Iodine, a reactant in the synthesis of thyroid hormones, is an essential micronutrient absorbed mainly through food and water to meet the needs of the human body(Reference Zimmermann and Boelaert1). The physiologic role of iodine is reflected by the thyroid hormones, which regulate the metabolism of substances and energy and promote growth and development(Reference Lind, Langsteger and Molnar2). Thyroid peroxidase antibody (TPOAb) and thyroglobulin antibody (TGAb) are two major thyroid antibodies that serve as important markers of thyroid autoimmunity. Among thyroid antibodies, TPOAb is a stronger indicator of autoimmune thyroid diseases (AITD)(Reference Ragusa, Fallahi and Elia3,Reference Mocellin, Walterfang and Velakoulis4) . Previous studies have found that TGAb and TPOAb indicate two different aspects of thyroid autoimmunity: TGAb reflects the innate thyroid immune response, whereas TPOAb characterises a later adaptive immune response(Reference Caturegli, De Remigis and Rose5). The association between iodine intake and the presence of thyroid antibodies is complex, and iodine intakes both below and above the recommended level are associated with elevated thyroid antibodies(Reference Rayman6,Reference Pedersen, Knudsen and Jørgensen7) . Although several studies have examined thyroid antibodies, all have used random urinary iodine concentrations (UIC) to distinguish the iodine nutrition status of the populations. Previous studies have investigated the relationship between thyroid antibodies and water iodine in the natural environment, but the levels of water iodine concentration were just mild or moderate iodine deficiency. In this study, we extended the data on the effects of higher water iodine concentration on the thyroid antibodies.
China’s geographical distribution is complicated. On the one hand, most areas in China are iodine deficient, and the residents have been severely affected by iodine deficiency disorders. To eliminate iodine deficiency disorder, a universal salt iodisation (USI) strategy has been implemented. After 26 years of USI, we sought to assess what the thyroid antibody status of the residents in these areas might be. On the other hand, in iodine adequate (IA) areas in China, natural iodine meets the physiological needs of residents, and salt iodisation interventions have not been implemented. Few studies have examined thyroid antibodies in residents in these areas. Finally, a total of 40·65 million residents live in areas with iodine excess (IE), with a high median water iodine concentration in China(Reference Wang, Wan and Liu8,Reference Wan, Qu and Wu9) . Does long-term residence in IE areas have any effect on thyroid antibodies among residents? The main objective of the current study was to explore the TPOAb and TGAb in three areas with differing water iodine concentrations and to describe the distribution of TPOAb and TGAb by age and sex and the effects on thyroid function and volume. Moreover, we discuss the relationship between these two thyroid antibodies and the occurrence of thyroid diseases in the three areas.
Materials and methods
Survey areas and participants
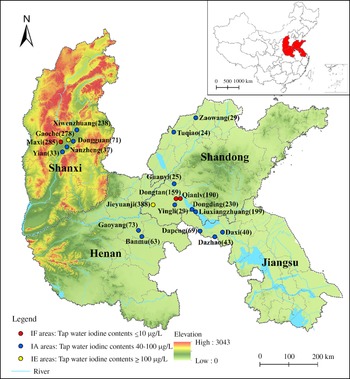
According to recent data from national iodine deficiency disorder surveillance and water iodine surveillance efforts in China(Reference Wang, Wan and Liu10), we chose twenty areas with different median water iodine in four provinces (Shandong, Shanxi, Jiangsu and Henan) to conduct an epidemiological study from April 2016 to June 2020. The classification was as follows: (1) Iodine fortification (IF) areas, with tap water iodine contents ≤ 10 μg/l and coverage rates of qualified iodised salt > 90 %; (2) IA areas, with tap water iodine contents 40–100 μg/l and non-iodised salt supplied; (3) IE areas, with tap water iodine contents ≥ 100 μg/l and non-iodised salt supplied. The information on the investigated areas is shown in Fig. 1. The inclusion criteria for participants were as follows: (1) age ≥ 18. (2) Residence in the selected areas for at least 5 years. (3) No pregnancy or lactation among women. (4) No iodine-containing medications and no history of radiation in the past year. On the basis of these criteria, we identified 2775 adults in twenty areas. The exclusion criteria were as follows: (1) Absence of basic information such as name, age or sex (n 77). (2) Absence of blood or urine samples (n 121). (3) Absence of thyroid ultrasonography measurement records (n 23). (4) Use of thyroid medicine, presence of congenital thyroid disease or a family history of thyroid disease (n 51). Finally, 2503 participants were included in this study. A standard questionnaire was designed to collect information on the demographic characteristics, including sex (male/female), name, age (≥ 18), BMI (height/weight2 (kg/m2)), smoking status (yes/no) and alcohol consumption (yes/no). The questionnaire was administered by trained research assistants in face-to-face interviews. The project was approved by the Ethics Review Committee of Harbin Medical University, and each participant provided signed informed consent before the investigation.

