Nuts contain a number of bioactives and health-promoting components(Reference Miraliakbari and Shahidi1, Reference Alasalvar, Shahidi, Amaral, Alasalvar and Shahidi2). They are highly nutritious and contain macronutrients (fat, protein and carbohydrate)(Reference Venkatachalam and Sathe3, 4), micronutrients (minerals and vitamins)(4), fat-soluble bioactives (MUFA, PUFA, monoacylglycerols, diacylglycerols, TAG, phospholipids, sterol esters, tocopherols, tocotrienols, phytosterols, phytostanols, squalene, terpenoids, sphingolipids, carotenoids, chlorophylls, alkyl phenols, essential oils and among others)(Reference Miraliakbari and Shahidi1, Reference Alasalvar, Shahidi, Amaral, Alasalvar and Shahidi2, 4–Reference Bolling, Oliver Chen and McKay11) and phytochemicals (phenolic acids such as hydroxybenzoic acid and hydroxycinnamic acid; flavonoids such as flavonols, flavones, flavanols or catechins, flavanones, anthocyanins, anthocyanidins and isoflavones; stilbenes; lignans; naphthoquinones; hydrolysable tannins (ellagitannins and gallotannins); condensed tannins or proanthocyanidins; ellagic acid; phenolic aldehydes; alkaloids; coumestan; phytates; terpenes; phytoestrogens and among others)(4, Reference Bolling, Oliver Chen and McKay11–Reference Alasalvar and Shahidi24). Moreover, nuts contain numerous types of antioxidants with different properties(Reference Wu, Beecher and Holden17, Reference Blomhoff, Carlsen and Andersen25).
Nuts are recognised for their health-promoting aspects, particularly for their role in reducing CVD risk. This may be due to the favourable lipid profile and low-glycaemic nature of nuts(Reference Li, Liu and Liu26, Reference Reis, Ribeiro and Costa27). Other evidence suggests that increased consumption of nuts increases antioxidant defences and reduces inflammation in populations with increased risk for CVD(Reference Jenkins, Kendall and Marchie28, Reference Liu, Liu and Chen29). Furthermore, emerging observational and/or clinical studies suggest that increased consumption of nuts may reduce risk for cancer(Reference Su, Tamimi and Collins30), benefit cognitive function(Reference Martínez-Lapiscina, Clavero and Toledo31), and reduce the risks of asthma(Reference Varraso, Kauffmann and Leynaert32, Reference Maslova, Granstrom and Hansen33) and inflammatory bowel disease(Reference Amre, D'Souza and Morgan34), and among others.
The present review reported the levels of major phytochemicals, fat-soluble bioactives as well as natural antioxidants present in commonly consumed nuts. Recent studies adding new evidence for the health benefits of nuts are also discussed.
Phytochemicals in nuts
Nuts are rich in phenolic phytochemicals and are identified among the richest sources of dietary polyphenols(Reference Pérez-Jiménez, Neveu and Vos35). A recent comprehensive review on tree nut phytochemicals has been published by Bolling et al. (Reference Bolling, Oliver Chen and McKay11). Therefore, a detailed compositional comparison was not intended for this review. It should be noted that quantitative values for phytochemicals in nuts vary widely in some instances. Proanthocyanidins and hydrolysable tannins are generally the most abundantly found polyphenols in some nuts (Table 1). Nuts also have a significant phytate content as well as flavonoid, phenolic acid and stilbene polyphenol classes. Fig. 1 shows the chemical structures of some phytochemicals present in nuts.
Table 1 Reported phytochemicals in nuts (mg/100 g)

ND, not detected.
* Data are expressed as mg of gallic acid equivalents/100 g and obtained from Rothwell et al. (Reference Rothwell, Urpi-Sarda and Boto-Ordoñez104), except heartnut.
† Data are obtained from USDA(105), except walnut that is obtained from Rothwell et al. (Reference Rothwell, Urpi-Sarda and Boto-Ordoñez104) and USDA(105).
