Glucagon-like peptide-1 (GLP-1) is a gastrointestinal hormone produced and released from enteroendocrine L cells. The primary function of GLP-1 is to enhance glucose-induced insulin secretion (the incretin effect), which maintains normal glucose homeostasis in the postprandial state( Reference Cho, Fujita and Kieffer 1 ). GLP-1 also has a proliferative effect on pancreatic β-cells, and multiple effects external to the pancreas, including the liver, adipose tissue, cardiovascular system and central nervous system( Reference Kim and Schuppan 2 ). Owing to the incretin effect, GLP-1 receptor agonists have been recently used for the treatment of type 2 diabetes( Reference Drucker, Dritselis and Kirkpatrick 3 , Reference Madsbad, Kielgast and Asmar 4 ), together with dipeptidyl peptidase-IV (DPP-IV) inhibitors that have been broadly used for the treatment of inflammatory diseases( Reference Yazbeck, Howarth and Abbott 5 ). Because GLP-1 and another incretin, glucose-dependent insulinotropic polypeptide (GIP), are immediately inactivated by degradation with plasma DPP-IV, inhibitors for DPP-IV can help preserve physiological activity of GLP-1 and GIP( Reference Cho, Fujita and Kieffer 1 , Reference Yazbeck, Howarth and Abbott 5 ). Increasing endogenous GLP-1 by possible dietary factors could be effective in improving glucose tolerance and preventing glucose intolerance. Recent studies have shown that the stimulatory effect on GLP-1 by single oral administration of dietary factors such as proteins/peptides( Reference Mochida, Hira and Hara 6 – Reference Jakubowicz, Froy and Ahrén 8 ) and an amino acid( Reference Reimann, Williams and da Silva Xavier 9 , Reference Greenfield, Farooqi and Keogh 10 ) effectively lowers the glycaemic response in animal models and/or human subjects.
Resistant maltodextrin (RMD) is a water-soluble, non-viscous and non-digestible saccharide( Reference Baer, Stote and Henderson 11 ). Its average molecular weight is approximately 2000 Da. Although RMD reportedly lowers the glycaemic response( Reference Livesey and Tagami 12 , Reference Hashizume, Kishimoto and Kanahori 13 ) and postprandial TAG elevation( Reference Kishimoto, Oga and Tagami 14 ), and also promotes mineral absorption( Reference Miyazato, Nakagawa and Kishimoto 15 ), the mechanisms involved in the improvement of glucose tolerance are poorly understood.
RMD is fermented by luminal microbiota, resulting in increased proportions of total bacteria and bifidobacteria, and enhanced SCFA production in animals( Reference Miyazato, Nakagawa and Kishimoto 15 ) and humans( Reference Baer, Stote and Henderson 11 , Reference Fastinger, Karr-Lilienthal and Spears 16 ). The continuous ingestion of fermentable, non-digestible saccharides such as fructo-oligosaccharides (FOS)( Reference Cani, Dewever and Delzenne 17 , Reference Cani, Daubioul and Reusens 18 ), inulin( Reference Delzenne, Cani and Daubioul 19 , Reference Reimer and Russell 20 ) and resistant starch( Reference Zhou, Martin and Tulley 21 ) increases GLP-1 levels in animal models and human subjects. The phenomenon is explained by the prebiotic effect of these fermentable saccharides. SCFA produced by the gut microbiota are capable of stimulating GLP-1 secretion, and the G protein-coupled receptor FFAR2 (GPR43) has been shown to function in L cells to sense luminal SCFA, and thereby induce GLP-1 secretion( Reference Tolhurst, Heffron and Lam 22 ). The fact that continuous ingestion of RMD improves glucose tolerance( Reference Livesey and Tagami 12 ) raises the possibility that GLP-1 secretion and/or production is associated with this effect.
In the present study, we examined whether continuous ingestion of RMD and FOS by rats affected their glucose tolerance, plasma GLP-1 levels and GLP-1 production in the small and large intestines. In addition, the stimulatory effect of single oral administration of RMD, and the direct effects of RMD on GLP-1 secretion were investigated in conscious rats, and in a GLP-1-producing enteroendocrine cell model, respectively.
