I is a trace element that is essential for the synthesis of thyroid hormones that are required for normal growth, and therefore important for the development of newborns. Adequate dietary I intake is critical for susceptible populations with high I requirements, such as pregnant and lactating women as well as infants. Several studies have shown that I deficiency during crucial periods of development in infants leads to growth retardation, impaired hearing capacity and reduced cognitive function( Reference Hetzel, Potter and Dulberg 1 – Reference Melse-Boonstra and Jaiswal 3 ). I intake is required not only for meeting the mother’s own synthesis of thyroid hormones but also for transferring the hormones to the infant through breast milk (BM) for maintaining normal thyroid function as well as growth and development, particularly of the brain. This additional loss of I is the main reason underlying I deficiency in lactating women( Reference Dorea 4 ). I deficiency can affect thyroid function during lactation, leading to adverse side-effects in the mother, and consequently pose a critical threat to growth and brain development in the breast-fed infant( Reference Hetzel, Potter and Dulberg 1 , 2 ). The current daily I intake of 250 μg/d for lactating women has been recommended by WHO/ICCIDD/UNICEF to ensure that I deficiency does not occur in the postpartum period, and the I content of milk is sufficient for infants’ requirements. In both lactating women and infants <2 years of age, median urinary iodine (MUI) concentration below 100 μg/l defines a population with I deficiency. However, limited data are available on the safe urinary iodine (UI) upper limit in lactating women( 2 ). Recent studies have shown that I excess in women (including lactating women) living in areas with high water I concentrations increases the risk of thyroid disease( Reference Liu, Liu and Shen 5 ). Other investigations have linked excessive I intake from BM to subclinical hypothyroidism in preterm Korean infants( Reference Chung, Shin and Yang 6 ). Accordingly, adequate I concentration in BM is considered particularly important for breast-fed infants to ensure optimal thyroid hormone storage and prevent neurological development impairment. Few studies to date have focused on the relationship between I intake and thyroid function in lactating women. The main objectives of the present study were to clarify the status of I nutrition and the prevalence of thyroid disease among lactating women from three regions with different water I contents in China, to ascertain the relationship between I intake and the status of I nutrition as well as thyroid function in lactating women and their infants and to simultaneously provide reference data for appropriate disease control and prevention measures.
Methods
Survey areas
Villages for survey were selected based on the national surveillance data on areas with high water I contents, along with historical iodine deficiency disorders (IDD) surveillance data obtained in recent years( Reference Shen, Liu and Sun 7 , Reference Sun, Xiao and Liu 8 ). The coastal area, Tieshangang district (Xinggang, Nankang and Yingpan Towns) of Beihai city, Guangxi province, was selected as the I-deficient region with median water iodine (MWI) ≤10 μg/l and with low coverage rates of iodised salt( Reference Shen, Sui and Ge 9 ). Luocheng village in Jiajiazhuang township and Xiaoguo village in Yangcheng township, both located in Fenyang City from Shanxi province, were selected as areas where residents have sufficient I nutrition (50 μg/l≤MWI≤150 μg/l), representing the control group. Chengzi, Donghe, Guxianzhuang and Dongshe villages in Jicun township and Jinghua and Ninggu villages in Pingyao township (Fenyang city of Shanxi province) were selected as areas with high I water content (MWI≥300 μg/l)( Reference Liu, Zhao and Zhu 10 ).
Survey subjects
From January to June 2014, a total of 343 lactating women (including 106, 104 and 133 subjects from the I-deficient, sufficient and excess regions, respectively) were recruited, excluding those taking anti-thyroid drugs or I supplements within a year of the study, consuming seafood at the time of the study and those with a family history of thyroid disease or having congenital thyroid disease. All the participants had lived in the region for >5 years. Infants whose breast-feeding occurred within a year of the study and with BM as the main source of food were selected.
Survey indicators
According to WHO recommendations, the following indicators were adopted to assess I status( 2 ): MUI, goitre rate (GR), thyroid-stimulating hormone (TSH) and thyroglobulin (Tg). Given the limitations of these indicators, free tri-iodothyronine (FT3), free thyroxin (FT4), thyroglobulin antibody (TgAb) and thyroid peroxidase antibody (TPOAb) were included as novel indicators for testing. Reference values are presented in Table 1.
