A high fruit and vegetable (FV) intake has been associated with a number of health benefits, including reduced risk for CVD and different types of cancer( Reference Liu 1 , Reference Boeing, Bechthold and Bub 2 ). Importantly, several observational studies have demonstrated that a higher fruit intake in childhood and adolescence might already be related to reduced cancer risk later in life( Reference Potischman, Weiss and Swanson 3 , Reference Maynard, Gunnell and Emmett 4 ). However, more recent data do not fully support the evidence for inverse FV–cancer relationships, which may to some extent be attributable to imprecise exposure assessment( Reference Key 5 , Reference Aune, Chan and Vieira 6 ). Another possible reason for this discrepancy may be that health benefits arise mainly from one component of FV, which is unevenly distributed across different FV subgroups. Polyphenols may represent this relevant component, as certain fruit polyphenols have been demonstrated to restrict cancer growth in vitro and in vivo ( Reference Noratto, Porter and Byrne 7 , Reference Aiyer, Warri and Woode 8 ).
Considering the long latency period between lifestyle (e.g. dietary) exposures and cancer diagnosis, intermediate markers related to cancer risk are particularly valuable in long-term observational studies. Components of the growth hormone (GH) insulin-like growth factor (IGF) axis, a major regulator of human growth, may represent such intermediate markers( Reference Juul 9 , Reference Jogie-Brahim, Feldman and Oh 10 ). Specifically, whereas higher IGF-1 levels seem to be associated with an increased cancer risk( Reference Juul 9 ), the antiproliferative and pro-apoptotic actions of its major binding protein IGFBP-3 suggest that higher IGFBP-3 levels might be related to lower cancer risk( Reference Jogie-Brahim, Feldman and Oh 10 ). The GH–IGF axis is also susceptible to nutritional influences( Reference Juul 9 ), and we and others have shown that diet during critical periods of early life may relevantly influence the GH–IGF axis in the longer term( Reference Ben-Shlomo, Holly and McCarthy 11 , Reference Joslowski, Remer and Assmann 12 ), thereby possibly explaining associations between childhood diet and later cancer risk. Thus far, epidemiological evidence for the possible longer-term relevance of dietary polyphenols, or their major subgroup flavonoids, for the GH–IGF axis is missing. Several in vitro ( Reference Koyama, Cobb and Mehta 13 , Reference Vijayababu, Arunkumar and Kanagaraj 14 ) and animal studies( Reference Akhtar, Meeran and Katiyar 15 , Reference Harper, Cook and Patel 16 ) have, however, demonstrated that administration of (fruit) polyphenols can reduce IGF-1 and/or elevate IGFBP-3 with a concurrent inhibition of tumour growth. Thus, higher IGFBP-3 and lower IGF-1 levels, attributable to a higher dietary polyphenol intake (from fruit and vegetables), may represent a plausible mechanism linking higher FV consumption to lower cancer risk. The GH–IGF axis is also closely linked to the metabolism of insulin, with higher IGFBP-2 concentrations potentially reflecting better long-term insulin sensitivity at lower insulin levels( Reference Arafat, Weickert and Frystyk 17 ). Higher flavonoid consumption from FV may improve insulin sensitivity( Reference Babu, Liu and Gilbert 18 ), thereby decreasing insulin levels, which could in turn contribute to decreased cancer risk( Reference Gallagher and LeRoith 19 ). The cancer-protective role of FV may therefore also be explained by their influence on the regulation of insulin and IGFBP-2, but further research is needed.
Thus, the aim of the present study was to investigate whether FV intake, fruit intake or dietary flavonoid intake from FV (FlavFV) during three distinct periods of growth (i.e. early life, adiposity rebound in mid-childhood and adolescence) is related to the GH–IGF axis in young adulthood in a general healthy population. To investigate these relationships in depth, exposure assessment was based on both the dietary intake data and the urinary biomarker hippuric acid (HA).
Methods
Study population
Data for the prospective analysis of dietary influences on the GH–IGF axis came from the Dortmund Nutritional and Anthropometric Longitudinally Designed (DONALD) Study, an open-cohort study that was initiated at the Research Institute of Child Nutrition in Dortmund, Germany, in 1985. The DONALD Study investigates relationships between diet, metabolism, growth and development from infancy until adulthood( Reference Kroke, Manz and Kersting 20 ). To this end, thirty-five to forty infants are newly recruited every year and are first examined at the age of 3 months. Three further visits are scheduled in the 1st and two visits in the 2nd year of life. Afterwards, annual assessments take place that generally include 3-d weighed dietary records, medical and anthropometric examinations as well as interviews on lifestyle. Beginning at the age of 3−4 years, 24-h urine samples are usually collected in parallel with the dietary records. In addition, since 2005, adult participants are invited for subsequent examinations including a fasting blood withdrawal. The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of the University of Bonn (Germany). All assessments were performed with parental and, later on, with the children’s written consent.
