Ageing is associated with changes in body composition, generally with decreased weight and fat-free mass but increased fat mass( Reference Forbes and Reina 1 – Reference Genton, Karsegard and Chevalley 4 ). The age-related loss of fat-free mass is of particular concern because it is related to a greater risk for functional impairment, disability, falls, impaired quality of life and mortality( Reference Genton, Karsegard and Chevalley 4 – Reference Janssen, Heymsfield and Ross 6 ). In particular, loss of appendicular skeletal muscle mass (ASMM) is one of the components in the assessment of sarcopenia as a geriatric syndrome( Reference Dutta 7 , Reference Cruz-Jentoft, Baeyens and Bauer 8 ). Sarcopenia is defined as the loss of muscle mass and strength with ageing and can increase the risk for functional limitation and mortality( Reference Janssen, Heymsfield and Ross 6 ). According to the results of the 2008–2009 Korea National Health and Nutrition Examination Survey (KNHANES), the prevalence of sarcopenia (class II) within the Korean population aged 40 years or older was 9·3 % among men and 7·6 % among women, when using a weight-adjusted definition( Reference Kim, Lee and Chung 9 ).
The decline in skeletal muscle mass associated with ageing has been known to depend on modifiable behavioural factors, such as diet and physical activity( Reference Cruz-Jentoft, Baeyens and Bauer 8 , Reference Scott, Blizzard and Fell 10 ). In particular, under-nutrition and the consumption of a less-varied diet with ageing( Reference Fox, Brummit and Ferguson-Wolf 11 ) are associated with less muscle mass. Among diet-related factors, a low vitamin D level has been known to influence declined muscle function and increase the risk of falling in the elderly( Reference Bischoff-Ferrari, Borchers and Gudat 12 , Reference Hamilton 13 ).
However, previous studies have reported an inconsistent relationship between vitamin D and muscle mass, in different sexes and age groups( Reference Verreault, Semba and Volpato 14 – Reference Visser, Deeg and Lips 20 ). In addition, there is a lack of studies on the relationship between vitamin D and muscle mass with consideration of both sex and age. The prevalence of vitamin D deficiency and the extent of loss of skeletal muscle mass were reported to be different between sexes, showing a higher prevalence of vitamin D deficiency among women compared with men( Reference Park and Kim 21 – Reference Hyppönen and Power 23 ) and a more rapid decreased rate of skeletal muscle mass after the age of 40 years in men compared with women( Reference Kim, Lee and Chung 9 , Reference Holick 24 – Reference Flynn, Nolph and Baker 25 ). Given the age- and sex-specific findings on vitamin D status and skeletal muscle mass, the relationship between vitamin D and muscle mass could be dependent on age and sex.
Therefore, this study aimed to examine whether a high serum 25-hydroxyvitamin D (25(OH)D) concentration was related to a high skeletal muscle mass, taking into account the effects of sex and age among the national representative subjects who were 40 years or older. The hypotheses were that high serum 25(OH)D concentration is related to high skeletal muscle mass, and the difference in skeletal muscle mass by serum 25(OH)D status could be dependent on age and sex.
Methods
Data sets and study participants
This was a cross-sectional study using data from the 2009 to 2010 KNHANES. The KNHANES has been conducted periodically since 1998 to assess the health and nutrition status study conducted by the Korea Centers for Disease Control and Prevention (KCDC). Subjects younger than 40 years of age were excluded for better comparability while examining the relationship between 25(OH)D and skeletal muscle mass. The included subjects were grouped under the following age groups: (1) 40–64 years and (2) 65 years and older. The total number of subjects who were 40 years or older in the KNHANES was 9800; subjects without dual-energy X-ray absorptiometry (DXA) data, anthropometric data or vitamin D values were excluded (n 1394). Data from 8406 subjects (3671 men and 4735 women) were finally analysed in this cross-sectional study, which included 85·8 % of the KNHANES population. All of the study participants provided informed consent, and the Institutional Review Board of the KCDC approved the study protocol.
Serum 25-hydroxyvitamin D assessment
Blood samples were collected from the subjects who had undertaken a more than 8-h overnight fast, immediately refrigerated, transported in cold storage to the Central Testing Institution in Seoul, Korea, and analysed within 24 h. Serum 25(OH)D concentrations were measured with RIA (DiaSorin Inc.) using a counter (1470 Wizard; PerkinElmer). Participants were classified as having hypovitaminosis if the level of serum 25(OH)D was <20 ng/ml( 26 ).