Table 1 Diagnostic criteria for thyroid disease

FT3, free tri-iodothyronine; FT4, free thyroxin; Tg, thyroglobulin; TgAb, thyroglobulin antibody; TPOAb, thyroid peroxidase antibody; TSH, thyroid-stimulating hormone.
* Reference values: FT4, 11·5–22·7 pmol/l; TSH, 0·27–4·2 mIU/l; FT3, 3·1–6·8 pmol/l; TPOAb, 0–60 U/ml; TgAb, 0–60 U/ml.
† Based on the diagnostic criterion of endemic goitre( Reference Liu, Li and Li 13 ).
Thyroid volume, UI, water I, salt I, BM I and thyroid function of lactating women were additionally examined. These indicators were matched for individual subjects. As some participants did not provide all three samples (urine, BM and blood), the number of each sample type differed from the total number of subjects. Urine samples were additionally collected from infants of the lactating study participants.
This project was approved by the Ethical Review Board of Harbin Medical University. Written informed consent was obtained from all the participants before the survey was conducted.
Survey methods, sample collections and measurements
A standard questionnaire was designed to acquire demographic information including name, age, personal or family history of thyroid disease (including type of thyroid disease), intake of supplements containing I, smoking habits, economic income, source of drinking water and drinking duration. The questionnaire was administered face-to-face and conducted by well-trained staff.
A single-spot urine sample was collected in the morning in clean plastic tubes and stored at 4°C until batch-analysed for I content using the China Health Standard Method for Determination of Iodine in Urine by As 3+ –Ce 4+ Catalytic Spectrophotometry ( Reference Yan, Zhang and Liu 11 ). Urine and standard samples (250 μl) were digested with 1000 μl (NH4)2S2O8 (1·0 mol/l) at 100°C for 60 min. Internal quality control samples for UI were provided by the Chinese National Reference Laboratory for Iodine Deficiency Disorders (NRLIDD).
In the pre-selected I-deficient area (Tieshangang district), each participant provided a water sample of at least 15 ml, whereas in the pre-selected I excess and sufficient villages, twenty-two water samples in total were collected from the central water supply. Water samples were stored at 4°C until analysis. The I concentration of drinking water was determined using the method recommended by NRLIDD, the Chinese centre for disease control and prevention.
The I content in table salt from households of lactating participants was determined using the general test method of the salt industry( Reference Tong and Huo 12 ). Each participant provided a table salt sample of at least 50 g. The standard salt I content in Guangxi province and Shanxi province is 25 mg/kg. Salt type was classified into the following categories: non-iodised salt (salt I content <5 mg/kg), qualified iodised salt (18 mg/kg≤salt I content≤33 mg/kg) and unqualified iodised salt (5 mg/kg<salt I content<18 mg/kg or >33 mg/kg).
An aliquot of at least 5 ml BM was collected from each lactating subject for determining the I content and stored at −20°C until analysis. I content was determined using arsenic–cerium catalytic spectrophotometry( Reference Liu, Li and Li 13 ), the National Standard method of the Chinese Ministry of Health. BM and standard samples (500 μl) were ashed with 1000 μl-K2CO3-NaCl and 1000 μl-ZnSO4-KClO3 in a Muffle furnace at 600°C for 4 h after drying and carbonisation.
Thyroid ultrasonography was performed by an experienced examiner using a 7·5 MHz transducer (mainly measuring the thyroid volume, nodule diameter and echo). Thyroid lobe volume was calculated by measuring the depth (d), width (w) and length (l) of each lobe using the following formula: V (ml)=0·479×d×w×l (mm)/1000, and was recorded as the sum of both lobes. The normal volume for female adults was <18 ml( Reference Liu, Chen and Jia 14 ).
Blood samples (5–6 ml) were collected from the cubital vein of lactating women. Serum samples were prepared by centrifugation at 3000 rpm for 10 min after allowing the samples to stand for 30 min and were subsequently frozen at −80°C until analysis. TSH, FT3, FT4, TPOAb and TgAb levels were determined using the chemiluminescent immunoassay (Bayer Healthcare Company, Siemens Medical Solutions Diagnostics). Tg levels were measured using electrochemiluminescence immunoassay, Elecsys and Cobas diagnostics (Bayer Healthcare Company, Roche).