Materials and methods
Animals and diets
Male Sprague–Dawley rats (5 weeks of age) were purchased from Japan SLC, Inc., and were fed an American Institute of Nutrition (AIN)-93G-based diet( Reference Reeves 23 ) (control diet) for a 1-week acclimatisation period. Each rat was housed in a separate cage, and had free access to the diet and water, except for the days preceding glucose tolerance test at week 6 and killing at week 7. The experiment was performed in a temperature-controlled room maintained at 23 ± 2°C with a 12 h light–12 h dark cycle (08.00–20.00 hours light period). Rats were divided into five groups and were fed the AIN-93G diet-based test diet (Table 1) for 7 weeks. All or half of the cellulose (50 g/kg diet) in the control diet was replaced with RMD (Fibersol-2; Matsutani Chemical Industry Company) or FOS (Meioligo-P; Meiji Company Limited) to yield final concentrations of 25 g/kg (2·5 %) and 50 g/kg (5 %) in the individual test diets. The study was approved by the Hokkaido University Animal Committee, and the animals were maintained in accordance with the Hokkaido University guidelines for the care and use of laboratory animals. Body weight and food intake were measured every 2–3 d.
Table 1 Composition of the test diet (g/kg diet)
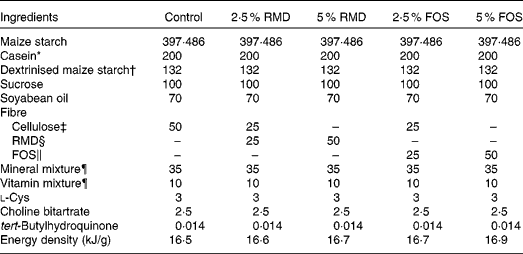
RMD, resistant maltodextrin; FOS, fructo-oligosaccharides; AIN, American Institute of Nutrition.
* Acid Casein (Fonterra Limited).
† TK-16 (Matsutani Chemical Industry Company Limited).
‡ Avicel PH102 (Asahi Kasei Chemicals Corporation).
§ RMD (Fibersol 2; 4·19 kJ/g; Matsutani Chemical Industry).
∥ FOS (Meioligo-P, 8·37 kJ/g; Meiji Company Limited).
¶ Mineral and vitamin mixtures were prepared according to the AIN-93G formulation.
Glucose tolerance test
An intraperitoneal glucose tolerance test (IPGTT) was performed on rats 6 weeks after feeding the test diets. Rats were fasted overnight, and basal (fasting) blood was collected from the tail vein for the measurements of glucose, insulin and total GLP-1 levels. After 15 min, the glucose solution was intraperitoneally injected (1 g/kg) and blood samples were collected from the tail vein at 0, 15, 30, 60, 90 and 120 min after injection. Blood samples were collected into tubes containing heparin (final concentration 50 IU/ml; Ajinomoto Company, Inc.), aprotinin (final concentration 500 Kallikrein inhibitor units (kIU)/ml; Wako Pure Chemical Industries Limited) and DPP-IV inhibitor (final concentration 50 μmol/l, DPP4-010; Millipore Corporation). Plasma was separated by centrifugation at 2500 g for 10 min at 4°C and frozen at − 80°C until glucose, insulin and GLP-1 measurements were taken. Plasma glucose concentration was measured using the Glucose CII Test Kit (Wako). Plasma insulin concentration was measured using the Rat Insulin ELISA (AKRIN-010T; Shibayagi Company Limited) and Multi Species GLP-1 Total ELISA (EZGLP1T-36K; Millipore), respectively. Homeostatic model assessment for insulin resistance (HOMA-IR)( Reference Cacho, Sevillano and de Castro 24 ) was calculated using the following equation:
where 1 mg insulin = 26 IU.
Portal blood and tissue collection
After 7 weeks, blood samples were collected from the portal veins of rats under sodium pentobarbital anaesthesia (50 mg/kg body weight, Somnopentyl injection; Kyoritsu Seiyaku Corporation) into a syringe containing heparin (final concentration 50 IU/ml), aprotinin (final concentration 500 kIU/ml; Wako Pure Chemical Industries Limited) and DPP-IV inhibitors (final concentration 50 μmol/l). Plasma was collected and stored as described above for the measurements of glucose, insulin, GLP-1 (total and active) and GIP. Active GLP-1 and total GIP levels were measured using the GLP-1 (Active) ELISA (EGLP-35K; Millipore) and Rat/Mouse GIP (total) ELISA (EZRMGIP-55K; Millipore), respectively. Active GLP-1 represents intact GLP-1 (GLP-17–36, GLP-17–37) that exerts insulin-releasing activity. Total GLP-1 includes both intact and inactivated forms of GLP-1 (GLP-19–36 and GLP-19–37 degraded by DPP-IV), and represents secreted GLP-1.
