or strain and breeding profile (previous environment) could be ascertained(Reference Daviau136). Furthermore, it is important to enquire about housing conditions and feeding/drinking paradigms (diet type – for open-formula diet, diet source and water source/supply method) of laboratory rodents to avoid interference with research methods particularly with neophobic strains. An indication of rodent history and origin is primary to the replicability of a research. In addition, during procurement, we recommend that the rodent undergoes relative physical examination for diseases (general and zoonotic).
Rodent physiology
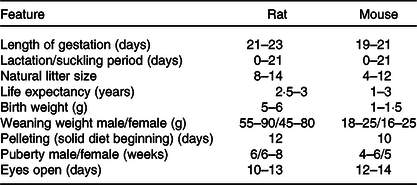
The laboratory mouse and rat may sometimes exhibit coprophagy and cannibalism(Reference Daviau136), which affects the outcome of research. These we believe arise when dams are stressed. Consequently, care and close monitoring must be adopted to reduce stress. Noteworthy, rodent’s eyes are exophthalmic and bear a periorbital harderian gland for the secretion of porphyrin, predominantly when stressed(Reference Otto, Franklin, Clifford, Fox, Anderson and Otto38). This secretion gives tears a reddish tinge which could be mistaken for bleeding. Though rodents lack sweat glands and emetic centre, they possess scent glands(Reference Otto, Franklin, Clifford, Fox, Anderson and Otto38), which hypothetically could detect foreign cues on pups post-handling and may trigger cannibalism via an unknown mechanism. Furthermore, Table 6 highlights some variable physiologic parameters of rats and mice. These may show deviations based on rodent source, stock or strain.
Table 6. Some physiological parameters to be noted for neonatal programming(Reference Pérez-Cano, Franch and Castellote11,Reference Otto, Franklin, Clifford, Fox, Anderson and Otto38,Reference Whary, Baumgarth, Fox, Fox, Anderson and Otto153)
Sexing of the pups
Though other methods exist, sexing of the pups is commonly done by comparing the anogenital distance of male and female littermates, as shown in Fig. 6. This distance is longer in males(Reference Lohmiller, Swing, Hanson, Suckow, Hankenson and Wilson137). Also, pre-weaned male pups have undescended testes, while the females have rudimentary mammae (appearing at PND 8–9) and an occluded vaginal opening(Reference Brower and Skinner82,Reference Vidal138) .
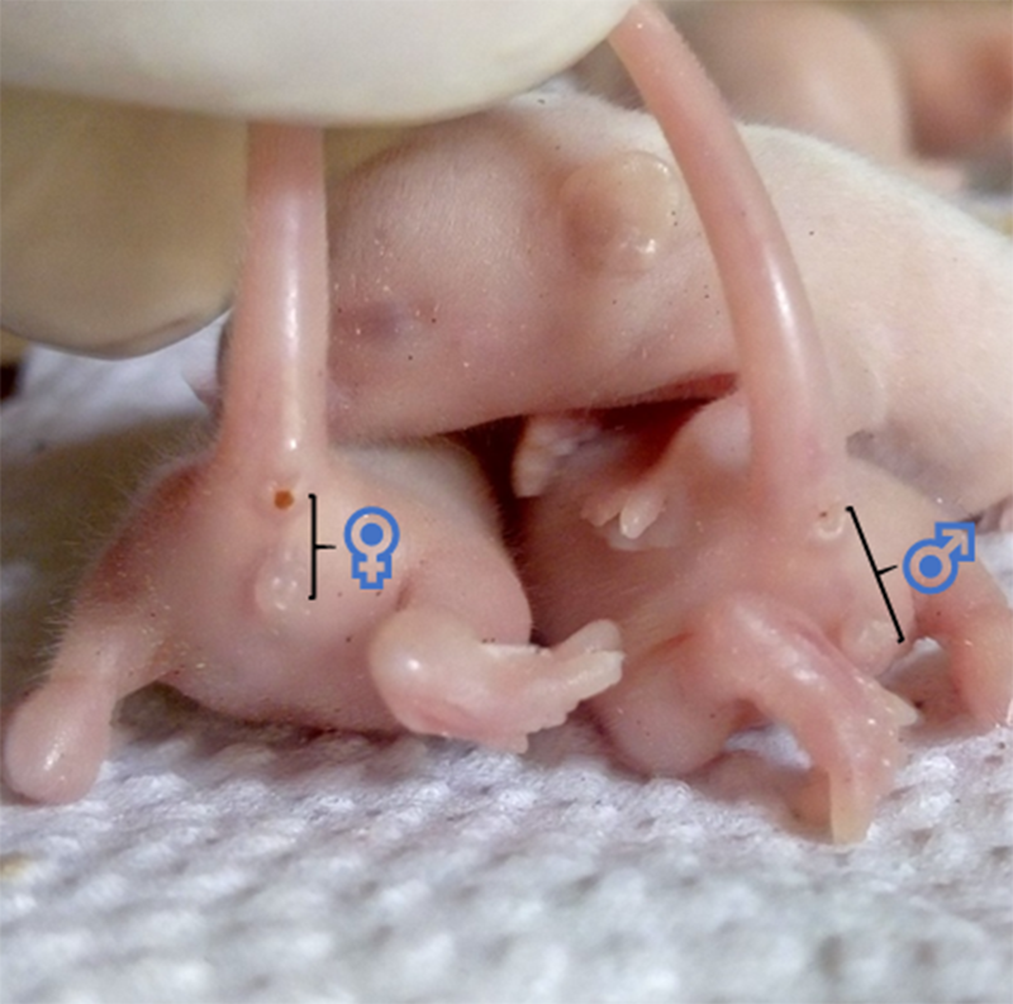
Fig. 6. Showing the anogenital distance of 7 d old Swiss mice (littermates). ♀, anogenital distance of a female pup ≈ 2·5 cm. ♂, anogenital distance of a male pup ≈ 4 cm.
Sexual differences (sexual dimorphism)