Thus far, >1500 children have participated in the DONALD Study, but the ages of the children initially recruited were quite variable, resulting in the fact that information on the first few years of life is not always available. Moreover, many participants have not yet reached adult age. The present analysis was based on a sample of 382 DONALD participants who were term (36–43-week gestation) singletons with a birth weight ≥2500 g and had provided a fasting blood sample for measurements of IGF-1, IGFBP-2 and IGFBP-3 between 18 and 39 years of age. Analyses in relation to FV intakes and flavonoid intakes estimated from dietary records were based on those who had additionally provided at least two plausible 3-d weighed dietary records to describe habitual diet in the age range of interest (i.e. early life: 0·5–2 years; approximate age of adiposity rebound: 3–7 years; adolescence: girls, 9–15 years; boys, 10–16 years). Dietary records were considered implausible if the ratio of reported total energy intake:predicted BMR was below age- and sex-specific cut-off values( Reference Sichert-Hellert, Kersting and Schoch 21 ). Participants who had consistently under-reported energy intake (i.e. all dietary records implausible or more implausible than plausible records) were not considered for the present analysis (n 15 in the adolescent data set). Furthermore, participants had to provide information on relevant covariates such as early life and socio-economic factors. Applying these criteria, study populations for the dietary analyses consisted of 191, 265 and 261 participants for prediction from the time frames of early life, adiposity rebound and adolescence, respectively.
Regarding the period of adolescence, additional analyses were based on the urinary biomarker HA. To address this aim, participants had to provide ≥3 complete 24-h urine samples in adolescence, resulting in a study population of 236 participants. Urine samples were considered as complete if body weight-related 24-h creatinine excretion was ≥0·1 mmol/kg( Reference Remer, Neubert and Maser-Gluth 22 ).
Preliminary power calculations indicated that available sample sizes were sufficient to detect correlations of 0·17–0·21 between dietary intakes and adult outcomes with a power of >80 %.
Dietary intake
Dietary intake was assessed using 3-d weighed dietary records. During 3 consecutive days, all foods and beverages consumed (including leftovers) are weighed and recorded to the nearest 1 g with the help of electronic food scales (initially Soehnle Digita 8000; Leifheit; now WEDO digi 2000; Werner Dorsch). If weighing is not possible, semi-quantitative recording (e.g. number of spoons) is allowed. For the present analysis, intakes of foods and nutrients were calculated as the individual means of the 3 recording days using our continuously updated in-house nutrient database LEBTAB (LEBensmittel TABelle)( Reference Sichert-Hellert, Kersting and Chada 23 ), which is based on German standard food tables and data obtained from commercial food products. The food group characterising general FV intake consisted of fruits and vegetables (including fresh, frozen and canned products) as well as fruit and vegetable juices, and is referred to as FV. Intake was calculated as the sum of (unprocessed) separately ingested fruits, vegetables or juices and ingredients of processed or prepared foods.
To estimate flavonoid consumption from FV, all composite foods were broken down to the ingredients and flavonoid assignment was performed on the recipe level. Aggregated values for flavonoids( 24 ), proanthocyanidins( 25 ) and isoflavones( 26 ) were taken from databases of the US Department of Agriculture (USDA). For all fruits, vegetables and juices consumed by DONALD participants included in the present analyses, available values from these three databases were assigned. If a consumed food item was not available in the USDA databases, a value for a similar food item (or the mean value of several similar food items) was assigned. In these cases, criteria for similarity included the botanical family as well as appearance (e.g. colour, size, texture). If data for a food item were only available in a different preparation (e.g. values for cooked pears or pear juice were needed, but the USDA database contains only values for raw pears), the value for the raw item was multiplied with published retention and/or yield factors( Reference Bhagwat, Haytowitz and Wasswa-Kintu 27 ). Flavonoid intake in the present study was calculated as the sum of flavonoids, isoflavones and proanthocyanidins reported in the three USDA databases. However, as proanthocyanidin monomers in the proanthocyanidin database( 25 ) and flavan-3-ols in the flavonoid database( 24 ) indicate the same compounds, the values for proanthocyanidin monomers were not considered to avoid data duplicity, as has been described previously( Reference Zamora-Ros, Andres-Lacueva and Lamuela-Raventos 28 ).