After the rats were killed by exsanguination, the jejunum, ileum, caecum and colon were isolated. Luminal contents of the jejunum, ileum and colon were washed with cold saline, then mucosa (5 cm segment) and 2 cm segments were collected from the middle region of these tissues for proglucagon mRNA analysis and GLP-1 content measurements, respectively. The tissue weight of the caecum as well as weight and pH of the caecal content were measured. After washing with cold saline, the caecal tissue was separated into halves; one half was collected for GLP-1 measurements and from the other half, the mucosa was scraped for proglucagon mRNA analysis. The intestinal segments were immediately frozen in liquid N2 and stored at − 80°C. Mucosa samples were immediately transferred into tubes containing buffer RLT (RNeasy Mini Kit; Qiagen) and frozen in liquid N2, and then stored at − 80°C. Mesenteric, retroperitoneal and epididymal adipose tissue weights were measured.
Measurement of glucagon-like peptide-1 content in intestinal tissues
Intestinal segments were homogenised in ethanol acid solution (ethanol–12 m-HCl–water 74:25:1; 5 ml/g tissue)( Reference Cani, Hoste and Guiot 25 ) and extracted for 24 h at 48°C. After centrifugation (2000 g for 20 min), the supernatants were collected and diluted with saline (1000-fold) to measure total GLP-1 levels by ELISA. Tissue protein contents were measured using Lowry's method.
Real-time PCR
Using a real-time PCR system( Reference Iwaya, Lee and Yamagishi 26 ), mRNA expression levels were determined. Total RNA was extracted using the RNeasy Mini kit (Qiagen), according to the manufacturer's instructions. Complementary DNA was synthesised using the ReverTra Ace qPCR Master Mix with gDNA Remover (Toyobo Company Limited), according to the manufacturer's instructions. Gene expression levels were determined using the Mx3000P Real-Time PCR System (Stratagene) and TaqMan Gene Expression Assay (Life Technologies Company) with rat gene-specific predesigned TaqMan primers and probe sets (Rn99999916_s1 for GAPDH (glyceraldehyde 3-phosphate dehydrogenase) and Rn00562293_m1 for proglucagon). Relative expression levels were calculated for each sample after normalisation to those of GAPDH as a reference gene using the standard curve method.
Glucagon-like peptide-1 secretion study using a glucagon-like peptide-1-producing cell line (GLUTag cells)
GLUTag cells (courtesy of Dr D. J. Drucker, University of Toronto, Toronto, Canada), a murine GLP-1-producing enteroendocrine cell line, were grown in Dulbecco's modified Eagle's medium (catalogue no. 12 100-038; GIBCO), supplemented with 10 % fetal bovine serum, 50 IU/ml of penicillin and 500 μg/ml of streptomycin in a humidified 5 % CO2 atmosphere at 37°C. Cells were routinely trypsinised and subcultured after reaching 80–90 % confluency. The GLUTag cells were grown in forty-eight-well culture plates at a density of 1·25 × 105 cells/well for 2 d until they reached 80–90 % confluency. Cells were washed twice with HEPES buffer (140 mm-NaCl, 4·5 mm-KCl, 20 mm-HEPES, 1·2 mm-CaCl2, 1·2 mm-MgCl2, 10 mm-glucose and 0·1 % bovine serum albumin, pH 7·4) to remove the culture media. The cells were then exposed to the test agents dissolved in the same buffer for 60 min at 37°C. Supernatants were collected from the wells, centrifuged at 800 g for 5 min at 4°C to remove the remaining cells, and then stored at − 50°C, until GLP-1 concentration was measured using a commercial enzyme immunoassay kit (Yanaihara Institute, Inc.).
Glucagon-like peptide-1 secretory responses after single oral administration of resistant maltodextrin in rats
To examine the acute effect of orally administered RMD on GLP-1 secretion, blood samples were collected from the jugular veins of conscious rats. Male Sprague–Dawley rats (7 weeks of age) equipped with jugular catheters were used in the experiment( Reference Higuchi, Hira and Yamada 7 ). After a 3–4 d recovery period, rats were fasted overnight. RMD, maltodextrin (PINEDEX#2; Matsutani Chemical Industry) and ZeinH( Reference Hira, Mochida and Miyashita 27 ) were each dissolved in deionised water that was orally administered (2 g/kg), by using a feeding tube (5 Fr; Atom Medical Company). Saline (8 ml/kg) was administered as a control treatment. Blood samples were collected before and after oral administration of the test solutions. Plasma total GLP-1 levels were measured as described above.
Statistical analyses
Data are expressed as means with their standard errors of the mean. Statistical analyses were performed using JMP Pro version 10.0 software (SAS Institute, Inc.). Statistical significance was assessed using one-way (Figs. 1–6) or two-way (Fig. 7) ANOVA. Significant differences (P< 0·05) between the mean values were determined using Tukey–Kramer or Dunnett's test, as appropriate.