Statistical analysis
All the data were recorded with EpiData3.1. Data processing and statistical analyses were performed using SPSS software (version 13.0). Normally distributed data were expressed as mean values and standard deviations, whereas non-normally distributed data were expressed as median with the 25th and 75th percentiles. ANOVA was used to compare normally distributed data, and the Mann–Whitney test was used to compare non-normally distributed data among the groups. The proportions among the three groups were analysed with the χ 2 test. Spearman’s method was used for correlation analysis of the variables. Binary logistic regression analysis was applied to calculate the OR and 95 % CI for abnormal TSH of lactating women from the three areas. Data were considered statistically significant at P<0·05.
Results
Subjects
A total of 343 lactating women were recruited for the survey. Demographic characteristics of the study subjects are presented in Table 2. Water I contents varied significantly among the three groups. No significant differences were observed with regard to age, height, weight and smoking habits among subjects from all three groups.
Table 2 Demographic characteristics of lactating women from three different water iodine groups (Normally distributed mean values and standard deviations; non-normal distributed 25th and 75th percentiles)
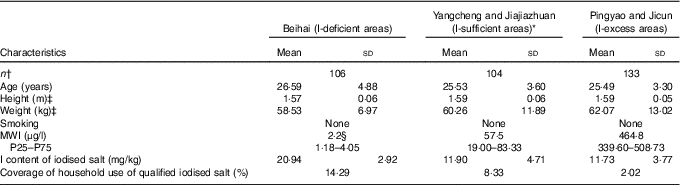
MWI, median water iodine.
* The control group.
† The sample sizes.
‡ Water I content varied significantly among the three groups (P<0·01).
Iodine nutritional status
MUI values of lactating women were 51·30, 282·42 and 822·51 μg/l for the I-deficient, sufficient and excess groups, respectively, as shown in Table 3. Compared with the I-sufficient group, MUI values were significantly lower in the I-deficient group (Z=−11·566; P=0·000) and significantly higher in the I-excess group (Z=−10·002; P=0·000). In parallel, MUI of breast-fed infants and median I content in BM differed significantly among the three groups.
Table 3 Status of iodine nutrition in lactating women and infants from the three water iodine groupsFootnote * (Medians, sample sizes and 25th to 75th percentiles)
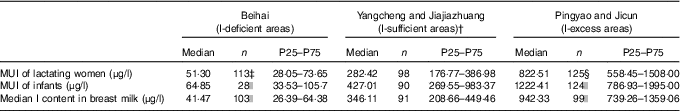
MUI, median urinary iodine.
* Test for difference among the three groups: Mann–Whitney test.
† The control group.
‡ Compared with the control group, MUI values were significantly lower in the I-deficient group (Z=−11·566; P=0·000).
§ Compared with the control group, MUI values were significantly higher in the I-excess group (Z=−10·002; P=0·000).
|| MUI of breast-fed infants and median I content in breast milk differed significantly among the three groups (P<0·01).
Positive correlations among BM I and UI of lactating women as well as UI of their breast-fed infants are depicted on scatter plots in Fig. 1.
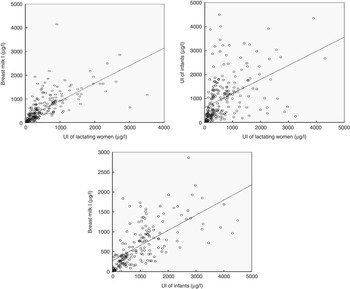
Fig. 1 Positive correlations among breast milk (BM) I, urinary iodine (UI) concentrations of lactating women and UI concentrations of their infants on a scatter plot. Positive correlations are evident between BM I and UI of lactating women, UI of infants and UI of lactating women and BM I and UI of infants (r 0·879, P=0·000; r 0·623, P=0·000; r 0·733, P=0·000).