‡ Detected, but by qualitative method or not quantified on whole nut basis. Data are obtained from Venkatachalam & Sathe(Reference Venkatachalam and Sathe3).
§ Data are obtained from Xie et al. (Reference Xie, Roto and Bolling50).
∥ Data are obtained from Bolling et al. (Reference Bolling, Oliver Chen and McKay11).
¶ Data are obtained from Xie & Bolling(Reference Xie and Bolling45).
** Data are obtained from John & Shahidi(Reference John and Shahidi42) and on a defatted kernel basis.
†† Data are obtained from Chandrasekara & Shahidi(Reference Chandrasekara and Shahidi41) and on a defatted kernel basis.
‡‡ Data are obtained from Abe et al. (Reference Abe, Lajolo and Genovese92).
§§ Data are obtained from Otles & Selek(Reference Otles and Selek106).
∥∥ Data are obtained from Pelvan et al. (Reference Pelvan, Alasalvar and Uzman107).
¶¶ Data are expressed as mg of gallic acid equivalents/100 g and obtained from Li et al. (Reference Li, Tsao and Yang108).
*** Data are obtained from de la Rosa et al. (Reference de la Rosa, Alvarez-Parrilla and Shahidi109).
††† Data are obtained from Mandalari et al. (Reference Mandalari, Bisignano and Filocamo110).
‡‡‡ Data are obtained from Gómez-Caravaca et al. (Reference Gómez-Caravaca, Verardo and Segura-Carretero111).
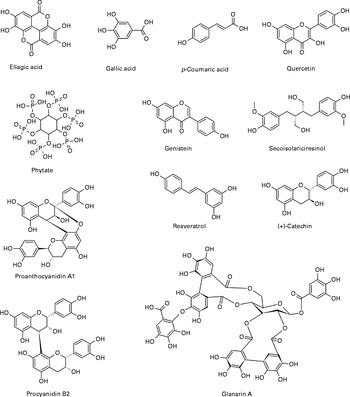
Fig. 1 Structures of some phytochemicals present in nuts.
Total phenols
Data from the Folin–Ciocalteu assay, a non-specific method to characterise the content of phenols, indicate that nuts have a significant phenolic content. Chestnut, pecan and pistachio have greater than 1 g gallic acid equivalents/100 g, while most nuts have >0·1 g gallic acid equivalents/100 g (Table 1). Thus, several nuts are among the top dietary sources of polyphenols(Reference Pérez-Jiménez, Neveu and Vos35). It should be noted that the Folin–Ciocalteu assay could be confounded with the differential contribution of individual flavonoid to total phenol values(Reference Bolling, Chen and Kamil36).
Proanthocyanidins
Proanthocyanidins are the most abundantly found class of polyphenols in almond, hazelnut, pecan and pistachio (Table 1). Nut proanthocyanidins are predominantly B-type, although A-type proanthocyanidins have been found in almond, peanut and hazelnut(Reference Monagas, Garrido and Lebron-Aguilar37). Nut proanthocyanidins are highly polymerised, with mean degrees of polymerisations ranging from 2·6 to 10·8(Reference Prior and Gu38).
Hydrolysable tannins
The hydrolysable tannins consist of glucose-bound gallic acid (gallotannins) or hexahydroxydiphenic acid (ellagitannins), which release gallic acid and ellagic acid upon acid hydrolysis, respectively. Most studies have quantified gallic or ellagic acid following hydrolysis of a nut extract. Thus, tannin structures are not well characterised for most nuts. Further analysis of hydrolysable tannins characterised in the presence of glansreginins A and B and glansrins A–D in walnut(Reference Ito, Okuda and Fukuda39). Most nuts likely have hydrolysable tannins, with almond, pecan and walnut having the highest contents. Gallic acid was also identified in extracts of hazelnut and was present at 22 mg/100 g of defatted cashew kernels(Reference Shahidi, Alasalvar and Liyana-Pathirana40, Reference Chandrasekara and Shahidi41). Brazil nut has 5·2 mg free and 8·2 mg bound gallic acid/100 g of defatted whole Brazil nut(Reference John and Shahidi42). By contrast, peanut does not appear to have a significant gallic acid or hydrolysable tannin content(Reference Francisco and Resurreccion43).