Fig. 1 Fasting glucose (A), insulin (B), glucagon-like peptide-1 (GLP-1) (D) levels and homeostatic model assessment for insulin resistance (HOMA-IR) (C) in the tail vein plasma of rats after 6 weeks of feeding the test diet. Blood samples were collected from the tail vein of rats fasted overnight after 6 weeks of feeding the test diet (15 min before intraperitoneal glucose administration). Glucose, insulin and total GLP-1 levels were measured in the plasma. HOMA-IR was calculated using glucose and insulin concentrations. To convert glucose in mg/dl to mmol/l, multiply by 0·0555. Values are means (n 7–8), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05; Dunnett's test). RMD, resistant maltodextrin; FOS, fructo-oligosaccharides.

Fig. 2 Plasma glucose levels in rats under the intraperitoneal glucose tolerance test (IPGTT) after 6 weeks. After 6 weeks of feeding the test diet, an IPGTT was performed in rats fasted overnight. Glucose solution was intraperitoneally injected (1 g/kg) at 0 min, and then blood samples were collected from the tail vein. Glucose levels were measured in the plasma (A). The AUC for changes in glucose levels relative to basal levels (B) was calculated using the trapezoidal rule. To convert glucose in mg/dl to mmol/l, multiply by 0·0555. Values are means (n 7–8), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control group at the same time point (P< 0·05; Tukey–Kramer test). a,bMean values with unlike letters were significantly different between the treatment groups (P< 0·05; Tukey–Kramer test). ![]() , Control;
, Control; ![]() , 2·5 % resistant maltodextrin (RMD);
, 2·5 % resistant maltodextrin (RMD); ![]() , 5 % RMD;
, 5 % RMD; ![]() , 2·5 % fructo-oligosaccharides (FOS);
, 2·5 % fructo-oligosaccharides (FOS); ![]() , 5 % FOS.
, 5 % FOS.

Fig. 3 Portal glucose (A), insulin (B), total glucagon-like peptide-1 (GLP-1) (C), active GLP-1 (D) and glucose-dependent insulinotropic polypeptide (GIP) (E) levels after 7 weeks of feeding the test diet. Blood samples were collected from the portal veins of rats fasted overnight after 7 weeks of feeding the test diet, and the levels of glucose, insulin, GLP-1 (total and active) and total GIP were measured in the plasma. To convert glucose in mg/dl to mmol/l, multiply by 0·0555. Values are means (n 7–8), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different between the treatment groups (P< 0·05; Tukey–Kramer test). RMD, resistant maltodextrin; FOS, fructo-oligosaccharides.

Fig. 4 Intestinal glucagon-like peptide-1 (GLP-1) concentrations after 7 weeks of feeding the test diet. Intestinal tissue samples were collected from the jejunum (A), ileum (B), caecum (C) and colon (D) of each rat. After acid–ethanol extraction, GLP-1 and total protein concentrations were measured. GLP-1 concentration was corrected according to the total protein content. GLP-1 content in the whole caecum (E) was calculated on the basis of the tissue weight of the whole caecum (Table 2). Values are means (n 7–8), with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different between the treatment groups (P< 0·05; Tukey–Kramer test). RMD, resistant maltodextrin; FOS, fructo-oligosaccharides.

Fig. 5 Proglucagon mRNA expression levels in the intestinal mucosa. Intestinal mucosa samples were scraped from the jejunum (A), ileum (B), caecum (C) and colon (D). Total RNA was used for real-time PCR analysis. Proglucagon mRNA expression levels were normalised to those of GAPDH (glyceraldehyde 3-phosphate dehydrogenase), and data are expressed as relative changes to the control group. Values are means (n 7–8), with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different between the treatment groups (P< 0·05; Tukey–Kramer test). RMD, resistant maltodextrin; FOS, fructo-oligosaccharides.

Fig. 6 Glucagon-like peptide-1 (GLP-1) secretion in glucagon-like peptide-1-producing cell line (GLUTag cells). Enteroendocrine GLUTag cells were exposed to various concentrations (1–20 mm) of resistant maltodextrin (RMD) or fructo-oligosaccharides (FOS) for 60 min. GLP-1 concentration in the supernatant was measured by ELISA. Values are means (n 8–20), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control (blank) treatment (P< 0·05; Tukey–Kramer test).