Several studies of metabolic programming have demonstrated that same stimulus elicits variable long-term effects based on the sex of the rodent offspring(Reference George, Draycott and Muir3,Reference Rodrigues, Moura and Bernardino80,Reference Kaczmarek, Mendoza and Kozak101,Reference Novaes Soares, Silva Tavares Rodrigues and Cherem Peixoto124) . The molecular mechanisms behind this dimorphism are not well understood. However, this unavoidable sexual differences appear long before embryo implantation, gonadal development, sex hormone differentiation or even programming(Reference Reichetzeder, Dwi Putra and Li19). This could be a result of unidentical sex chromosomes (i.e. Y and X chromosomal genes), which give rise to dissimilar transcripts whose expression can also alter the transcription of autosomal genes (in a process of genetic imprinting: in which only a paternal or maternal copy of an allele is exclusively expressed), thus amplifying the sexual difference(Reference Bermejo-Alvarez, Rizos and Lonergan139). Possibly, the early differential expression of proteins influences molecular pathways (glucose and protein metabolism) and impacts on epigenetic mechanisms (especially DNA methylation). The result is a sex-based difference with susceptibility to environmental cues leading to distinct outcomes in adult health independent of neonatal programming. Consequently, a number of outcomes have been attributed to sexual dimorphism. For instance, though runts are rare, male pups are slightly heavier than female littermates and maternal behaviour has been shown to also vary with the sex of the pup. Due to such dimorphisms, several studies show sex preference for male than female pups(Reference Liu, Mahmood and Srinivasan73,Reference Serrano, Asnani-Kishnani and Couturier75,Reference Mathias, Miranda and Barella95,Reference Richard, Lewis and Goruk140) . However, sexual bias should be used only with genuine justifications, as new discoveries could be masked by sex.
In altered diet models of low protein, researchers tend to use male pups due to sexual differences in insulin levels and glucose tolerance that have been observed in earlier studies(Reference Mathias, Miranda and Barella95). Others prefer male rodents due to susceptibility to changes in adiposity and body weight in the context of metabolic programming(Reference Butruille, Marousez and Pourpe59). Conversely, some articles specific for either male or female sex fail to report appropriate justification for usage(Reference Fante, Simino and Reginato32). This raises serious concerns, as no satisfactory background is laid. Consequently, single sex research should carry appropriate justification for usage.