Flavonoids
Pecan, walnut, hazelnut and pistachio are rich sources of flavonoids (Table 1). Almond contains catechins, as well as flavonols such as naringenin, quercetin and kaempferol, predominantly as glycosides or rutinosides(Reference Bolling, Dolnikowski and Blumberg44). Flavonoids such as hazelnut, pistachio and pecan are mainly sources of catechins and gallocatechins, and pistachio has additional flavanone and flavone polyphenols.
Phenolic acids
Walnut and Brazil nut have the highest phenolic acid content among nuts with 36 and 11 mg/100 g, respectively, although this polyphenol class has not been well characterised in most nuts (Table 1). Peanut has p-coumaric acid and protocatechuic acid, with caffeic acid only in Spanish peanut(Reference Francisco and Resurreccion43). When gallic acid (free or derived from hydrolysable tannins) is included with phenolic acids, chestnut, pecan and almond have a significant phenolic acid content ranging from 14 to 900 mg/100 g (Table 1).
Stilbenes
The presence of stilbenes has been reported in almond, peanut and pistachio. These nuts have comparable levels of stilbenes, presented in the μg/100 g range. Almond contains resveratrol-3-O-glucoside, concentrated in the skin(Reference Xie and Bolling45). The variability of resveratrol content among 109 US peanut cultivars over 2 years was 0·003–0·026 mg/100 g peanut(Reference Wang, Chen and Tonnis46).
Free and bound phenolics
Nuts contain solvent-extractable phenolics as well as non-extractable and covalently bound phenolics. Bound phenolics are associated with dietary fibre, lignins or other cell wall components. Nuts are composed of 3–13 % of dietary fibre, with almond and pistachio having the highest content(4). Bound phenolics may contribute 10–60 % of the total in vitro antioxidant capacity of nut extracts(Reference Pellegrini, Serafini and Salvatore47). Notably, macadamia has the highest level of bound phenolics among nuts, but has proportionally lower extractable phenolics and flavonoids(Reference Yang, Liu and Halim48). Almond contains 1–2 mg of bound proanthocyanidins and approximately 8 mg of bound phenolic acids per 100 g(Reference Mandalari, Tomaino and Arcoraci49, Reference Xie, Roto and Bolling50). Few studies have characterised the nature and types of bound phenolics in nuts, and analysis is inherently complicated by the use of harsh acidic or alkaline conditions used in their extraction.
Phytates
Phytates have been found in most nuts. Phytates are composed of various inositol phosphates and range from inositol phosphate-1 to inositol phosphate-6 in almond and hazelnut(Reference Liu, Villalta and Sturla51). Nuts have similar phytate contents ranging from 150 to 290 mg/100 g(Reference Venkatachalam and Sathe3).
Other classes
Juglone, a naphthoquinone, has been reported to be present in significant quantity in walnut, which had 7–19 mg/100 g among ten cultivars(Reference Colaric, Veberic and Solar52).
Fat-soluble bioactives in nuts
There has been an increasing interest in discussing the functional characteristics of nut oils as they seem to be an interesting source of bioactive constituents. Fig. 2 shows chemical structures of some fat-soluble bioactives present in nuts. Table 2 summarises the fat-soluble bioactives in nuts (lipid, tocols, phytosterols, sphingolipids, carotenoids, chlorophylls and alkyl phenols).
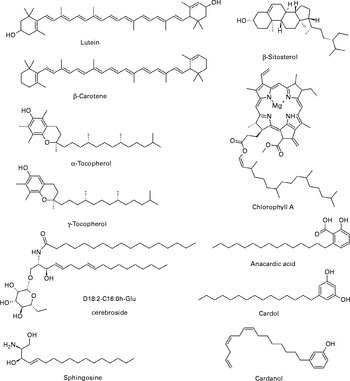
Fig. 2 Structures of some fat-soluble bioactives present in nuts.