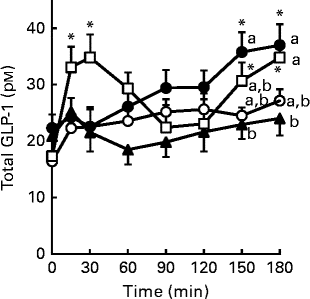
Fig. 7 Glucagon-like peptide-1 (GLP-1) secretion in response to single oral administration of resistant maltodextrin (RMD) in conscious rats. Saline (8 ml/kg, control;![]() ) or 2 g/kg of either RMD (
) or 2 g/kg of either RMD (![]() ), maltodextrin (
), maltodextrin (![]() ) or ZeinH (
) or ZeinH (![]() ) were orally administered to the fasted rats. Blood samples were collected from the jugular vein before and after oral administration. Plasma total GLP-1 concentrations were measured by ELISA. Two-way ANOVA P values were < 0·01 for time and treatment, and < 0·068 for the interaction between time and treatment. Values are means (n 6–8), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the basal (0 min) values within each treatment group (P< 0·05; Dunnett's test). a,b,cMean values with unlike letters were significantly different between the treatment groups at the same time point (P< 0·05; Tukey–Kramer test).
) were orally administered to the fasted rats. Blood samples were collected from the jugular vein before and after oral administration. Plasma total GLP-1 concentrations were measured by ELISA. Two-way ANOVA P values were < 0·01 for time and treatment, and < 0·068 for the interaction between time and treatment. Values are means (n 6–8), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the basal (0 min) values within each treatment group (P< 0·05; Dunnett's test). a,b,cMean values with unlike letters were significantly different between the treatment groups at the same time point (P< 0·05; Tukey–Kramer test).
Results
After 6 weeks of feeding the test diet, rats were fasted overnight and basal glucose (Fig. 1(A)) and insulin (Fig. 1(B)) levels were measured. No significant differences among the treatment groups were observed; however, a slight decline in insulin levels and HOMA-IR (Fig. 1(C)) was observed in the RMD and FOS groups. Plasma total GLP-1 levels (Fig. 1(D)) increased dose-dependently in both the RMD and FOS groups, and the 5 % RMD group showed a significantly (P= 0·036) higher GLP-1 concentration, compared with the control group (P< 0·05; Dunnett's test).
Glucose solution was injected intraperitoneally 15 min after the fasting blood samples were collected. The RMD group showed a dose-dependent reduction in the overall glycaemic response, and glucose level after 15 min was significantly lower in the 5 % RMD group than in the control group (P= 0·047; Fig. 2(A)). The 2·5 % FOS group had a significantly lower glycaemic response after 30 min (P= 0·035), compared with the control group. The AUC for changes in glucose concentration was significantly lower (P= 0·028) in the 5 % RMD group, and the other three groups had lower tendency (as indicated by ‘a,b’ in the figure), compared with the control group (Fig. 2(B)).
After 7 weeks of feeding the test diet, caecal tissue weights were significantly increased by the RMD (2·5 and 5 %, both P< 0·01) and FOS (5 %, P< 0·01) treatments (Table 2). The weights of caecal content were increased significantly by the RMD treatments compared with the control group (P= 0·023 for the 2·5 % RMD group and P= 0·01 for the 5·0 % RMD group, respectively). Conversely, the pH of caecal content was significantly decreased in a dose-dependent manner in rats fed the RMD (2·5 and 5 %, both P< 0·01) and FOS (5 %, P< 0·01) diets. The final body weights of the rats and their daily food intake did not differ among the treatment groups. The weights of the mesenteric, retroperitoneal and epididymal adipose tissues were slightly lower in the RMD and FOS groups, but significant difference was not detected. In the early period of the experiment, slightly soft faeces but not diarrhoea were observed in both the RMD and FOS groups.
Table 2 Body weight, food intake, adipose weight, caecal weight and caecal content pH after 7 weeks of feeding the test diet (Mean values with their standard errors, n 7–8)

RMD, resistant maltodextrin; FOS, fructo-oligosaccharides.
a,b,cMean values with unlike superscript letters were significantly different between the treatment groups (P< 0·05; Tukey–Kramer test).
Fasting portal glucose levels did not differ among the treatment groups (Fig. 3(A)), but insulin levels were significantly lower in the 5 % RMD (P= 0·011) and 5 % FOS (P= 0·024) groups (Fig. 3(B)) than in the control group. Portal GLP-1 levels increased dose-dependently in response to the RMD and FOS treatments, and the 5 % RMD (P= 0·007) and 5 % FOS (P= 0·041) groups had significantly higher active GLP-1 levels than the control group (Fig. 3(C)). Total GLP-1 level was also significantly higher in the 5 % RMD group (P= 0·006, Fig. 3(D)). Portal GIP levels were similar in all the groups (Fig. 3(E)).