Fig. 1. Geographical distribution of twenty survey areas and the number of subjects in Shandong, Shanxi, Henan and Jiangsu provinces of China. MWI, median water iodine.
Laboratory testing and clinical diagnosis
In this study, the non-central water supply was sampled in IF areas (three villages). Five samples each were collected from east, west, north, south and central locations in each village. For IA (fifteen villages) and IE (two villages), the area’s central water supply was sampled, and two parallel samples were collected from each village. The median water iodine was determined with the As3+-Ce4+ catalytic spectrophotometry method. Certified reference material (GBW09113 and GBW09114) from the National Reference Laboratory for Iodine Deficiency Disorders (NRLIDD) in China was used to control measurement quality, and the target values were 9·5 ± 1·2 μg/l and 61 ± 6 μg/l. The inter-assay CV were 2·7 and 2·5 %, and the intra-assay CV were 2·3 and 2·7 %. Urine samples were collected in the morning and measured with the As3+-Ce4+ catalytic spectrophotometry method according to the China Health Standard Method (WS/T107·1-2016). Certified reference materials (GBW09108, GBW9109 and GBW9110) from the NRLIDD in China were used to control measurement quality, and the target values were 69·5 ± 9·0 μg/l, 134 ± 10 μg/l and 239 ± 15 μg/l. The intra-assay CV were 2·7, 1·4 and 2·3 % and the inter-assay CV were 2·3, 2·5 and 2·4 %. According to the WHO/United Nations International Children’s Emergency Fund (UNICEF)/Iodine Global Network (IGN), median UIC < 100 μg/l was defined as iodine deficiency; 100 μg/l ≤ median UIC ≤ 199·99 μg/l was defined as iodine adequacy; 200 μg/l ≤ median UIC ≤ 299·99 μg/l was defined as iodine above requirements and median UIC ≥ 300 μg/l was defined as iodine excessive. Venous blood was collected from the participants after they had fasted for 8 h. The levels of free triiodothyronine (FT3), free thyroxine (FT4), thyroid stimulating hormone (TSH), TPOAb and TGAb in serum were measured with chemiluminescent immunoassays (Siemens Healthcare Diagnostics Inc.). The sensitivity of assay were FT3 (0·30 pmol/l), FT4 (1·3 pmol/l), TSH (0·01 mIU/l), TPOAb (15 IU/ml) and TGAb (10 IU/ml). The intra-assay CV and inter-assay CV were FT3 (2·4 % and 2·89 %), FT4 (2·2 % and 1·6 %), TSH (2·4 % and 2·1 %), TPOAb (1·3 % and 2·8 %) and TGAb (2·9 % and 2·0 %). According to the test kit manufacturers, the reference ranges of thyroid function were as follows: 0·27 mIU/l < TSH < 4·20 mIU/l; 12 pmol/l < FT4 < 22 pmol/l; 2·8 pmol/l < FT3 < 7·1 pmol/l; thyroid antibodies were detected when they are above a certain level, TPOAb(−) and TGAb(−) were defined as TPOAb < 34 IU/ml and TGAb < 115 IU/ml; TPOAb(+) was defined as TPOAb ≥34 IU/ml; TGAb(+) was defined as TGAb ≥ 115 IU/ml; TPOAb(+) and TGAb(+) were defined as TPOAb ≥ 34 IU/ml and TGAb ≥ 115 IU/ml; TPOAb(+) or TGAb(+) was defined as TPOAb ≥ 34 IU/ml or TGAb ≥ 115 IU/ml. The levels of TPOAb and TGAb were classified on the basis of a previous study as follows(Reference Teng, Yang and Shi11): (1) TPOAb: level 1: TPOAb < 34 IU/ml; level 2:34 IU/ml ≤ TPOAb < 200 IU/ml; level 3:200 IU/ml ≤ TPOAb < 400 IU/ml; and level 4: TPOAb ≥ 400 IU/ml. (2) TGAb: level 1: TGAb < 115 IU/ml; level 2:115 IU/ml ≤ TGAb < 200 IU/ml; level 3:200 IU/ml ≤ TGAb < 400 IU/ml and level 4: TGAb ≥ 400 IU/ml. Thyroid ultrasonography was performed by experienced radiologists using portable 7·5 MHz ultrasound instruments. Thyroid volume (TV) was defined as length × width × thickness × 0·479(Reference Zhai, Zhang and Chen12). The normal reference values for TV were < 18 ml for women and < 25 ml for men.