Table 2 Reported fat-soluble bioactives in nut oils (mg/100 g)

ND, not detected.
* Data are obtained from USDA(4) (European chestnut and English walnut), except heartnut that is obtained from Li et al. (Reference Li, Tsao and Yang54).
† Data are compiled from Alasalvar & Pelvan(Reference Alasalvar and Pelvan10).
‡ Data are compiled from Alasalvar & Pelvan(Reference Alasalvar and Pelvan10), except sweet chestnut that is obtained from Zlatanov et al. (Reference Zlatanov, Antova and Angelova-Romova112).
§ Data are obtained from Miraliakbari & Shahidi(Reference Miraliakbari and Shahidi73). The oils of nuts were extracted using chloroform/methanol.
∥ Data are mean value of β-carotene, lutein and zeaxanthin and obtained from Trox et al. (Reference Trox, Vadivel and Vetter77). Carotenoid content in cashew oil was calculated based on oil content of cashew (43·85 g/100 g).
¶ Data are obtained from Trevisan et al. (Reference Trevisan, Pfundstein and Haubner80). The total amount of alkyl phenols in cashew oil was calculated based on oil content of cashew (43·85 g/100 g).
** Data are mean value of lutein (27th week after flowering date) and obtained from Bouali et al. (Reference Bouali, Trabelsi and Abdallah78).
†† Data are obtained from Cheikh-Rouhou et al. (Reference Cheikh-Rouhou, Hentati and Besbes79).
‡‡ Data are mean value of lutein from three ripe pistachio samples and obtained from Bellomo & Fallico(Reference Bellomo and Fallico76). Carotenoid content in pistachio oil was calculated based on oil content of pistachio (45·39 g/100 g).
§§ Data are mean value of chlorophylls b and a from three ripe pistachio samples and obtained from Bellomo & Fallico(Reference Bellomo and Fallico76). Chlorophyll content in pistachio oil was calculated based on oil content of pistachio (45·39 g/100 g).
∥∥ Data are obtained from Saitta et al. (Reference Saitta, Giuffrida and La Torre81).
Lipid
The oil content of nuts ranges from 2·26 % for European chestnut to 75·77 % for macadamia(4). The benefits of including nuts in human diet are partly related to their fat components. MUFA is the predominant fat-soluble bioactive in most nut oils, except Brazil nut, European chestnut, heartnut, pine nut and walnut that are rich in PUFA(Reference Alasalvar and Pelvan10).
Tocols
The mean content of total tocols range from 6·15 mg/100 g for macadamia oil to 59·60 mg/100 g for chestnut oil (Table 2)(Reference Alasalvar and Pelvan10). Oils extracted from various nuts have been reported to show significant tocol (tocopherols and tocotrienols) differences and patterns(Reference Miraliakbari and Shahidi1, 4, Reference Maguire, O'Sullivan and Galvin5, Reference Ryan, Galvin and O'Connor9, Reference Alasalvar, Amaral and Shahidi53–Reference Gómez-Caravaca, Verardo and Caboni61). Data for tocol levels and patterns in twelve nuts (almond, Brazil nut, cashew, chestnut, heartnut, hazelnut, macadamia, peanut, pecan, pine nut, pistachio and walnut) have been reported elsewhere(Reference Alasalvar and Pelvan10). Vitamin E, consisting of α-, β-, γ- and δ-tocopherols as well as tocotrienols, is a fat-soluble vitamin that generally functions as a potent antioxidant activity via chain-breaking reactions during peroxidation of unsaturated lipids(Reference Trumbo, Schlicker and Yates62). It is considered to be a significant health-promoting component of nuts and affords health benefits to those who routinely consume nuts(Reference Kris-Etherton, Hu and Ros63). Compared with other nut oils, hazelnut oil has been reported to serve as an excellent source of vitamin E(Reference Alasalvar, Amaral and Shahidi53), followed by pistachio, almond, pine nut(Reference Miraliakbari and Shahidi1) and peanut(Reference Jonnala, Dunford and Dashiell56), respectively. Vitamin E content in nut oils range from 1·13 to 41·92 mg/100 g, being lowest in European chestnut and highest in hazelnut(Reference Alasalvar and Pelvan10).