Laboratory measurements
Annual 24-h urine collections are usually performed at the 3rd day of the 3-d dietary records. During the 24-h collection period, all micturitions are immediately (i.e. at home) stored frozen ≤−12°C in Extran-cleaned (Extran MA03; Merck), preservative-free 1 litre plastic containers before being transferred to the Research Institute where they are further stored at ≤−20°C until analysed. After thawing and stirring, urine volume is documented and creatinine excretion is determined by the kinetic Jaffé’s method on a creatinine analyser (Beckman-2; Beckman Instruments) in all urine samples. Urea was analysed photometrically using the Urease-Berthelot method (Randox Laboratories Ltd). Urinary HA, used as a biomarker for polyphenol consumption from fruits and vegetables( Reference Frank, Netzel and Kammerer 29 , Reference Krupp, Doberstein and Shi 30 ), was quantified colourimetrically according to Tomokuni & Ogata( Reference Tomokuni and Ogata 31 ) with minor modifications( Reference Krupp, Doberstein and Shi 30 ). HA excretion has been reported to increase markedly after the ingestion of foods rich in flavonoids and phenolic acids( Reference Frank, Netzel and Kammerer 29 , Reference Rechner, Kuhnle and Bremner 32 ); moreover, although HA excretion may also be influenced by dietary intakes of protein and the food preservative benzoic acid as well as by the individual intestinal microbiota( Reference Krupp, Doberstein and Shi 30 ), robust correlations with calculated FlavFV were observed in the DONALD cohort( Reference Bring, Penczynski and Krupp 33 ).
Venous blood samples were drawn after an overnight fast and were separated by centrifugation at 4°C for 15 min. Blood samples were stored frozen at −80°C until shipped to the Laboratory for Translational Hormone Analytics in Paediatric Endocrinology at the University of Giessen, where IGF-1 and IGFBP-3 were analysed by RIA( Reference Blum, Ranke and Bierich 34 ) and IGFBP-2 was determined using an ELISA.
Anthropometric measurements and calculations
Anthropometric measurements were performed during each visit at the study centre according to standard procedures by trained nurses undergoing annual quality control. For determination of height and weight, participants were dressed in underwear only with no shoes. Until 2 years of age, recumbent length was measured using a Harpenden stadiometer (Holtain Ltd). For children older than 2 years, standing height was determined to the nearest 0·1 cm with a digital telescopic wall-mounted stadiometer (Harpenden; Holtain Ltd). Body weight was measured to the nearest 0·1 kg with the help of an electronic scale (Seca 753E; Seca Weighing and Measuring Systems). Skinfold thickness was measured at four different sites (biceps, triceps, suprailiacal, subscapular) at the right side of the body to the nearest 0·1 mm using a Holtain caliper (Holtain Ltd).
Body surface area (BSA) in m2 was calculated according to the formula of DuBois & DuBois( Reference DuBois and DuBois 35 ): 0·007184×height (cm)0·725×weight (kg)0·425. Body weight and height were also used to calculate participants’ BMI (kg/m2), and age- and sex-independent standard deviation scores for BMI were determined according to German reference curves( Reference Neuhauser, Schienkiewitz and Schaffrath Rosario 36 ). Calculation of body fat percentage (BF%) during early life and adiposity rebound was based on all four skinfolds( Reference Deurenberg, Pieters and Hautvast 37 ), whereas pubertal BF% was estimated using the equations of Slaughter et al.( Reference Slaughter, Lohman and Boileau 38 ) for pubescent children, which consider triceps and subscapular skinfolds. Fat mass index (FMI, kg/m2) was then calculated using the following equation: FMI=weight×BF%/height2.
Parental characteristics and additional information
On admission of their children to the DONALD Study, parents were interviewed about familial characteristics (e.g. educational status, number of smokers in the household), and anthropometric measurements were performed with the same equipment as used for the children. Information on the child’s birth characteristics was abstracted from the ‘Mutterpass’, a standardised document given to all pregnant women in Germany. The duration of full breast-feeding (i.e. no solid foods or liquids except breast milk, tea or water) was enquired during the first visit until complementary feeding was initiated.
Statistical analyses
SAS procedures (version 9.2; SAS Institute) were used for all analyses and a P value<0·05 was considered significant in all statistical tests.