Assessment of thyroid hormones and antibodies
Compared with the I-deficient group, the FT3 levels of lactating women were significantly higher in the I-sufficient and excess groups (5·25 and 5·25 v. 4·79 pmol/l; P=0·049, 0·020).
We additionally observed a trend for increased serum FT4 concentrations with increasing water I content. In the I-excess group, FT4 levels of lactating women were significantly higher compared with the I-deficient group (14·10 v. 12·71 pmol/l; P=0·010).
Median TSH concentrations of lactating women in the I-deficient group were significantly lower than those in the I-sufficient group (Z=−6·828; P=0·000) and were higher in the I-excess group compared with the I-sufficient group (Z=−1·864; P=0·062), although this difference was not statistically significant.
Serum Tg levels varied markedly among the three groups and were higher in the I-deficient and excess groups compared with the I-sufficient group (18·36 and 16·25 v. 10·53 μg/l; Z=−4·286, P=0·000; Z=−2·579, P=0·01).
We observed no significant differences in positive rates for TgAb, TPOAb and the combined antibodies among the three groups (7·55, 13·46 and 15·79 % for TgAb; 13·21, 9·62 and 12·79 % for TPOAb; and 5·66, 6·73 and 9·02 % for TgAb and TPOAb for the I-deficient, sufficient and excess groups, respectively; Table 4).
Table 4 Thyroid parameters of lactating women in the three water iodine groupsFootnote * (Numbers and percentages; mean values and standard deviations; medians and 25th to 75th percentiles)
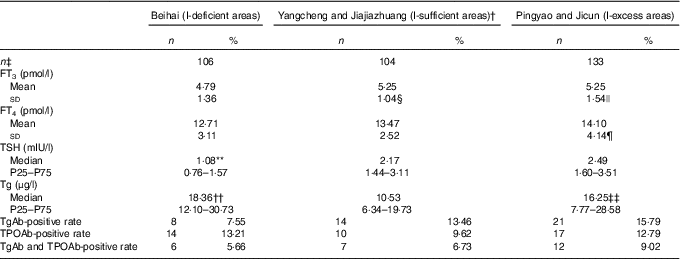
FT3, free tri-iodothyronine; FT4, free thyroxin; Tg, thyroglobulin; TgAb, thyroglobulin antibody; TPOAb, thyroid peroxidase antibody; TSH, thyroid-stimulating hormone.
* Differences among the three groups were analysed by ANOVA or the Mann–Whitney test.
† The control group.
‡ The sample sizes.
§ Compared with the I-deficient group, the FT3 levels of lactating women were significantly higher in the I-sufficient groups (P=0·049).
|| Compared with the I-deficient group, the FT3 levels of lactating women were significantly higher in the I-excess groups (P=0·020).
¶ In the I-excess group, FT4 levels of lactating women were significantly higher compared with the I-deficient group (14·10 v. 12·71 pmol/l; P=0·010).
** Median TSH concentrations of lactating women in the I-deficient group were significantly lower compared with the I-sufficient group (Z=−6·828, P=0·000).
†† Serum Tg levels were higher in the I-deficient group than the I-sufficient group (Z=−4·286, P=0·000).
‡‡ Serum Tg levels were higher in the I-excess group than the I-sufficient group (Z=−2·579, P=0·01).
Non-linear correlations among TSH and Tg and MUI of lactating women were observed, their relationships are depicted on scatter plots in Fig. 2.
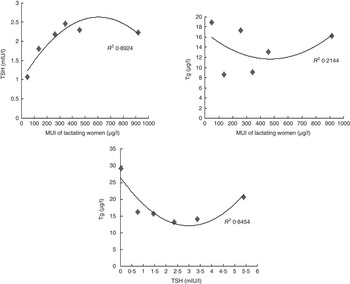
Fig. 2 Median urinary iodine (MUI) was divided into six groups (<100, 100–199·9, 200–299·9, 300–399·9, 400–499·9 and >500 μg/l). Thyroid-stimulating hormone (TSH) levels tended to increase and subsequently decrease with increased levels of MUI. TSH was divided into six groups (<0·27, 0·27–0·99, 1·00–1·99, 2·00–2·99, 3·00–4·19 and >4·20 mIU/l). Thyroglobulin values showed a decrease and subsequent increase with increased levels of TSH, forming a ‘U curve’ relationship in lactating women.