Phytosterols
Phytosterols, which are lipophilic plant-synthesised steroids, are one of the important bioactive classes of constitutes in nut oils. Phytosterol is a collective term, and saturated sterols are known as stanols. Sterols and stanols are differentiated by an alkane bond at the C1 position on the B ring. The total phytosterol content of nut oils expressed as mg/100 g is given in Table 2. Sweet chestnut oil contains the highest amount of total phytosterols (800 mg/100 g), followed by walnut (307 mg/100 g), peanut (284 mg/100 g), pecan (283 mg/100 g) and almond (271 mg/100 g). By contrast, macadamia oil contains the lowest total phytosterol content (128 mg/100 g)(Reference Alasalvar and Pelvan10). To the best of our knowledge, no phytosterol data on heartnut exist in the literature. Despite high level of phytosterols in chestnut oil, it may not be appreciable due to the presence of small amount of fat (0·81–2·26 %). Of the phytosterols, nuts are mainly composed of β-sitosterol but are also composed of a number of minor sterols, sterol esters and stanols(Reference Bolling, Oliver Chen and McKay11). Data on phytosterol levels and patterns in nuts (almond, Brazil nut, cashew, chestnut, heartnut, hazelnut, macadamia, peanut, pecan, pine nut, pistachio and walnut) have been reported elsewhere(Reference Miraliakbari and Shahidi1, Reference Alasalvar, Shahidi, Amaral, Alasalvar and Shahidi2, 4, Reference Maguire, O'Sullivan and Galvin5, Reference Ryan, Galvin and O'Connor9–Reference Bolling, Oliver Chen and McKay11, Reference Alasalvar, Amaral and Shahidi53, Reference Jonnala, Dunford and Dashiell56, Reference Bada, León-Camacho and Prieto60, Reference Kaijser, Dutta and Savage64–Reference da Costa, Ballus and Teixeria-Filho71).
Sphingolipids
Two recent reviews discussing the sphingolipid characteristics of certain nuts have been published(Reference Alasalvar and Pelvan10, Reference Wang, Tan, Ho, Alasalvar and Shahidi72). Studies about sphingolipids in nuts are quite sparse. Miraliakbari & Shahidi(Reference Miraliakbari and Shahidi73) have measured sphingolipid levels in seven hexane- or chloroform–methanol extracted nut oils (almond, Brazil nut, hazelnut, pecan, pine nut, pistachio and walnut); values ranged from 20 mg/100 g (hazelnut oil) to 330 mg/100 g (pistachio oil) in chloroform–methanol extracted oils and are presented in Table 2. The majority of the sphingolipid data have been derived by Miraliakbari & Shahidi(Reference Miraliakbari and Shahidi1, Reference Miraliakbari and Shahidi73, Reference Miraliakbari and Shahidi74), utilising a non-specific method of quantification. Thus, more effort is needed to gain reliable data on the sphingolipid content in nuts. Compared with other foods such as milk, egg, soyabeans, meat (chicken, beef and pork) and cereal (wheat), nuts have a comparatively low sphingolipid content.
Carotenoids
In contrast to other plant foods, certain nuts contain very low amounts of carotenoids such as α- and β-carotene, β-cryptoxanthin, lutein, lycopene and zeaxanthin(Reference Bolling, Oliver Chen and McKay11, Reference Kornsteiner, Wagner and Elmadfa75–Reference Trox, Vadivel and Vetter77). Pistachio oil presents only traces of β-carotene and lutein ( < 5/100 g oil)(Reference Kornsteiner, Wagner and Elmadfa75). Among twelve nuts (Table 2), only cashew oil (0·09 mg/100 g), pecan oil (0·014 mg/100 g) and pistachio oil (6·70 mg/100 g) have been reported to contain carotenoids(Reference Bellomo and Fallico76, Reference Trox, Vadivel and Vetter77, Reference Bouali, Trabelsi and Abdallah78). Trox et al. (Reference Trox, Vadivel and Vetter77) have reported that raw cashew contains traces of β-carotene, lutein and zeaxanthin. Bolling et al. (Reference Bolling, Oliver Chen and McKay11) have mentioned that as most nuts are not significant dietary sources of carotenoids, an effort to improve carotenoid data may not be worthwhile.