For prospective analyses of potential relationships between dietary intakes of FV, fruits, FlavFV and HA excretion and IGF-1, IGFBP-2 and IGFBP-3, multiple linear regression models were used. Nutritional variables were energy adjusted using the residual method( Reference Hu, Stampfer and Rimm 39 ) and standardised by age group and sex (mean=0 (sd 1)) to account for age-dependent changes in intake. Similarly, because no information on adolescent energy intake in the urinary data set was available and because of the close correlation between BSA and energy intake (r 0·58 in our adolescent data set with dietary data), urinary HA was calculated as residuals on individual BSA and standardised by age group and sex. Dietary and urinary predictors were included in the regression analyses as individual arithmetic means of the repeated measurements during the respective time frames (i.e. early life, adiposity rebound and adolescence) to provide estimates on habitual intake or excretion levels. To achieve normal distribution, all outcome variables except for IGF-1 were log transformed before analyses. Initial models (model A in Tables 2–5) included the respective dietary or urinary predictor, sex, age at outcome assessment and a dummy variable for year of blood measurement. This dummy variable was assigned because measurements of the GH–IGF axis were conducted in two separate series (2011 and 2014). Interaction analyses in these initial models indicated no differences in the predictor–outcome relationships between males and females.
For adjusted models (model B in Tables 2–5), the following covariates were additionally considered as potential confounders: gestational age, birth weight, full breast-feeding (>2 weeks, yes/no), maternal overweight (BMI≥25 kg/m2, yes/no), high maternal educational status (≥12 years of schooling, yes/no), any smokers in the household (yes/no) and BMI or FMI at baseline (i.e. the first measurement in the respective time window). Dietary intakes of energy, protein (animal), SFA and fibre from sources other than FV were additionally considered in models with dietary predictors. Urine volume, 24-h creatinine excretion and 24-h urea excretion (as a biomarker of protein intake( Reference Bihuniak, Simpson and Sullivan 40 )) were tested in the models with the predictor 24-h HA excretion. Covariates were only included in the final models if they modified the regression coefficient of the main predictor by ≥10 %. Adjusted means (i.e. the least square means of IGF-1, IGFBP-2 and IGFBP-3 predicted by the model when the other variables were held constant) are presented with their 95 % CI by tertiles of the respective predictors in Tables 2–5. For reasons of comparability, the same adjustment was used for all dietary predictors within the same period for a given outcome. This adjustment was usually derived from the regression analyses with the predictor FV.
Results
Socio-economic, dietary (or urinary) and anthropometric characteristics of the study samples available for early life, adiposity rebound and adolescence (dietary and urinary data set) are presented in Table 1, together with information on relevant early life factors and characteristics in young adulthood. Participants obtained a higher percentage of energy from fat and SFA in early life compared with mid-childhood and adolescence, whereas percentage energy consumption from carbohydrates was highest in adolescence. Although total energy intake was more than twice as high in the pubertal sample compared with the early life sample, absolute consumption of FV and FlavFV differed less markedly with age, with 69 % higher FV intake and 83 % higher median FlavFV in adolescence compared with early life. Absolute median fruit intake was almost constant across the age groups. In those subjects providing dietary intake data in all three growth periods (n 150), FV intake and fruit intake correlated moderately between the different age ranges (r 0·34–0·55, data not shown).
Table 1 Characteristics of the study samples during early life (0·5–2 years), adiposity rebound (3–7 years) and adolescence (boys: 10–16 years, girls: 9–15 years) (Dortmund Nutritional and Anthropometric Longitudinally Designed Study, Germany)(Medians and interquartile ranges (IQR); frequencies and percentages)

FV, fruits and vegetables including juices; FlavFV, flavonoid intake from FV; SDS, standard deviation score.
* Defined as full breast-feeding >2 weeks.
† BMI≥25 kg/m2.
‡ Dietary, urinary and anthropometric data are presented as the arithmetic means of ≥2 repeated measurements (or ≥3 repeated measurements for urinary data) for each individual in the respective time frame.
§ SDS derived from German reference values for children and adolescents( Reference Neuhauser, Schienkiewitz and Schaffrath Rosario 36 ).
During early life, adjusted linear regression models (Table 2, model B) revealed no associations of FV, fruits or FlavFV with IGF-1 or IGFBP-3, but higher intakes of FV in this age group tended to be related to higher IGFBP-2 concentrations in young adulthood (P=0·07).
Table 2 Prospective associations of fruits and vegetables including juices (FV), fruit and flavonoid intake from FV (FlavFV) during early life (0·5−2 years) and insulin-like growth factor (IGF-1) and its binding proteins (IGFBP-2 and IGFBP-3) in young adulthoodFootnote *(Mean values and 95 % confidence intervals; medians and interquartile ranges (IQR); n 191)

T, tertile.