Diagnostic criteria for thyroid diseases
AITD: pure TGAb, TPOAb positivity and double antibody positivity indicated a diagnosis of autoimmune thyroid disease(Reference Wan, Qu and Wu9); overt hypothyroidism: TSH > 4·20 mIU/l and FT4 < 12·0 pmol/l; subclinical hypothyroidism: TSH > 4·20 mIU/l and FT4 within the reference range; overt hyperthyroidism: TSH < 0·27 mIU/l FT4 > 22 pmol/l and FT3 > 7·1 pmol/l; subclinical hyperthyroidism: TSH < 0·27 mIU/l, and FT3 and FT4 within the reference range; hypothyroxinaemia: FT4 < 12·0 pmol/l and TSH within the reference range; and goitre: TV > 25 ml (men) and > 18 ml (women).
Statistical analysis
We used Microsoft Office Excel 2016 to record data and SPSS 23·0 software for statistical analysis. The Kolmogorov–Smirnov method was used to test for the normal distribution of the data. The data following a normal distribution are represented by the mean ± standard deviation (mean ± sd), and the differences in means were compared with one-way ANOVA. If a difference was found between groups, the Least-Significant Difference (LSD-t) test was performed accordingly. Non-normally distributed data are described with the median and 25th and 75th percentiles. Comparisons of data among different groups were performed with the Kruskal–Wallis H test. The chi-square (χ2) test was used to compare the rates between groups with categorical outcomes. Bonferroni correction was used for pairwise comparisons. To evaluate the trends in the prevalence of thyroid antibodies with increasing age, we used the SPSS module for trend analysis (P for trend). Binary logistic regression models were used to assess the association between thyroid antibody levels and subclinical hypothyroidism. The results are reported as adjusted OR with 95 % CI. P < 0·05 was considered statistically significant.
Results
Description of the participants
As shown in Table 1 compared with those in IA and IF areas, the levels of UIC, TSH and FT4 were significantly higher in IE areas. In IA areas, the UIC values were significantly higher than that in IF areas (all adjusted P < 0·050) (P was adjusted for the Bonferroni correction). In IF areas, the level of FT3 was significantly higher than those in IA and IE areas (both adjusted P < 0·050). The TV in IF and IE areas was significantly higher than that in IA areas (P < 0·010). We observed significant differences in the prevalence of AITD and subclinical hypothyroidism among the three areas (both P < 0·001).
Table 1. Demographic characteristics and the prevalence of thyroid diseases among three areas
(Numbers and percentages; median and percentiles; mean values and standard deviations)

IF, Iodine fortification areas; IA, iodine adequate areas; IE, iodine excess areas; UIC, urinary iodine concentration; MWI, median water iodine; TSH, thyroid stimulating hormone; FT3, free triiodothyronine; FT4, free thyroxine; TV, thyroid volume.
Age, BMI and TV are represented by mean ± sd; UIC, median water iodine, TSH, FT3 and FT4, are represented by the median and 25th and 75th percentiles; The prevalence of thyroid diseases is represented by n (%).
* Significant differences between IF and IA areas.
† Significant differences between IE and IA areas.
‡ Significant differences between IE and IF areas.