GLP-1 concentration, corrected according to the total tissue protein concentration (pmol/mg protein), was increased overall, across the intestinal region from the proximal to the distal. GLP-1 concentration was significantly decreased in the jejunum of rats fed the FOS diet, compared with the control group (P= 0·004 for the 2·5 % FOS group and P= 0·033 for the 5·0 % FOS group, respectively; Fig. 4(A)), but was not increased by any test diet in the other intestinal regions (Fig. 4(A)–(D)). However, the GLP-1 content in the whole caecum (Fig. 4(E)) of the 2·5 and 5 % RMD groups tended to be higher than that of the control group, due to the increased caecal tissue weight (Table 2). In contrast, the FOS groups had similar or slightly lower GLP-1 content in the whole caecum compared with the control group.
Although the jejunum is not a major source of GLP-1 production, proglucagon expression levels were decreased in this tissue by the RMD and FOS treatments (Fig. 5(A)). In contrast, the RMD and FOS treatments increased proglucagon expression levels in the caecum (P= 0·025 for the 2·5 % RMD group, P< 0·001 for the 5·0 % RMD group and the 5·0 % FOS group v. the control group, respectively) and colon (P= 0·007 for the 5·0 % RMD group and P= 0·036 for the 5·0 % FOS group v. the control group, respectively) in a dose-dependent manner (Fig. 5(C) and (D)).
GLP-1 secretion was markedly increased by ZeinH (P< 0·001), a dietary peptide used as a positive control( Reference Hira, Mochida and Miyashita 27 ). RMD at 20 mm induced a significant increase in GLP-1 secretion (P< 0·001; Fig. 6), whereas FOS at 20 mm had only a mild (P= 0·097) effect. Both RMD and FOS at concentrations lower than 20 mm did not induce GLP-1 secretion.
To examine its acute effect on GLP-1 secretion in vivo, RMD was orally administered to fasted rats in a separate experiment. Single oral administration of RMD gradually increased plasma GLP-1 levels, with significant elevations observed at 150 min (P= 0·040) and 180 min (P= 0·022), compared with the basal (0 min) value (Fig. 7). In contrast, (digestible) maltodextrin, which has a similar molecular weight as RMD, had little effect on plasma GLP-1 levels. ZeinH immediately (15–30 min) increased plasma GLP-1 levels (P= 0·040 for 15 min and P= 0·004 for 30 min, respectively), compared with the basal (0 min) value, followed by gradual and significant elevations at 150 min (P= 0·013) and 180 min (P= 0·005), compared with the basal value.
Discussion
Endogenous GLP-1 is a potential therapeutic target to improve glucose homeostasis, and possibly to prevent glucose intolerance. Although postprandial luminal nutrient-triggered GLP-1 secretion is well understood, studies related to the regulation of fasting GLP-1 levels by dietary factors are limited. In the present study, we examined whether supplementation of water-soluble non-digestible saccharides RMD and FOS in a standard diet affected fasting GLP-1 levels, intestinal GLP-1 production and glucose tolerance in normal rats.
Basal GLP-1 levels after 6 and 7 weeks of feeding the test diet were higher in the 5 % RMD group than those in the control group (Figs. 1 and 3). Because rats were fasted overnight, elevated GLP-1 levels could reflect enhanced spontaneous GLP-1 secretion, rather than induced by the remaining chyme in the intestinal lumen. The elevation of portal GLP-1 levels, with no change in GIP levels, in both the RMD and FOS groups indicates that the effect of these non-digestible saccharides was selective for GLP-1, but not for both the incretins.
Improved glucose tolerance was observed in the RMD groups, as indicated by the IPGTT (Fig. 2). Consistent with this finding, fasting insulin levels were reduced in the RMD groups (Fig. 3). In the present study, the IPGTT was conducted as a glucose tolerance test, rather than as the conventional oral glucose tolerance test, because an oral glucose load could stimulate GLP-1 secretion, and the glycaemic response, after an oral glucose load, could be affected by the gastric emptying rate of the glucose solution and glucose absorption in the small intestine( Reference Muramatsu, Hira and Mitsunaga 28 ). The lower glycaemic response, shown in the present study, did not involve either reduced intestinal glucose absorption or luminal glucose-induced GLP-1 secretion. This could rather be due to improved insulin sensitivity in the RMD group. As GLP-1 receptor agonists improve glucose tolerance, when used as a treatment for type 2 diabetes( Reference Drucker, Dritselis and Kirkpatrick 3 , Reference Madsbad, Kielgast and Asmar 4 ), enhanced basal GLP-1 levels could have contributed to the observed improvement in glucose tolerance in the present study.