* Dietary predictors were included in the models as residuals on energy intake, standardised by age group and sex.
† Adjusted for sex, adult age and dummy variable for year of blood measurement.
‡ Model B for IGFBP-3: model A additionally adjusted for baseline BMI.
§ n 190 for IGFBP-2.|| Model B for IGFBP-2: model A additionally adjusted for intake of SFA.
¶ Model B for IGF-1: model A additionally adjusted for intakes of SFA and fibre from other sources than FV, for gestational age, high maternal education and full breast-feeding.
In models adjusted for dietary and early life factors (Table 3, model B), higher FV as well as fruit consumption around adiposity rebound were significantly related to higher adult IGFBP-2 levels (P=0·045 and P=0·03, respectively), whereas no similar associations were observed for FlavFV. However, a higher FlavFV was in trend (P=0·08) related to higher IGFBP-3 in young adulthood in the adjusted model (Table 3, model B). Moreover, FlavFV showed a significant inverse association with the IGF-1:IGFBP-3 ratio (P=0·04 in the adjusted model; data not shown), thought to reflect (biologically active) free IGF-1 concentrations( Reference McGreevy, Hoel and Lipsitz 41 ). Nevertheless, also for the age range around adiposity rebound, none of the investigated dietary predictors was associated with IGF-1.
Table 3 Prospective associations of fruits and vegetables including juices (FV), fruit and flavonoid intake from FV (FlavFV) during adiposity rebound (3–7 years) and insulin-like growth factor (IGF-1) and its binding proteins (IGFBP-2 and IGFBP-3) in young adulthoodFootnote *(Mean values and 95 % confidence intervals; medians and interquartile ranges (IQR); n 265)

T, tertile.
* Dietary predictors were included in the models as residuals on energy intake, standardised by age group and sex.
† Adjusted for sex, adult age and dummy variable for year of blood measurement.
‡ Model B for IGFBP-3: model A additionally adjusted for standardised energy intake, intake of SFA and maternal overweight (BMI≥25 kg/m2, yes/no).
§ n 264 for IGFBP-2.
|| Model B for IGFBP-2: model A additionally adjusted for intake of SFA and full breast-feeding.
¶ Model B for IGF-1: model A additionally adjusted for intake of SFA, birth weight and full breast-feeding.
With respect to dietary intakes during adolescence, a trend for an association with higher adult IGFBP-2 was observed for higher FV intake (P=0·09) and a significant association for higher fruit intake (P=0·045) (Table 4, model B). Again, no associations were observed between FlavFV and IGFBP-2 as well as between any of the dietary predictors and adult IGF-1 or IGFBP-3. For the biomarker 24-h HA excretion in adolescence (Table 5), a trend (P=0·056) for a direct association was detected for adult IGFBP-2, but not for IGFBP-3 or IGF-1. Sensitivity analyses in a subgroup providing at least two dietary records with parallelly collected 24-h urine samples during adolescence (n 224) indicated that HA-IGFBP-2 associations remained stable after adjustment for intakes of energy, protein (animal), SFA or fibre from other sources than FV (data not shown).
Table 4 Prospective associations of fruits and vegetables including juices (FV), fruit and flavonoid intake from FV (FlavFV) during adolescence (boys: 10–16 years, girls: 9–15 years) and insulin-like growth factor (IGF-1) and its binding proteins (IGFBP-2 and IGFBP-3) in young adulthoodFootnote *(Mean values and 95 % confidence intervals; medians and interquartile ranges (IQR); n 261)

T, tertile.
* Dietary predictors were included in the models as residuals on energy intake, standardised by age group and sex.
† Adjusted for sex, adult age and dummy variable for year of blood measurement.
‡ Model B for IGFBP-3: model A additionally adjusted for intakes of SFA and protein, for birth weight and maternal overweight (BMI≥25 kg/m2, yes/no).
§ n 260 for IGFBP-2.
|| Model B for IGFBP-2: model A additionally adjusted for intake of SFA, high maternal educational status and maternal overweight (BMI≥25 kg/m2, yes/no).
¶ Model B for IGF-1: model A additionally adjusted for intake of SFA.