Chlorophylls
The chlorophyll pigments are important quality parameters as they correlate with colour, which is a basic attribute for evaluating oil quality. Among commonly consumed nuts, only pine nut oil (0·007 mg/100 g) and pistachio oil (24·09 mg/100 g) have been reported to contain the lipid subclasses of chlorophylls(Reference Bellomo and Fallico76, Reference Cheikh-Rouhou, Hentati and Besbes79). In ripe pistachio samples, chlorophyll a is approximately 3-fold higher than chlorophyll b counterpart(Reference Bellomo and Fallico76). No other published information on the chlorophyll content of other nuts is available.
Alkyl phenols
Cashew oil contains 146–242 mg/100 g of anacardic acids and cardols(Reference Trevisan, Pfundstein and Haubner80), while pistachio oil has sixteen different cardanols (44 mg/100 g)(Reference Saitta, Giuffrida and La Torre81). To the best of our knowledge, alkyl phenols in other nuts have not been characterised and reported. A series of twelve components (anacardic acids, cardols, 2-methylcardols and cardanols) in cashew shell oil were isolated(Reference Kubo, Komatsu and Ochi82). Different levels and profiles of alkyl phenols in various cashew products (such as apple, nut, cashew nut shell liquid and fibre) have also been reported(Reference Trevisan, Pfundstein and Haubner80).
Natural antioxidants in nuts
Natural antioxidants present in nuts are in the form of nutrient and non-nutrient (phytochemicals) antioxidants. Every food plant including nuts contains numerous types of natural antioxidants with different properties. In addition to well-known nutrient antioxidants (e.g. vitamins A, C and E and the mineral Se), there are numerous non-nutrient antioxidants (e.g. carotenoids such as β-carotene and lycopene and phenolics) in food plants(Reference Alasalvar and Shahidi24).
Nuts are good sources of nutrient antioxidants (such as vitamin E and Se)(4, Reference Alasalvar and Shahidi24, Reference Sathe, Monaghan, Kshirsagar, Alasalvar and Shahidi83). Among antioxidant vitamins (A, C and E), vitamin E is the most abundantly found vitamin in most nuts. At suggested consumption level (1·5 oz or approximately 42·5 g/d)(84), almond and hazelnut provide up to 74·8 and 72·7 % vitamin E, respectively, of the daily 15 mg recommended for adult males and females(Reference Alasalvar and Shahidi24). The other nuts contain much lower amount of vitamin E compared with almond and hazelnut. In general, nuts are not good sources of vitamins A and C. With respect to Se, Brazil nut itself serves as an excellent source of this mineral compound. Only one kernel of Brazil nut (approximately 5 g) supplies 174 % of Se for RDA(Reference Alasalvar and Shahidi24).
With regard to non-nutrient antioxidants, several thousands of phytochemicals, some of which possess strong antioxidant activities (e.g. catechin, quercetin, tannins, ellagic acid, chlorogenic acid and cyanidin), have been reported(Reference Bolling, Oliver Chen and McKay11, Reference Shahidi and Naczk13, Reference Alasalvar, Karamać and Kosińska23, Reference Alasalvar and Shahidi24, Reference Lui85–Reference Yang, Martinson and Liu88). Several studies have also reported that phenolic compounds possess much stronger antioxidant activities than nutrient antioxidants(Reference Shahidi and Naczk13).