Thyroid antibody status in three areas
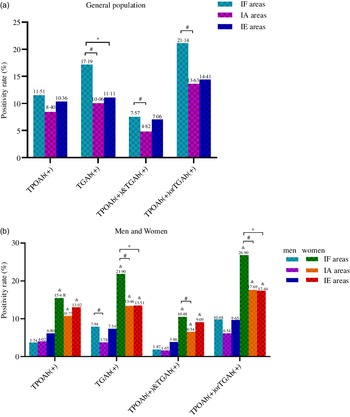
As shown in Fig. 2(a), in the general population, the positivity rate of TGAb(+), TPOAb(+) and TGAb(+) and TPOAb(+) or TGAb(+) in IF areas was significantly higher than those in IA areas (all P < 0·001) and TGAb(+) in IF areas was higher than that in IE areas. In Fig. 2(b), all thyroid antibody groups were significantly higher in women than in men among the three areas (all P < 0·001). The positivity rates of TGAb(+) and TPOAb(+) or TGAb(+) in women from IF areas were significantly higher than those in IA and IE areas (all P < 0·001). In IF areas, the prevalence of TGAb(+) in men and TPOAb(+) and TGAb(+) in women were significantly higher than that in IA areas (both P < 0·010). As shown in Fig. 3, in IF and IE areas, the positivity rate of TPOAb(+), TGAb(+) and TPOAb(+) or TGAb(+) significantly increased with age (all P for trend < 0·05). In Fig. 3(a), in IA areas, the positivity rate of TPOAb(+) significantly increased with age until the age of 50–59 years and decreased from the 50–59 group to the ≥ 60 group (P for trend = 0·035).

Fig. 2. The positivity rate of thyroid antibodies among three areas. (a) Thyroid antibodies in general population; (b) Thyroid antibodies in men and women. # Indicates significant differences between IF and IA areas; * Indicates significant differences between IF and IE areas; & Indicates significant differences between men and women. IF, iodine fortification; IA, iodine adequate; IE, iodine excess.

Fig. 3. The positivity rate of thyroid antibodies divided by age groups among three areas. (a)TPOAb(+); (b)TGAb(+); (c)TPOAb(+) and TGAb(+); (d)TPOAb(+) or TGAb(+). a Indicates significant differences between IF and IA areas. b Indicates significant differences between IF and IE areas. *Indicates the trend of thyroid antibodies is significant in this area. TPOAb, thyroid peroxidase antibody; TGAb, thyroglobulin antibody; IF, iodine fortification; IA, iodine adequate; IE, iodine excess.
Thyroid function and thyroid volume in different thyroid antibody groups among the three areas
Table 2 shows that, compared with those in the TPOAb(-) and TGAb(-) groups, the levels of TSH, FT3 and TV were significantly higher in all thyroid antibody-positive groups (except TSH in TPOAb(+) group in IA areas) among the three areas (all adjusted P < 0·050). In the TPOAb(−) and TGAb(−) group, the levels of TSH, FT3, FT4 and TV in IE areas were significantly higher than those in IA areas and the levels of TSH and FT4 were significantly higher than those in IF areas at the same time (all P < 0·050). In the TGAb(+) groups, the levels of FT4 in IE areas were significantly higher than those in IF and IA areas (all adjusted P < 0·05) and in the TPOAb(+) and TGAb(+) group was significantly higher than that in IA areas (adjusted P = 0·010).
Table 2. Thyroid function and thyroid volume in different thyroid antibody groups among three areas
(OR and 95 % CI)

IF, Iodine fortification areas; IA, iodine adequate areas; IE, iodine excess areas; TPOAb, thyroid peroxidase antibody; TGAb, thyroglobulin antibody; TSH, thyroid stimulating hormone; FT3, free triiodothyronine; FT4, free thyroxine; TV, thyroid volume..
* Significant differences compared to negative group.
† Significant differences compared to IA areas.
‡ Significant differences compared to IF areas.
§ P: Kruskal–Wallis H test for TSH, FT3, FT4 and ANOVA test for TV among different thyroid antibody groups.
|| P: Kruskal–Wallis H test for TSH, FT3, FT4 and ANOVA test for TV among three areas.
The prevalence of thyroid diseases in different thyroid antibody groups among the three areas
As shown in Table 3 compared with that in the TPOAb(−) and TGAb(−) groups, the prevalence of overt hypothyroidism, subclinical hypothyroidism and goitre was significantly higher in all thyroid antibody-positive groups in three areas (all P < 0·010). In the TPOAb(−) and TGAb(−) groups, the prevalence of subclinical hypothyroidism in IE areas was significantly higher than that in IA and IF areas (both P < 0·001). In the IA areas, the prevalence of overt hyperthyroidism was significantly higher in all positive thyroid antibody groups than in the TPOAb(-) and TGAb(-) group (all P < 0·010).