Table 5 Associations of 24-h hippuric acid excretion during adolescence (boys: 10–16 years, girls: 9–15 years) and insulin-like growth factor (IGF-1) and its binding proteins (IGFBP-2 and IGFBP-3) in young adulthoodFootnote *(Mean values and 95 % confidence intervals; medians and interquartile ranges (IQR); n 236)
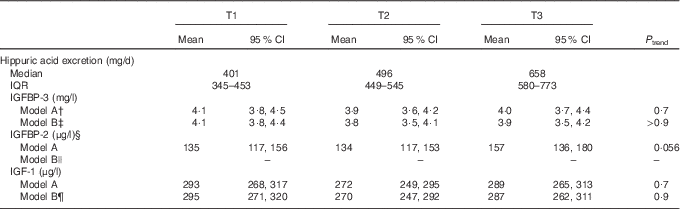
T, tertile.
* Hippuric acid excretion was included in the models as residuals on individual body surface area, standardised by age group and sex.
† Adjusted for sex, adult age and dummy variable for year of blood measurement.
‡ Model B for IGFBP-3: model A additionally adjusted for 24-h urine volume, 24-h urea excretion, maternal overweight (BMI≥25 kg/m2, yes/no) and smokers in the household (yes/no).
§ n 235 for IGFBP-2.
|| Model B for IGFBP-2: see model A (no additional confounders identified).
¶ Model B for IGF-1: model A additionally adjusted for baseline fat mass index, 24-h urine volume, 24-h creatinine excretion and gestational age.
Repeating the analyses using FV intake without juices as a predictor yielded results that were very similar to those reported in Tables 2–4 (FV intake including juices). Moreover, additional adjustment for vegetable intake in the models with fruit intake as the predictor changed the results only marginally (data not shown).
To examine whether the observed associations between fruit or FV intake in childhood and adolescence with the adult GH–IGF axis are independent of adult intake levels, we repeated our analyses in smaller data sets (n 150 in early life, n 203 around adiposity rebound, n 196 in adolescence) of subjects who had also provided 3-d dietary records at the time of blood sampling (see online Supplementary Tables S1–S3). Although the prospective association between fruit intake during mid-childhood and adult IGFBP-2 levels was attenuated by adjustment for fruit intake in young adulthood, trends for higher IGFBP-2 levels associated with higher FV intakes in early life or mid-childhood were independent of adult intake levels. In the smaller adolescent sample (with consequently reduced statistical power), relations of FV or fruit intakes to adult IGFBP-2 levels were no longer discernable, regardless of adult intake levels.
As a lower dietary glycaemic index (GI), which has been related to higher fruit consumption( Reference Du, van der and van Bakel 42 ), might be one explanation for the observed relations between fruit or FV and an improved insulin sensitivity (as indicated by higher levels of IGFBP-2), we considered dietary GI as an additional covariate in our analyses. In the adjusted models including GI, the associations of fruit intake and FV intake around adiposity rebound with IGFBP-2 in young adulthood were attenuated (P=0·06 and P=0·1), whereas GI adjustment did not relevantly affect the fruit IGFBP-2 associations for the adolescent sample (data not shown).
Discussion
Our findings suggest that a habitually higher FV intake during critical periods of childhood and adolescence may be related to higher levels of IGFBP-2 in young, healthy adults. These were additionally supported by our analyses based on the urinary polyphenol biomarker HA during adolescence, but not by the results for estimated FlavFV. In contrast to our findings for IGFBP-2, we did not observe any associations of the investigated dietary (and urinary) predictors during growth with adult IGF-1 levels, and a direct relation with adult IGFBP-3 concentrations was only observed in trend for FlavFV in mid-childhood.
With respect to previous evidence on the relevance of FV intake for IGFBP-2 concentrations, a few observational studies have been conducted, but it has been reported that levels of IGFBP-2 (and IGFBP-1) were substantially higher in British women consuming a vegan diet compared with those eating meat or following a vegetarian diet( Reference Allen, Appleby and Davey 43 ). As the partial attenuation of FV–IGFBP-2 associations in our pathway analyses indicated, a lower dietary GI may be one relevant aspect of FV intake contributing to its beneficial effect on IGFBP-2. Alternatively, the high flavonoid content of FV could probably also explain their associations with IGFBP-2 and the extent to which it reflects insulin sensitivity, because a recent intervention study demonstrated that long-term flavonoid administration was able to reduce insulin resistance in post-menopausal diabetic women( Reference Curtis, Sampson and Potter 44 ).