Recently, Vinson & Cai(Reference Vinson and Cai89) measured the levels of free and total polyphenols (μmol catechin equivalents/g) in both raw and roasted nuts (almond, Brazil nut, cashew, macadamia, peanut, pecan, pistachio and walnut). Walnut had the highest levels of free and total polyphenols than in both the combined raw and roasted nut samples. Roasting had some effect on either free or total polyphenols in nuts and total polyphenols were slightly higher in roasted than in raw nuts. They also measured the efficacy of the antioxidants in the nuts. The order of decreasing the efficacy (increasing inhibitory concentration 50% (IC50)) in raw nuts is walnut>cashew>hazelnut>pecan>almond>macadamia>pistachio>Brazil nut>peanut. Roasting causes a decline in efficacy. The total antioxidant activity of nuts has been assessed by different researchers using different assays (such as ferric-reducing ability of plasma, total oxyradical scavenging capacity, 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity and 2,2-azino-bis (3-ethylbenz-thiazoline-6-sulfonic acid) radical scavenging activity); walnut possess the highest antioxidant activity in all assays among nuts(Reference Blomhoff, Carlsen and Andersen25, Reference Yang, Liu and Halim48, Reference Halvorsen, Holte and Myhrstad90–Reference Abe, Lajolo and Genovese92). In all nuts, most of the antioxidants are located in the skin (pellicle) and less than 10 % is retained in the walnut when skin is removed(Reference Blomhoff, Carlsen and Andersen25). In other words, the removal of skin from nuts reduces the antioxidant activity considerably(Reference Arcan and Yemenicioğlu91). When using an oxygen radical absorbance capacity assay of nuts, pecan has the highest total oxygen radical absorbance capacity value of 179·40 μmol of trolox equivalents/g(Reference Wu, Beecher and Holden17), total phenolics 20·16 mg of gallic acid equivalents/g(Reference Wu, Beecher and Holden17) and total flavonoids 34·01 mg/100 g(15), whereas pistachio has the highest total isoflavone value of 176·9 μg/100 g, lignans 198·9 μg/100 g and phytoestrogens 382·5 μg/100 g(Reference Thompson, Boucher and Liu19). In addition, hazelnut contains the highest total content of proanthocyanidins or condensed tannins (500·7 mg/100 g)(14, Reference Gu, Kelm and Hammerstone16), among nuts.
Based on USDA available data, the total nut consumption (tree nuts and peanut) in the US is 162 mg/d. In the European Union (EU), 5·0 g of peanut/d is consumed, contributing 71·5 mg of polyphenols. Tree nuts have 86·9 mg of polyphenols and thus nut contribution of polyphenols to the EU diet amounts to 158 mg/d, which is very similar to that of the US diet(Reference Vinson and Cai89).
Health benefits of nuts
Traditionally, nuts have been used for improving immunity, digestion, wound healing and circulation, as well as analgesics(Reference Casas-Agustench, Salas-Huetos and Salas-Salvado93). Today, nut consumption is recognised for its potential to reduce CVD risk. In 2003(84) and 2004(94), the US Food and Drug Administration granted a qualified health claim that consumption of 1·5 oz (approximately 42·5 g)/d of most nuts, or specifically walnut, may reduce the risk of heart disease as part of a diet low in saturated fat and cholesterol. In 2011, European Food Safety Authority (EFSA) allowed an Article 13 claim that the consumption of 30 g of walnuts in the context of a balanced diet leads to improvement of endothelium-dependent vasodilation(95). Claims can also be made for LDL-cholesterol reduction based on the MUFA/PUFA and linoleic/α-linoleic content of nuts(95).
Recent observational studies further support the association of increased nut consumption and reduced mortality. Data from the Nurses’ Health Study and the Health Professionals Follow-up Study, two prospective cohort studies of 118 962 adults with 24 and 30 years of follow-up, respectively, were used to examine the association between nut consumption and mortality(Reference Bao, Han and Hu96). Increasing frequency of nut consumption was associated with lower hazard ratios for death (P< 0·001) with 0·80 (95 % CI 0·73, 0·86) for individuals consuming nuts seven or more times per week(Reference Bao, Han and Hu96). Rates of death from cancer, CVD, heart disease and respiratory disease were inversely related to frequency of nut consumption(Reference Bao, Han and Hu96). Observational data from the Australian Blue Mountains Eye Study also supported this finding. Higher frequency of nut consumption was associated with age- and sex-adjusted rates of inflammatory disease activity (P= 0·02), but this trend is insignificant upon multivariable adjustment(Reference Gopinath, Buyken and Flood97).