Handling of the pups
When investigators handle pups, the goal is to accomplish the ideal task with the least amount of restraint to avoid agitation, reduce stress and avoid experimental variations by relaxed handling. It is also important that appropriate personal protective equipment’s are used to minimise exposure to hazardous agents and allergens. Unlike adult rodents, the neonates are rather fragile and a major concern when handling is the contact which may incite cannibalism by the dam(Reference Raineki, Lucion and Weinberg123). Also, males and females are housed separately after weaning to avoid aggression and dominance(Reference Kaczmarek, Mendoza and Kozak101).
Identification of the pups
Besides the use of cage and nontoxic tail colour tags for identifying pup groups(Reference Nyakudya, Mukwevho and Nkomozepi43,Reference Nyakudya, Isaiah and Ayeleso104) , researchers also adopt toe clipping and tattooing. Toe clipping represents the removal of digits for identification and genotyping(Reference Kao, DePeters and Van Eenennaam34). It is useful for identifying genetically modified mice at the suckling stage. Though, perceived to be painful for routine usage, toe clipping has proven to be a fast, safe and easy method. Along established protocols, short- or long-term effects of toe clipping are shown to be completely avoidable(Reference Paluch, Lieggi and Dumont64). Manual and micro-tattoo systems create tattoo markers on the tail, toes and footpads of pups to aid identification. Electronic microchip devices are also available(Reference Duke Boynton, Dunbar and Koewler121). Pup identification is important for determining the origins of unexpected breeding errors.
Sample collection
Several methods of neonatal euthanasia have been described(Reference Danneman and Mandrell141–Reference Pritchett-Corning143). Acceptable techniques include the injection of chemical anaesthetic, cervical dislocation and decapitation(Reference Valentim, Guedes and Pereira144). Euthanasia is done at the same period of the day to avoid the circadian influence on metabolic outcomes(Reference Martin Agnoux, El Ghaziri and Moyon74). Some articles fail to clearly highlight the samples obtained after euthanasia(Reference Jennings, Ozanne and Dorling87,Reference Arreguín, Ribot and Mušinović106) , which is detrimental to research reporting. Furthermore, the collection of specimens such as blood, milk (from pup stomach), is unavoidable parts of most developmental research. Consequently, given that neonatal rats and mice are perceptive to pain, suitable procedures that initiate the least possible harm are encouraged.
Blood represents a vast repertoire of developmental parameters and is a window to the physiological status of the neonate. Considerations regarding the method of blood collection include anaesthesia to be used and impact of the assessment to be conducted(Reference Duke Boynton, Dunbar and Koewler121). Given the difficulty associated with serial blood sample collection, particularly of mice, decapitation is most commonly adopted(Reference Martin Agnoux, El Ghaziri and Moyon74,Reference Serrano, Asnani-Kishnani and Couturier75,Reference Otani, Mori and Koyama98) . However, cardiac puncture has been specially adopted for collecting blood samples from neonatal rats by Grazer(Reference Grazer145). Notably, though neonates metabolise drugs slowly, they display relative resistance to hypoxia(Reference Valentim, Guedes and Pereira144). Thus, for anaesthesia, age represents another consideration because neonatal rodents show relatively greater resistance to CO2 compared with adults. Such resistance causes delayed time of death (sometimes 50 min at PND0 for mice), which gradually decreases with increasing age; 3 min decrease per day between PND 0–10 for rats(Reference Pritchett, Corrow and Stockwell142,Reference Pritchett-Corning143) . Consequently, moderate considerations must be made when euthanising the suckling mice or rats with CO2. In addition, to confirm death following anaesthesia, secondary techniques such as bilateral pneumothorax and immersion in liquid nitrogen (for hairless neonates) may be used(Reference Valentim, Guedes and Pereira144). The weight and genetic background (stocks and strains) of neonatal rodents are also important considerations when euthanising(Reference Pritchett, Corrow and Stockwell142–Reference Valentim, Guedes and Pereira144).