Apart from these mechanistic considerations, it is possible that the FV–IGFBP-2 associations observed in our study reflect shorter-term influences of current intake rather than longer-term adaptations of the IGF axis to intake levels during growth. We thus performed sensitivity analyses adjusting for adult FV or fruit intake levels in young adulthood, which indeed suggest that the benefits associated with fruit intake in mid-childhood may be partly attributable to tracking of fruit intake into young adulthood. However, interpretation is hampered by the reduced power in the smaller subsamples available for these analyses. Yet, it is of interest that these sensitivity analyses did not refute the potential protective link between FV intake in early life or mid-childhood and adult IGFBP-2 levels.
In our analyses, we found a direct association between the polyphenol biomarker HA in adolescence and adult IGFBP-2 levels. However, these findings were not corroborated with respect to estimated flavonoid consumption from FV. These conflicting results may be partly due to methodological problems in flavonoid estimation. As has been previously stated, estimation of flavonoid intake from dietary protocols is difficult due to the great variation of flavonoid content in natural products, differing bioavailability of the ingested compounds and incomplete or missing information in food composition databases( Reference Linseisen and Rohrmann 45 , Reference Cassidy, O’Reilly and Kay 46 ). Although several prospective studies calculating flavonoid intakes from the USDA databases observed meaningful associations of these dietary compounds with hypertension incidence( Reference Cassidy, O’Reilly and Kay 46 ) as well as CVD mortality( Reference Mink, Scrafford and Barraj 47 ), our findings strengthen the importance of combining intake data with biomarker analyses in epidemiological studies on diet–disease relationships.
The elevated IGFBP-2 concentrations related to higher FV intake may have distinct implications for future cancer risk, because unlike IGFBP-2’s clearly favourable role in insulin metabolism( Reference Arafat, Weickert and Frystyk 17 ), conflicting results have been reported regarding the relevance of circulating IGFBP-2 levels for cancer. On the one hand, two previous studies found a reduced risk of colorectal cancer( Reference Kaaks, Toniolo and Akhmedkhanov 48 ) as well as post-menopausal breast cancer( Reference Krajcik, Borofsky and Massardo 49 ) in those individuals with higher IGFBP-2 concentrations, probably related to its inverse regulation by insulin and its role in restricting IGF-1 bioavailability. On the other hand, it has been reported that IGFBP-2 levels are frequently elevated in individuals with different types of cancer and that IGFBP-2 might be a marker of tumour differentiation( Reference Hoeflich, Reisinger and Lahm 50 ). These findings implicate that IGFBP-2 may exert a different role in cancer initiation compared with the already-established disease.
In contrast to the results for IGFBP-2 in our study populations, no consistent prospective associations were discernible between the investigated dietary predictors and IGF-1 or IGFBP-3. This is in contrast with in vitro and animal studies that have quite consistently reported up-regulation of IGFBP-3 and down-regulation of IGF-1 concurrently with diminished tumour growth upon administration of different plant polyphenols( Reference Koyama, Cobb and Mehta 13 – Reference Harper, Cook and Patel 16 , Reference Suh, Kim and Sung 51 ). The effects of polyphenol-rich extracts or single polyphenolic compounds administered in pharmacological doses in these studies may, however, not be transferable to polyphenol levels achievable with normal human diets.
To our knowledge, long-term associations between flavonoid intakes and the GH–IGF system have not been investigated in epidemiological studies, but cross-sectional studies in adults on (biomarkers of) FV, as a flavonoid-rich food group, have reported inconsistent results, with some studies supporting( Reference McGreevy, Hoel and Lipsitz 41 , Reference Gunnell, Oliver and Peters 52 ) and other studies opposing( Reference Suzuki, Ito and Hashimoto 53 ) the findings from the above-mentioned in vitro and animal data. Regarding available evidence during growth, a cross-sectional analysis in 521 7–8-year-old children found that IGFBP-3 levels were not associated with the intakes of fruits, vegetables or tomatoes, whereas higher IGF-1 concentrations were unexpectedly observed in those boys with the highest fruit intake( Reference Rogers, Gunnell and Emmett 54 ). In addition to the difficulties of interpreting these contradictory results from studies examining different age groups with different FV intake levels as well as varying dietary assessment tools, no causal relations can be deduced from cross-sectional data.