Intervention studies have also provided new data suggesting health benefits of nut consumption. The Prevención con Dieta Mediterránea intervention study evaluated the effect of consuming nuts or olive oil with participants adhering to a Mediterranean diet, relative to a low-fat control diet (n 7447 adults)(Reference Estruch, Ros and Salas-Salvadó98). Participants in the nuts group consumed 30 g of mixed walnut, hazelnut and almond per d. After a median follow-up of 4·8 years, both the Mediterranean diet with olive oil and nuts reduced the rates of cardiovascular events. The hazard ratio was 0·72 (95 % CI 0·54, 0·96) for the nut-supplemented group compared with the control diet group(Reference Estruch, Ros and Salas-Salvadó98). The nut-supplemented group also had reduced risk of all-cause mortality. Those who consumed nuts more than three servings per week had the lowest rates of total mortality risk (HR 0·36, 95 % CI 0·22, 0·66)(Reference Guasch-Ferré, Bulló and Martínez-González99). Furthermore, cognitive improvement occurred in a subset of Prevención con Dieta Mediterránea participants at high vascular risk (n 522)(Reference Martínez-Lapiscina, Clavero and Toledo31). Assessments of cognitive function by the mini-mental state examination and clock drawing test were higher after 6·5 years of the intervention in both nuts and olive-oil supplemented groups(Reference Martínez-Lapiscina, Clavero and Toledo31).
Other recent studies suggest that components in nuts beyond lipids contribute to mechanisms of cardioprotection. For example, apoE− / − mice fed a high-fat diet reduced atherosclerotic plaque development by 55 %, whereas walnut oil supplemented mice did not(Reference Nergiz-Unal, Kuijpers and de Witt100). Also, acute consumption of walnut skins (5·6 g) for 3 d decreased the postprandial reactive hyperaemia index compared with baseline, in a crossover trial of n 15 healthy overweight and obese moderate hypercholesterolaemic adults(Reference Berryman, Grieger and West101).
Further evidence supports the emerging role of nuts in reducing risk for insulin resistance and type 2 diabetes mellitus. Almond consumption (56 g/d for 4 weeks) by Chinese adults with type 2 diabetes mellitus with mild hyperlipidaemia decreased C-reactive protein, IL-6 and TNF-α levels and increased resistance to Cu-induced LDL-oxidation relative to the control diet group(Reference Liu, Liu and Chen29). Inclusion of 42·5 g of peanut butter in a carbohydrate-rich breakfast meal in a crossover study of n 15 women with high type 2 diabetes mellitus risk reduced postprandial glucose and enhanced gut satiety hormone secretion(Reference Reis, Ribeiro and Costa27). Nut polyphenols could also limit glycaemia by inhibiting carbohydrate digestion. Almond and chestnut tannins were identified as potent α-amylase inhibitors and were confirmed to reduce blood glucose in rats fed maize (corn) starch(Reference Tsujita, Yamada and Takaku102, Reference Tsujita, Shintani and Sato103).
Conclusions
Nuts, which contain phytochemicals, fat-soluble bioactives, minor components as well as nutrient and non-nutrient antioxidants beyond their basic nutritional functions, offer an excellent choice for heart-healthy snack food and food additive. Nuts should be consumed with their skin (pellicles), whenever possible, because of their high phytochemical content as well as antioxidant activity. The present report provides a detailed and up to date summary and scientific review of the available data on nut antioxidant components and health.
Acknowledgements
The authors’ contributions are as follows: C. A. contributed introduction, fat-soluble bioactives in nuts, natural antioxidants in nuts and conclusions; B. W. B. contributed introduction, phytochemicals in nuts, health benefits of nuts and conclusions.
All authors declare that there are no conflicts of interest.