Table 3. The prevalence of thyroid diseases in different thyroid antibody groups among three areas
(Number and percentages)

TPOAb, thyroid peroxidase antibody; TGAb, thyroglobulin antibody; IF, Iodine fortification areas; IA, iodine adequate areas; IE, iodine excess areas.
* Significant differences compared to negative group.
† Significant differences compared to IA areas.
‡ Significant differences compared to IF areas.
§ P: χ2 test for overt hypothyroidism, subclinical hypothyroidism, overt hyperthyroidism, subclinical hyperthyroidism and goitre among different thyroid antibody groups.
|| P: χ2 test for overt hypothyroidism, subclinical hypothyroidism, overt hyperthyroidism, subclinical hyperthyroidism and goitre among three areas.
Relationship between thyroid antibody titre and subclinical hypothyroidism among the three areas
As shown in Table 4, in the three areas, subjects with positivity for any thyroid antibodies had increased risks of subclinical hypothyroidism and those positive for only TPOAb had higher risks than those positive for only TGAb. In IF and IE areas, the risk of subclinical hypothyroidism in thyroid antibody-positive patients are higher than that in IA areas. Figure 4 shows the prevalence of elevated serum TSH at different combinations of TPOAb and TGAb in three areas. The frequency of elevated serum TSH increased dramatically with the increasing level of TPOAb in IF areas (all P for trend < 0·05), but it was not evident in IA and IE areas. In three areas, the association between an elevated TSH and the level of TgAb was not obvious. We only observed a significant increase in the prevalence of elevated TSH following the levels of TGAb when TPOAb was at level 1 (P for trend < 0·001) in IF areas.
Table 4. Relationship between thyroid antibody titre and subclinical hypothyroidism among three areas
(OR and 95 % CI)

IF, iodine fortification areas; IA, iodine adequate areas; IE, iodine excess areas; aOR, adjusted OR; TPOAb, thyroid peroxidase antibody; TGAb, thyroglobulin antibody.
TPOAb: level 1: TPOAb < 34 IU/ml; level 2:34 IU/ml ≤ TPOAb < 200 IU/ml; level 3:200 IU/ml ≤ TPOAb < 400 IU/ml; and level 4: TPOAb ≥ 400 IU/ml.
TGAb: level 1: TGAb < 115 IU/ml; level 2:115 IU/ml ≤ TGAb < 200 IU/ml; level 3:200 IU/ml ≤ TGAb < 400 IU/ml; and level 4: TGAb ≥ 400 IU/ml.
* Adjusted age, gender, BMI, urinary iodine concentration (UIC) and TGAb.
† Adjusted age, gender, BMI, UIC and TPOAb.

Fig. 4. The prevalence of elevated TSH at different levels of TPOAb and TGAb. (a) IF, iodine fortification areas; (b) IA, iodine adequate areas; (c) IE, iodine excess areas. TPOAb: level 1: TPOAb < 34 IU/ml; level 2:34 IU/ml ≤ TPOAb < 200 IU/ml; level 3:200 IU/ml ≤ TPOAb < 400 IU/ml; and level 4: TPOAb ≥ 400 IU/ml. TGAb: level 1: TGAb < 115 IU/ml; level 2:115 IU/ml ≤ TGAb < 200 IU/ml; level 3:200 IU/mL ≤TGAb < 400 IU/ml; and level 4: TGAb ≥ 400 IU/ml. TSH, thyroid stimulating hormone; IF, iodine fortification; IA, iodine adequate; IE, iodine excess; TPOAb, thyroid peroxidase antibody; TGAb, thyroglobulin antibody.