Thyroid diseases
Compared with the I-sufficient group, the prevalence of subclinical hypothyroidism was significantly higher (χ 2=4·486; P=0·034) in the I-excess group. However, we observed no significant differences in the prevalence of other thyroid diseases examined among the three groups (Table 5).
Table 5 Prevalence of thyroid disease among lactating women from the different water I groupsFootnote * (Number of cases and percentages)
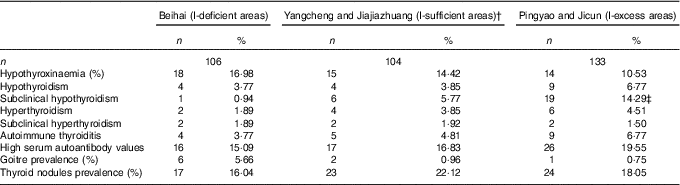
* Differences among the three groups were analysed by χ 2 test.
† The control group.
‡ Compared with the I-sufficient group, the prevalence of subclinical hypothyroidism was significantly higher (χ 2=4·486; P=0·034) in the I-excess group.
Analysis of risk factors for abnormal thyroid-stimulating hormone in lactating women
Rates of abnormal TSH values (including cases of hypothyroidism, subclinical hypothyroidism, hyperthyroidism and subclinical hyperthyroidism) differed significantly among the three groups (χ 2=14·511; P=0·001). Further analysis of associated factors revealed that water I, positive rates of TPOAb and TgAb, serum Tg level and UI are risk factors for abnormal TSH. Logistic regression analysis showed that high water I areas (OR 2·295; 95 % CI 1·067, 4·932; P=0·033), high TPOAb (OR 11·033; 95 % CI 5·033, 24·185; P=0·001) and high Tg levels (OR 1·014; 95 % CI 1·005, 1·024; P=0·002) are risk factors for abnormal TSH levels (Table 6).
Table 6 Association of abnormal thyroid-stimulating hormone (TSH)Footnote * of lactating women with water I and other risk factors (Odds ratios and 95 % confidence intervals)
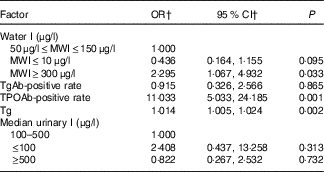
MWI, median water iodine; Tg, thyroglobulin; TgAb, thyroglobulin antibody; TPOAb, thyroid peroxidase antibody.
* Abnormal TSH values including cases of hypothyroidism, subclinical hypothyroidism, hyperthyroidism and subclinical hyperthyroidism.
† Binary logistic regression analysis was used to calculate the OR and 95 % CI for abnormal TSH in lactating women from the three areas.
Discussion
Thyroid hormones play an important role in growth and development of the human body, particularly in the brain. The I intake of breast-fed infants relies solely on concentrations in BM, the only source of I after birth, to meet the infant’s requirements for the synthesis of thyroid hormones( Reference Laurberg, Andersen and Knudsen 15 ). Thus, brain development in infants is positively associated with the status of I nutrition and thyroid function of the mother. Previously, IDD were widespread in China. In recent years, IDD has been effectively controlled using the Universal Salt Iodization (USI) programme. However, pregnant, lactating women and women of reproductive age remain at higher risk( Reference Yan, Chen and Yang 16 – Reference Laurberg, Pedersen and Hreidarsson 18 ). In China, thirty-one million people are currently living in high water I areas( Reference Shen, Liu and Sun 7 ). Considering the complicated geographical environment of China, we evaluated the status of I nutrition and the prevalence of thyroid disease among lactating women in three regions with different water I contents.
This study aimed to assess I nutrition in lactating women in areas with different water I contents. Areas where salt was rarely used were selected to avoid interference from I in salt, thus ensuring minimal contribution to UI. Coverage of qualified iodised salt was 14·29, 8·33 and 2·02 % in deficient, adequate and excess areas, respectively. Notably, coverage of qualified iodised salt in Beihai was rather low, as cheaper sea salt was used by the residents in this coastal city. The use of iodised salt has been forbidden in I-excess areas( 19 ). Both Jiajiazhuang and Yangcheng townships are in Fenyang city, located adjacent to high water I areas; therefore, the I content of iodised salt and coverage of qualified iodised salt were relatively low owing to the usage of non-iodised salt.