Breeding
To facilitate timed and synchronised births, prime considerations are made about the reproduction of pups from parental rodents. Knowledge of how to identify and control rodent mating, gestation and parturition is priceless in this pursuit. For the selection of a mating regime, it is advisable to consider three conditions of the dams: the Whitten effect (synchronised oestrus when a new male is added), the Bruce effect (delayed embryo implantation when a new male is added) and the Lee-Boot effect (absent oestrus when females are caged together). All three conditions are more pronounced with mice, while the Whitten effect is completely lacking in rats(Reference Lohmiller, Swing, Hanson, Suckow, Hankenson and Wilson137). In addition, due to their prolific nature, these rodents exhibit fertile postpartum oestrus and special assistance is needed to curb overpopulation. Notably, normal gestation that lasts about 21 days is extended by suckling. Also, under the right conditions, a pair of housed mice can generate a progeny of approximately 50,000 members in 1 year(Reference Daviau136).
Conclusions
An appropriate understanding of the differences that lie in model usage and protocols is gainfully essential. The five models recognised in this review are useful to varying degrees for the investigation of neonatal programming and several variabilities accompanying their usage have been identified. They may be used singly or in combination, and the metabolic outcomes from one model may vary from that of another. For instance, neonatal programming for adult-onset obesity had been shown to be reversible, by Liu et al. (Reference Liu, Srinivasan and Mahmood40) and not reversible by energy restriction by Srinivasan et al. (Reference Srinivasan, Mahmood and Patel146) Explanation for these opposing findings may lie in the models adopted as well as different levels of caloric restriction employed in these two studies. While Liu et al. (Reference Liu, Srinivasan and Mahmood40) adopted the ‘altered litter size model’, Srinivasan et al. (Reference Srinivasan, Mahmood and Patel146) adopted the ‘model of altered pup diet’, both arriving at scientifically acceptable results. That the animal models differ but prime for similar metabolic outcomes such as obesity, advocates for improved mechanistic evaluation to elucidate outcome differences. Also, choosing the right model for research should be accompanied with relevant considerations which when clearly stated aid the reproducibility of an investigation and allow for the interpretation of comparative reports and experimental variability. Furthermore, it is evident that the rat and mouse are excellent models for investigations focused at understanding the metabolic consequences of genetics, epigenetics, nutrient availability, natural lactation and/or substituted suckling. Thus, for example, given that prematurity is associated with neonatal programming(Reference Reichetzeder, Dwi Putra and Li19), studies using these models could help in developing optimal nutritional strategy for preterm mammals. Overall, these models are useful for studying the pathogenesis of metabolic disorders and developing strategies for their prevention and treatment.
Statements of Significance
-
According to DOHaD (developmental origins of adult health and disease), the neonatal period is a critically responsive time to cues that may trigger adaptation of the metabolic phenotype.
-
Rodent-based investigations remain the predominant means of studying the results of this ‘neonatal programming’ on metabolic health.
-
Here we establish, for the first time, five approach-based models of rodents useful to neonatal programming.
-
Novel considerations to guide the design and conduct of newer experiments are also highlighted.
-
This will provide background and guide to biomedical and nutritional researchers focused on the aetiology, mechanisms, prevention and treatment of metabolic disorders.
Acknowledgements
We appreciate the intellectual support and resource provided by staff and postgraduate members of the Centre for Advanced Medical Research and Training (CAMRET), Usmanu Danfodiyo University, Sokoto, Nigeria. D. U. acknowledges the postgraduate scholarship awarded to him (CAMRET/2019/MSc/SCH003) by CAMRET.
No specific funding was received to support this work.
K. G. I. conceptualised the review. D. U.: composed the outline, drafted the manuscript and drew the figures. K. G. I., M. B. B., I. M., B. A., M. B. A. and M. U. I.: edited drafts and contributed to the organisation of the manuscript. K. G. I., M. B. A. and M. U. I.: validated the review and revised the manuscript. The manuscript was written through contributions of all authors. All authors have read and approved the final manuscript. Figures 1–5 were created using Biorender.
The authors have no relationship with industry and no potential financial conflicts of interest relevant to the submitted manuscript.