Discussion
In this study, we investigated 2503 adults in twenty villages from three areas. According to the recommendations from WHO/UNICEF/IGN, the iodine status in IF areas was adequate (median UIC: 177·65 μg/l), and that in IA areas was above the requirements (median UIC: 213·07 μg/l), whereas that in IE areas was excessive (median UIC: 408·70 μg/l). These results indicated that after the 26-year USI policy, the iodine nutritional status of residents in IF areas in China has significantly improved and stabilised. It is no doubt about the overall benefits of USI and our research provides support for further implementation of USI. However, USI could be further fine tuned, such as starting with small doses of iodine in iodine-deficient areas and gradually reaching the optimal fortification dose of iodine over several stages. Our data show that the optimal level of iodine fortification is a balance between avoiding iodine deficiency disorders and excessive iodine supplements that induce hypothyroidism. Despite the supply of non-iodised salt in IE areas, the iodine nutritional status was still excessive; this situation has attracted the attention of Chinese scholars.
Many studies have shown that after iodine fortification, the prevalence of thyroid antibodies among residents increases(Reference Pedersen, Knudsen and Jørgensen7,Reference Wang, Li and Teng13) . A cohort study in Denmark has indicated that the prevalence of both TPOAb and TGAb remains elevated 4–5 years after iodine fortification in areas with moderate or mild iodine deficiency(Reference Pedersen, Knudsen and Carlé14). However, no report has been published on the relationship between thyroid antibodies and iodine in people living in areas with naturally high water iodine levels. Many reports have suggested that excessive iodine consumption is a risk factor for the development of thyroid autoimmunity(Reference Rayman6,Reference Luo, Kawashima and Ishido15,Reference Ferrari, Fallahi and Antonelli16) . Some researchers disagree and have suggested that increased iodine intake is not associated with thyroid antibodies(Reference Hong, Stokes and Otahal17,Reference Khattak, Ittermann and Nauck18) . In our study, in IF areas, the elevated levels of TGAb were clearer than the TPOAb levels, and the high prevalence of TGAb in IF areas was probably due to enhanced iodination of TG by the iodine provided in salt. TG is an iodinated molecule, and sudden iodine supplementation after iodine deficiency can cause TG iodisation. Experimental animal and human data suggest that iodinated TG is more immunogenic than poorly iodinated TG(Reference Rose, Saboori and Rasooly19,Reference Rasooly, Burek and Rose20) . Human T lymphocytes are more likely to respond to iodinated TG than poorly iodinated TG(Reference Sundick, Bagchi and Brown21), thus suggesting that TGAb is more sensitive to iodine fortification after long-term iodine deficiency, whereas the sensitivity of TPOAb is not clear. In our study, the thyroid antibody positivity rate in IE areas was only slightly elevated, and iodised salt was not provided in IE areas. The iodine intake was mainly from drinking water. In recent years, the Chinese government has gradually improved the drinking water of residents in IE areas, and the iodine nutritional status of some residents has changed, thus potentially influencing the prevalence of thyroid antibody, a possibility requiring further study.
A survey in an iodine-deficient community in southern Italy has reported that the prevalence of thyroid antibody increases from childhood to 46–55 years of age(Reference Aghini-Lombardi, Antonangeli and Martino22). A study from Denmark has shown that thyroid antibodies in the oldest age groups residing in moderately iodine deficient or mildly iodine deficient areas increased after the Danish iodine fortification program(Reference Andersen, Iversen and Terpling23). These findings are consistent with our results. Notably, in IF and IE areas after the age of 50, the prevalence of TPOAb and TGAb increased sharply. On the one hand, before USI (1995), this group in IF areas experienced at least 25 years of iodine deficiency, followed by approximately 25 years of iodine supplementation. On the other hand, residents in this age group in IE areas have been exposed to an IE for many years. This finding indicated that older residents in IF and IE areas might have experienced iodine deficiency or IE for longer times, and therefore might have had a higher prevalence of thyroid antibodies (TGAb and TPOAb). The thyroid function status of the older residents in areas with IE or those who have experienced iodine supplement after iodine deficiency deserves special attention. Interestingly, we found that the prevalence of thyroid antibodies in IA areas appeared to follow an inverted U-shape, and nearly no change in the positivity rate of TGAb was observed after the age of 30 years. The