Another factor that may have contributed to the inconsistent results regarding dietary influences on circulating components of the GH–IGF axis is the ability of these blood levels to reflect the biologically active concentrations: in rats, oral administration of the flavonoid genistein and the stilbene resveratrol effectively reduced tumour growth and tissue expression of IGF-1, whereas serum levels of IGF-1 were unaffected by the treatment( Reference Harper, Cook and Patel 16 ). In addition, a human study reported that IGFBP-3 expression in the colonic mucosa, but not plasma IGFBP-3 levels, was lower in patients with colorectal adenomas compared with healthy controls( Reference Keku, Sandler and Simmons 55 ). These studies( Reference Harper, Cook and Patel 16 , Reference Keku, Sandler and Simmons 55 ) indicate that – at least in the short term – circulating levels of IGF-1 and its binding proteins may not reflect the relevant tissue levels. Furthermore, heterogeneous findings in epidemiological studies may arise from the assay used, as previous studies demonstrated that at least for IGF-1 and IGFBP-3 disease risk estimates can in great part depend on the method of measurement( Reference Muller, Wallaschofski and Brabant 56 , Reference Rinaldi, Kaaks and Zeleniuch-Jacquotte 57 ). In our study, the same in-house RIA were used for both measurement series (2011 and 2014) of IGF-1 and IGFBP-3, which preclude bias due to a change in methodology. Nevertheless, subtle changes between the measurement series could have contributed to dilution of possible influences of fruit, FV and FlavFV on these outcomes.
Our study has several additional limitations including the comparatively small sample sizes as well as the fact that only one blood sample for each individual could be used for measurements of the GH–IGF axis in young adulthood. Despite the fact that we used the mean of at least two dietary records, which were also checked for plausibility, to obtain stable estimates of usual dietary intake in each age range, we cannot exclude the possibility that the higher percentage of dietary records filled in by the adolescents themselves affects the comparability of the data between the age groups. However, previous analyses indicated that the number of autonomously recorded dietary protocols did not differ between adolescents with plausible and implausible records( Reference Sichert-Hellert, Kersting and Schoch 21 ). Flavonoid intake in our analyses was only calculated from the food groups of fruits, vegetables and juices. Thus, relevant flavonoid intake from other foods might have influenced our results. However, non-FV food groups reported to relevantly contribute to flavonoid intake in other population-based studies, such as tea, red wine and coffee( Reference Zamora-Ros, Andres-Lacueva and Lamuela-Raventos 28 , Reference Zamora-Ros, Forouhi and Sharp 58 ), were consumed in only minimal amounts by our DONALD children and adolescents. Moreover, additional adjustment for the consumption of coffee or tea as well as cocoa products yielded very similar results compared with those reported in Tables 2–4 (data not shown).
Strengths of our study include its prospective design covering different periods of childhood and adolescence to identify potentially vulnerable age ranges in which diet may influence the GH–IGF axis in the longer term. Furthermore, repeated detailed dietary data were available to reliably describe habitual diet in the time frames of interest. Although the DONALD cohort is characterised by a relatively high socio-economic status and extremes of dietary behaviour may not be represented( Reference Kroke, Manz and Kersting 20 ), FV intake in DONALD children seems comparable with data from other German representative paediatric populations( Reference Behrendt and Krawinkel 59 ). Finally, in our adolescent sample, comparative analyses could be performed using urinary HA excretion, a polyphenol biomarker that reflects potentially bioavailable polyphenols and does not share the same potential biases as dietary assessment( Reference Linseisen and Rohrmann 45 ). We were, however, not able to control for the influence of benzoic acid added as a food preservative, which also constitutes a potentially important precursor of urinary HA excretion( Reference Krupp, Doberstein and Shi 30 ).
To conclude, a higher fruit and FV intake during growth may beneficially affect adult insulin metabolism and restrict IGF-1 bioavailability as indicated by higher levels of IGFBP-2. As suggested by our biomarker analyses, these associations might be partly attributable to the high polyphenol contents of FV. Our study does not support a major relevance of fruit or FV for adult IGF-1 or IGFBP-3 concentrations, but methodological considerations hamper a definite conclusion on these biological plausible associations.
Acknowledgements
The Dortmund Nutritional and Anthropometric Longitudinally Designed Study is supported by the Ministry of Science and Research of North Rhine Westphalia, Germany. This analysis was funded by the Wereld Kanker Onderzoek Fonds (WCRF NL), as part of the WCRF international grant programme (grant no. 2013/975). The funders had no role in the design, analysis or writing of this article.
The authors’ contributions are as follows: D. K., T. R. and A. E. B. conceived the project. D. K. carried out the statistical analyses and drafted the manuscript. T. R. and A. E. B. contributed to the study design, the manuscript drafting and data interpretation. K. J. P. contributed to the flavonoid assignment procedure and data analyses. K. B. contributed to the statistical analyses and data interpretation. Measurements of IGF-1 and IGFBP were carried out in the laboratory of S. A. W. All authors critically revised the manuscript for important intellectual content.
The authors declare that there are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114515004742