GLP-1 content in the whole caecum was highest in the RMD groups (Fig. 4(E)), due to the increased caecal tissue weight (Table 2). This could partially explain why the highest plasma GLP-1 levels were observed in the 5 % RMD group (Figs. 1 and 3). Proglucagon mRNA expression was markedly increased by the RMD and FOS treatments (Fig. 5), but such changes were not directly reflected by GLP-1 concentrations in the caecum and colon. Previous studies have demonstrated that GLP-1 content in the distal intestine was increased by inulin-type fructans, together with increased proglucagon mRNA expression( Reference Cani, Dewever and Delzenne 17 , Reference Cani, Daubioul and Reusens 18 , Reference Cani, Hoste and Guiot 25 ). However, in these studies, fibre contents in the diet were higher (10 %) than those used in the present study (5 %). Possibly, RMD at 5 % was not sufficient to increase GLP-1 content in each L cell or to increase L-cell numbers. Another mechanism such as post-translational regulation might be involved in the increment of tissue GLP-1 content. Prohormone convertase 1 (PC1) plays an important role in the liberation of GLP-1 from proglucagon protein in enteroendocrine L cells. It has previously been demonstrated that oligofructose treatment increased GLP-1 content and PC1 mRNA level in the colon of diabetic rats( Reference Cani, Daubioul and Reusens 18 ). Interestingly, GLP-1 concentration and proglucagon mRNA expression level in the jejunum were significantly reduced in the FOS groups, and slightly reduced in the RMD groups (Fig. 5(A)). Similarly, a previous study has demonstrated that GLP-1 content in obese mice was decreased in the upper small intestine, but increased in the lower small intestine and colon, by chronic ingestion of voglibose, an α-glucosidase inhibitor( Reference Moritoh, Takeuchi and Hazama 29 ). The mechanism of the reduction in upper small-intestinal GLP-1 production is totally unknown. However, these changes appear to have a relatively small impact on plasma GLP-1 levels because the jejunum is not a major source of GLP-1 production.
In vitro experiments, using enteroendocrine GLUTag cells, suggest that RMD directly stimulates GLP-1 secretion in L cells in the intestine (Fig. 6). Because the same concentration of FOS failed to increase the secretion, these results cannot be attributed to the osmotic effects. Previous studies using GLUTag cells have demonstrated that potent GLP-1 secretion by glucose at 10 mm ( Reference Reimann, Williams and da Silva Xavier 9 , Reference Parker, Adriaenssens and Rogers 30 – Reference Hira, Muramatsu and Okuno 32 ), and the luminal concentration of RMD, can be estimated at about 12·5–25 mm (25–50 mg/ml), when rats consumed a 5–10 g diet, after fasting( Reference Chen, Hira and Nakajima 33 ). Therefore, the concentration used in the present study can also be applied in the intestinal lumen. The direct effects of dietary fibres on GLP-1 secretion have not been demonstrated previously. RMD may be the first fibre having such a direct effect on GLP-1 secretion. RMD is composed of glucose polymers having not only 1–4 and 1–6 linkages, but also 1–2 and 1–3 linkages with a branching structure. FOS is mainly composed of 1-kestose (glucosyl-fructosyl-fructose), nystose (glucosyl-fructosyl-fructosyl-fructose) and fructofuranosylnystose (glucosyl-fructosyl-fructosyl-fructosyl-fructose), all of which have a linear structure. As glucose, but not sucrose, is well known to stimulate directly GLP-1 secretion in L cells( Reference Kokrashvili, Mosinger and Margolskee 34 , Reference Shirazi-Beechey, Daly and Al-Rammahi 35 ), glucose-related or branching structure in RMD might be recognised by cells via pattern recognition receptor(s) such as Toll-like receptor(s), or orphan G protein-coupled receptor(s), that trigger GLP-1 secretion. For example, although FOS was not effective in GLUTag cells in the present study, a recent study has demonstrated that Toll-like receptor 2 is activated by β2 → 1-fructans in the intestinal epithelium( Reference Vogt, Meyer and Pullens 36 ). Because RMD does not provide any taste, the sweet taste receptor (T1R2/T1R3) is unlikely to be involved in the stimulatory effect of RMD on GLP-1 secretion( Reference Kokrashvili, Mosinger and Margolskee 34 ).