previous studies have described the U-shaped curve. In our study, the levels of iodine intake in IA areas (median UIC:213·07 μg/l) are higher than those of previous studies in which the lower end of the U-shaped curve, thus we now add data to extend this curve at the iodine intake levels at the higher end. Many studies have found that after the age of 60, the positivity rate begins to decrease, and at the age of 60–65, people have the lowest prevalence of TPOAb and TGAb, in agreement with our findings in IA areas(Reference Zhai, Zhang and Chen12). Residents of areas with naturally suitable iodine conditions have not experienced iodine supplementation after iodine deficiency or long-term IE, and their thyroid autoimmunity is relatively stable; thus, living in such areas may be beneficial to thyroid health. Some studies indicate that it is a benefit to longevity when living in an area with a stable and recommended iodine intake(Reference Riis, Pedersen and Danielsen24). Similarly to those of previous studies(Reference Aghini Lombardi, Fiore and Tonacchera25,Reference Bjoro, Holmen and Krüger26) , our results indicated higher positivity rates of both TGAb and TPOAb in women than men, thus suggesting that women are more likely to have abnormal thyroid antibodies irrespective of the area. There are two possible reasons for the high positivity rates of antibodies in women. On the one hand, for women, the physiologic changes associated with different life stages affect the occurrence of AITD. For example, during pregnancy, the immune system of women is suppressed to protect the fetus and rebound after delivery, so about 5 % of women develop thyroiditis after delivery(Reference Alexander, Pearce and Brent27). On the other hand, the occurrence of AITD in women may be influenced by the prolactin and estrogen in their bodies. Some studies found that AITD is associated with hyperprolactinaemia and the production of thyroid autoantibodies in women seemed to be related to increased levels of prolactin(Reference Sayki Arslan, Sahin and Topaloglu28). Estrogen can exert multiple regulations of the immune response, an in vitro experiment has indicated that the metabolite of endogenous estrogen caused the apoptosis of thyroid cells which resulted in the release of TPOAb(Reference Wang, Myc and Koenig29).
A cross-sectional study in 1205 Chinese residents has indicated that TPOAb or TGAb positivity is significantly and positively associated with TV(Reference Liu, Huang and Zeng30), thereby suggesting a strong association between thyroid antibodies and enlarged TV. In our study, regardless of area, the TV levels and the incidence of goitre in people with thyroid antibody positivity were higher than those in people with thyroid antibody negativity. Because thyroid antibody positivity is an independent risk factor for the occurrence of goitre, there is a close association between goitre and thyroid autoimmunity, the latter of which may stimulate goitre development and serve as an effector in the maintenance of goitre(Reference Vanderpump, Tunbridge and French31). Another study has shown that iodine supplementation to a level more than adequate in a region in which iodine intake was previously mildly deficient may accelerate the development of goitre in people with thyroid autoantibody positivity(Reference Yu, Fan and Shan32). However, we did not find the same result in our study. Because of the limitations in the diagnostic methods during the investigation and the sample size, we did not further analyse the relationship between different subtypes of goitre and positive thyroid antibodies.
We observed the relationship between thyroid antibody positivity and TSH in residents of three areas. The results showed that the TSH in thyroid antibody-positive residents increased. Thyroid antibodies are closely associated with TSH; however, the effects of thyroid antibody subtypes are controversial. Hollowell and others have proposed that TGAb positivity and TPOAb positivity can cause the levels of TSH to rise, especially in women and older people(Reference Hollowell, Staehling and Flanders33). However, Vanderpump and colleagues conducted a 20-year follow-up survey in 2779 British community residents indicating that the risk of elevated TSH increases with the concentrations of TPOAb, but the effect is not obvious in TgAb-positive people(Reference Vanderpump, Tunbridge and French31). In this study, we graded thyroid antibody titre and analysed its relationship with TSH. We found that positivity for thyroid antibodies significantly increased the levels of TSH and was associated with abnormally elevated levels of TSH in the three areas. In addition, we found that the frequency of abnormally elevated TSH increased dramatically with the amount of TPOAb in the three areas (Fig. 4). Our results are closer to the results of Vanderpump, and TPOAb positivity was more closely associated with TSH.