A number of international researchers and the WHO have recommended that I nutrition from BM is optimal at concentrations of 100–200 μg/l to ensure normal development in infants( Reference Neville, Keller and Seacat 20 – Reference Semba and Delange 22 ). When the MUI of infants <2 years of age is above 100 μg/l, the current I nutrition status of the population is considered adequate. In our study, the median I concentration in water from Beihai in Guangxi province was only 2·2 μg/l, clearly reflecting the lack of I in the external environment. In addition, median BM I was 41·47 μg/l and MUI was 64·85 μg/l in infants, which were lower than the recommended values. These results clearly reveal deficient I nutritional status among lactating women and infants from Beihai. In contrast, MUI was as high as 822·51 and 1222·41 μg/l, respectively, in lactating women and infants from Pingyao and Jicun villages in Shanxi province, indicating excessive I nutritional status in these cases. The above findings support the recommendation that UI concentration should be monitored in lactating women and infants, especially for populations living in high or low water I areas.
Owing to loss of I in BM and urine during lactation, dietary I requirements are increased in lactating women, and I metabolism is enhanced with increasing I intake. The BM I content increased with UI concentration in lactating women in our study. The I intake of breast-fed infants relies solely on concentrations in BM. BM I and UI of breast-fed infants were positively correlated, confirming that the I nutrition status of infants is affected by the I nutrition status of their mothers( Reference Wang, Zhang and Ge 23 , Reference Caldwell, Jones and Hollowell 24 ).
Previous studies have reported increasing TSH levels with I intake in adults( Reference Laurberg, Pedersen and Hreidarsson 18 , Reference Wang, Jin and Teng 25 ). Consistent with earlier data, we observed an increase in the median concentration of TSH with increasing water I concentrations in lactating women from three regions with different water I contents. The FT3, FT4, TSH and UI concentration were low in Beihai, an area with low I levels in drinking water. However, GR was >5 %, which was consistent with a report documenting high GR in lactating women in I-deficient areas( Reference Azizi and Smyth 26 ). Accordingly, we speculated that Beihai city is a mild I-deficient area with a low prevalence of thyroid disease.
We compared the utility of TSH and Tg as biomarkers of I status in lactating women using two methods. (1) Groups were classified based on the three different I nutrition areas (Table 4). In this case, TSH increased with the water I content. However, median Tg levels of lactating women in both I-deficient and excess groups (18·36 and 16·25 μg/l, respectively) were significantly higher than that in the I-sufficient group (10·53 μg/l), showing lower values in the middle and higher values on both sides (approximate ‘V shape’). (2) Groups were classified based on the different UI concentration levels (Fig. 2). In this study, the TSH level was increased and subsequently decreased with increased levels of MUI, similar to data from a previous study( Reference Meng, Zhao and Liu 27 ) (approximate inverted ‘U curve’). In children, a ‘U curve’ relationship between TSH and UI has been reported( Reference Meng, Zhao and Liu 27 ). However, no obvious correlation was evident between median Tg and MUI. I nutrition in children is sufficient at Tg<13 μg/l. A ‘U curve’ relationship has been demonstrated between UI concentration and median Tg level in children, suggesting that Tg is a useful biomarker for I deficiency and excess I intake( Reference Ma and Skeaff 28 , Reference Zimmermann, Aeberli and Andersson 29 ). However, we assumed that children and lactating women are different populations, the latter being affected by the external environment, metabolism of the body itself, diet and small sample size, and further research on a larger scale is warranted. Our preliminary data indicate that TSH and Tg are not suitable as biomarkers for evaluating I nutrition in lactating women.