In the single oral administration experiment, RMD gradually increased plasma GLP-1 levels throughout the experimental period (Fig. 7). Because RMD is resistant to luminal digestion, it should be gradually accumulated in the lower intestine after single oral administration. Therefore, GLP-1 secretion was likely to be caused by the direct action of RMD, gradually concentrated in the middle–distal small intestine. Further studies are needed to elucidate the molecular mechanisms by which RMD stimulates GLP-1 secretion. The dietary protein hydrolysate ZeinH induced biphasic GLP-1 secretion, probably reflecting indirect and direct stimulation of GLP-1-producing cells( Reference Hira, Mochida and Miyashita 27 , Reference Rocca and Brubaker 37 ). The first phase was induced conceivably by indirect stimulation, triggered in the upper small intestine via the vagal pathway, and the second by a direct action in the lower small intestine( Reference Hira, Mochida and Miyashita 27 ). Zein protein is an ethanol-soluble prolamin fraction of maize protein, and well known as a low-digestible protein( Reference Sarwar 38 ). ZeinH was prepared in our previous study from zein protein by in vitro papain digestion, and further in vitro digestion by pancreatin, which did not abolish its GLP-1-releasing effect ( Reference Higuchi, Hira and Yamada 7 ). This supports the notion that the second phase of GLP-1 secretion was caused by the direct action of ZeinH-derived peptide fragments that remained and accumulated in the lower small intestine. In addition, the differential response of GLP-1 secretion to ZeinH and RMD raises the interesting possibility that the induction of GLP-1 secretion in the upper small intestine is sensitive to dietary peptides, but not to RMD. The direct and acute effect of RMD on GLP-1 secretion, as shown in Figs. 6 and 7, could have enhanced the daily postprandial GLP-1 secretory response in rats fed the RMD-supplemented diet, compared with those fed the control or FOS-supplemented diet.
Both the RMD and FOS diets lowered luminal pH in the caecum, suggesting the enhancement of gut fermentation by continuous feeding of these fibres. This observation is consistent with previous reports, and a recent review has concluded that the gut fermentation of non-digestible carbohydrates is associated with the secretion of GLP-1( Reference Everard and Cani 39 ). SCFA produced by the gut microbiota stimulate GLP-1 secretion and production( Reference Tolhurst, Heffron and Lam 22 , Reference Nøhr, Pedersen and Gille 40 ). The decreased pH of the caecal content in the RMD and FOS groups suggests that SCFA production is increased by enhanced gut fermentation( Reference Miyazato, Nakagawa and Kishimoto 15 , Reference Cani, Hoste and Guiot 25 , Reference Matsukawa, Matsumoto and Shinoki 41 , Reference Campbell, Fahey and Wolf 42 ). Thus, SCFA production was likely to be increased in the caecum by RMD and FOS, which could stimulate GLP-1 production and secretion. Previous reports have demonstrated the promotive effect of non-digestible saccharides (e.g. inulin, FOS and resistant starch) on luminal fermentation, and GLP-1 production in animal and human studies( Reference Everard and Cani 39 , Reference Mansour, Hosseini and Larijani 43 ). In animal studies, these saccharides have been supplemented in the diet at levels of 9–30 %, which are much higher than those in the present study (2·5–5 %). It is expected that dietary RMD exerts promotive effects on GLP-1 and glycaemia at a relatively lower dose compared with other non-digestible saccharides, with less risk of causing diarrhoea( Reference Kishimoto, Kanahori and Sakano 44 ). Further studies are necessary to investigate the effect of RMD on GLP-1 secretion/production in obese and diabetic models as well as in human subjects. Thus, the direct and indirect (through increased gut microbial fermentation) effects of RMD on both GLP-1 secretion and production possibly improve glucose tolerance (Fig. 2) through multiple action of GLP-1( Reference Kim and Schuppan 2 ).
In summary, rats fed a normal diet containing 5 % RMD for 6 weeks showed elevated fasting GLP-1 levels, and a suppressed glycaemic response as determined by the IPGTT. Fasting GLP-1 levels in the portal vein were also elevated, and the insulin level was reduced in the 5 % RMD group. Enhanced gut fermentation was suggested by the reduction of luminal pH in the caecum of rats treated with RMD and FOS, but a significant increase in GLP-1 content was observed in the RMD group only. Furthermore, RMD directly stimulated GLP-1 secretion in the enteroendocrine cell model, and single oral administration of RMD induced GLP-1 secretion in conscious rats. These results demonstrate that stimulatory effects of RMD, a soluble dietary fibre, on GLP-1 secretion and production are caused by its indirect (enhanced gut fermentation) and direct effects on GLP-1-producing cells. Ingestion of RMD can be a novel strategy to increase endogenous GLP-1, which probably contributes to the prevention of glucose intolerance.
Acknowledgements
The present study was supported by JSPS KAKENHI (grant no. 22380070) and Matsutani Chemical Industry.
The authors' contributions are as follows: T. H., A. I., Y. K. and H. H. designed the research; T. H. and A. I. conducted the research and analysed the data; T. H., Y. K., S. K. and H. H. wrote the paper; T. H. had primary responsibility for the final content. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest, although Y. K. and S. K. are employees of Matsutani Chemical Industry.