An early follow-up study has indicated that, among patients with baseline positive antibodies, 5% per year develop hypothyroidism. At baseline, positive thyroid antibodies are powerful predictors of future hypothyroidism(Reference Tunbridge, Brewis and French34). Our current findings regarding whether TPOAb or TGAb caused the increased prevalence of overt hypothyroidism and subclinical hypothyroidism supported the results of previous studies. Interestingly, in the thyroid antibody negative group, the prevalence of subclinical hypothyroidism in IE areas was significantly higher than that in the other two areas, thus indicating that in addition to the thyroid autoimmune state, external environmental iodine factors also strongly influence the health status of the thyroid, a possible explanation could be the inhibitory effect on thyroid function by some humic substances coexisting with iodine as indicated(Reference Andersen, Petersen and Laurberg35). Adersen and his colleagues found that the iodine content of tap water was present not as iodide but mostly bound in humic substances and approximately 85 % of iodine bound in humic substances in tap water was bioavailable on a population level(Reference Laurberg, Andersen and Pedersen36,Reference Andersen, Pedersen and Iversen37) . However, there are few researches on this in China and it needs to be confirmed in future studies. Because this study used a cross-sectional design, whether the prevalence of overt hypothyroidism and subclinical hypothyroidism in these positive patients will increase over time is worthy of further study.
Both TPOAb and TGAb are known to associate with damage to the thyroid gland, and the effect of TPOAb is greater than that of TGAb(Reference McIntosh, Asghar and Weetman38–Reference McLachlan and Rapoport40). In clinical settings, TPOAb positivity is a well-known risk factor for developing hypothyroidism. In patients who have circulating TPOAb, there is a greater risk of progression from subclinical to overt hypothyroidism(Reference Biondi, Cappola and Cooper41). Therefore, we analysed the association of the levels of TPOAb and TGAb with the prevalence of subclinical hypothyroidism. Our results indicated that TPOAb(+) or TGAb(+) adults had a higher risk of subclinical hypothyroidism than those who were negative for either antibody, and the risk increased with the titre of TPOAb and TGAb. The OR for TPOAb were slightly higher than those for TGAb in the three areas, thus suggesting that TPOAb positivity is associated with a higher risk of subclinical hypothyroidism than TGAb positivity. This result is consistent with findings from several studies(Reference McIntosh, Asghar and Weetman38,Reference McLachlan and Rapoport40) . In residents in IE and IF areas, the OR (95 % CI) for TPOAb and TGAb were higher than those in IA areas, thereby suggesting that iodine supplementation after iodine deficiency or living in IE areas for long time periods aggravates the risk of subclinical hypothyroidism in antibody-positive individuals. In our investigation, the numbers of cases of overt hyperthyroidism and subclinical hyperthyroidism were small. The relationships between thyroid antibody positivity and overt hyperthyroidism and subclinical hyperthyroidism in areas with different water-iodine levels require further study in an expanded sample size. Since our study is a cross-sectional study in which the thyroid function of the subjects in our study was measured only at this point in time, so it is difficult to distinguish between temporary and permanent thyroid dysfunction in patients. This limitation in our study should be improved in future studies.
In conclusion, in IF areas, after USI both the TPOAb and TGAb positivity rates were higher than IA areas, especially the rate of TGAb positivity, which is sensitive to iodine fortification after long-term iodine deficiency. In areas with different median water iodine, positivity for both TPOAb and TGAb was associated with increased TSH and TV (goitre). As the levels of TPOAb increased, the frequency of abnormally elevated TSH increased dramatically in the three areas. The risk of subclinical hypothyroidism in all three regions increased with increasing levels of TPOAb and TGAb. Compared to residents in IA areas, experiencing iodine supplementation after iodine deficiency or living in IE areas for long time periods aggravates the risk of subclinical hypothyroidism in antibody-positive individuals, and the effect of the former is greater than that of the latter. Our study supports the notion that the association between iodine intake and thyroid disorders is a U-shaped curve, and it is important to monitor IF and IE areas to uphold the optimal iodine nutrition of the population.
Acknowledgements
We are grateful for the assistance provided by the Institute for Prevention and Treatment of Endemic Disease of Shandong, Shanxi, Jiangsu and Henan Province for collecting epidemiological data and samples and contributions and support from all participants.
This study was supported by grants from the National Natural Science Foundation of China (82073490 and 81830098).
The contribution of each author is as follows: H. S. designed the study; H. S., Z. Z., L. L., M. J., B. R., F. M., D. W., J. L., B. L., Y. H., F. L. conducted the research. Z. Z. analysed the data. Z. Z. and L. L. drafted the manuscript. All authors revised the report and approved the final version before submission.
The authors declared that they have no conflict of interest.