In our comparative analysis of I-deficient and sufficient areas, increased prevalence of thyroid disease (hypothyroidism, subclinical hypothyroidism, hyperthyroidism and subclinical hyperthyroidism, referred to as abnormal TSH) of lactating women was observed in I-excess areas. In high water I areas, high TPOAb and high Tg levels were determined as risk factors for abnormal TSH among lactating women. However, the influence of I deficiency on abnormal TSH was not established in this study, possibly because only mild I deficiency was present in our survey area of Beihai, Guangxi. Notably, our results are analogous with previous findings of increased incidence of subclinical hypothyroidism in pregnant women under high I conditions( Reference Teng, Shan and Teng 30 , Reference Sang, Wei and Zhao 31 ). The prevalence of hypothyroxinaemia was evaluated as 14·42 % in I-sufficient areas, compared with an earlier study showing 10·54 % in six I-sufficient areas in China( Reference Meng, Zhao and Liu 27 ). Taking into consideration the complicated geographical environment of China, household coverage of adequately iodised salt and demographic characteristics in the scope of the current survey, we propose that excessive I intake may lead to thyroid disease, especially subclinical hypothyroidism.
I deficiency results in a wide spectrum of adverse effects throughout the life cycle. Among these, the effect of I deficiency on infant intellectual development is of the greatest concern. No clear results on the association between loss of intelligence and I excess have been obtained to date. In this study, we focused on the status of I nutrition in relation to the prevalence of thyroid disease in lactating women. Urine samples were additionally collected from breast-fed infants to determine UI levels (as urine samples were difficult to collect for infants, we were unable to obtain all the counterpart samples from both lactating women and their infants). Moreover, the infants examined in this study were too young to obtain blood samples, and therefore we planned to use experimental animals for further research. Another limitation of this study was the lack of dietary assessment in individuals (e.g. water consumption was not measured).
Monitoring the I status of schoolchildren alone is not adequate. The Ministry of Health in China recommended careful monitoring of the I nutrition status in lactating women as part of the state monitoring for preventing and controlling tasks programme in 2011( Reference Sun, Xiao and Liu 8 ). UI concentrations among lactating women were monitored in thirty-one provinces and corps. According to the monitoring outcomes, MUI values among most lactating women were at the national level. However, both water I content and household coverage of adequately iodised salt were low in Beihai, Guangxi, where the prevalence of thyroid disease was not high but UI levels were low in lactating women and infants. These results clearly indicate that the status of I nutrition in lactating women and infants remains deficient in a number of regions in China. To ensure that brain development in infants is not affected as a result of I deficiency, the relevant governmental departments and the salt industry should increase co-operation and supervision to put effective targeted measures of I supplementation in place. Analogously, it is important to prevent excessive I intake through stopping the provision of iodised salt and implementing effective measurements for water improvement (such as finding other drinking water sources with no excessive I) in high water I areas. Public awareness of the adverse effects of inadequate I intake on infant development needs to be enhanced. Further implementation of scientific I supplementation strategies composed of measures that are contextually appropriate, diverse for the general population and specific for the key ones is necessary.
Conclusion
Excessive I intake may induce subclinical hypothyroidism in lactating women. Moreover, adequate I nutrition is essential for lactating women and infants, especially those living in I-deficient areas. Enhanced monitoring of I status is important to avoid adverse effects of I deficiency or excess, particularly in susceptible populations such as pregnant or lactating women and infants.
Acknowledgements
The authors are grateful to the organisations that participated in this survey, including the Institute for Prevention and Treatment of Endemic Disease of Shanxi Province and the Center for Disease Control and Prevention of GuangXi Province for technical assistance. They also thank the colleagues from the above-mentioned organisations who took part in the survey for their dedication and support.
This work was supported by the National Natural Science Foundation of China (grant no. 81273012) and Specialized Research Fund for the Doctoral Program of Senior Education (SRFDP, 20122307110010).
The contribution of each author is as follows: H. S. and L. L. designed the study; H. S., L. L., D. W., P. L. F., M. D. W., Q. J., J. L., X. Z. and P. J. conducted the research; L. L. analysed the data; L. L., D. W. and P. L. wrote the paper; H. S. took the primary responsibility for final content of the manuscript. All the authors read and approved the final version of the manuscript.
There are no conflicts of interest to declare.
The authors have nothing to disclose.