Appendicular skeletal muscle mass assessment
Whole-body fat-free mass (kg) excluding the skeleton was based on the whole-body DXA scan using fan beam technology (Hologic). The muscle mass was estimated by measuring the appendicular skeletal muscle mass index (ASMMI, kg), which is one of the indices to identifying sarcopenia( Reference Baumgartner, Koehler and Gallagher 27 , Reference Janssen and Ross 28 ) and is defined as the sum of the body mass (kg) from the arms and legs, based on the method of Heymsfield et al.( Reference Heymsfield, Smith and Aulet 29 ). Because absolute muscle mass is correlated with height, the relative muscle mass was considered so that the height-adjusted ASMMI was regarded as an index of relative skeletal muscle mass( Reference Baumgartner, Koehler and Gallagher 27 ). In this study, the ASMMI (kg/m2) defined as ASMM (kg) divided by height squared (m2) was used as an outcome.
Covariate assessment
Information on socio-demographics and health-related behaviour was collected by the trained interviewers or self-reported. On the basis of education the subjects were categorised as follows: less than elementary school; middle school; high school; and college degree or higher. On the basis of occupation, subjects were categorised into the following groups: non-manual; manual; and no job. The income level was divided into quartiles based on the equivalent income of the household (monthly household income/
![]() $$\sqrt {{\rm number}\,{\rm of}} \,{\rm a}\,{\rm household}\,{\rm member}$$
). Information on history of diabetes, osteoarthritis, rheumatoid arthritis, myocardial infarction, angina, chronic obstructive pulmonary disease, tuberculosis, stroke and cancer was provided by the participants. Smoking and drinking statuses were categorised into current, previous and never. Participants were also asked whether they had taken dietary supplements for 2 weeks or more in the previous year. Status regarding marriage, menopausal status and year of participation in the survey were also examined. The participants’ physical activity status was also classified into groups based on whether they met or did not meet the recommended criteria for physical activity: (1) 20 min or more/session of vigorous-intensity activity 3 d or more/week; or (2) 30 min or more/session of moderate-intensity physical activity 5 d or more/week.
$$\sqrt {{\rm number}\,{\rm of}} \,{\rm a}\,{\rm household}\,{\rm member}$$
). Information on history of diabetes, osteoarthritis, rheumatoid arthritis, myocardial infarction, angina, chronic obstructive pulmonary disease, tuberculosis, stroke and cancer was provided by the participants. Smoking and drinking statuses were categorised into current, previous and never. Participants were also asked whether they had taken dietary supplements for 2 weeks or more in the previous year. Status regarding marriage, menopausal status and year of participation in the survey were also examined. The participants’ physical activity status was also classified into groups based on whether they met or did not meet the recommended criteria for physical activity: (1) 20 min or more/session of vigorous-intensity activity 3 d or more/week; or (2) 30 min or more/session of moderate-intensity physical activity 5 d or more/week.
BMI was calculated as weight/height2 (kg/m2). Measurement of intact parathyroid hormone (PTH) was carried out in the same laboratory using a chemiluminescence assay (DiaSorin).
Dietary intakes were assessed on the basis of a 24-h recall in the nutrition survey. Daily intakes of total energy, carbohydrate, fat, protein, antioxidants (vitamin A and vitamin C) and Ca, which is known to relate to skeletal muscle mass and 25(OH)D( Reference Robinson, Cooper and Sayer 30 ), were estimated from the consumed food items.
Statistical analysis
For the representativeness of the study sample, the excluded samples were included in the data sets, and, after stratification, sample weights were assigned to a stratified group. Values were presented as mean values with their se or as percentage with se because the values were based on KNHANES-supplied sample weights and adjusted for age. Either the Student’s t test or the χ 2 test was applied to evaluate the sex differences for general characteristics. The ASMMI was depicted by sex, age group and vitamin D status, all of which were examined by the χ 2 test. To explore the factors related to vitamin D status, the participants’ characteristics were compared according to their vitamin D status and the age group for each sex, using the Student’s t test or the χ 2 test. The difference in ASMMI due to 25(OH)D status (<20 and ≥20 ng/ml) was assessed by the general linear model, and the results were presented as least squares means after adjusting for confounding factors. The confounding factors that are related to lean body mass and serum 25(OH)D level were selected on the basis of a literature review. Only those confounding factors with a statistically significant relationship with 25(OH)D status within each sex and age group were included in the model. Regarding the nutrient variables, protein was highly correlated with carbohydrate and fat and hence it was excluded from the model because of multicollinearity problems. The analysis was stratified by sex and age groups. All of the analyses were weighted to allow for oversampling, nonresponse and the Korean population in the survey period, considering the complex sampling design. A type I error rate of 0·05 was used to validate the tests of hypothesis, and all analyses were performed using SAS version 9.2 (SAS Inc.).
Results
The general characteristics of the study participants, according to sex, are shown in Table 1. Among those who were 40–64 years of age, 81·5 % were men and 75·9 % were women. The mean value of ASMMI was higher among men and younger adults than among women and older adults. The difference in ASMMI by age group was greater in men than in women. Women had a greater proportion of hypovitaminosis (71·1 %) compared with men (53·2 %), whereas the mean value of 25(OH)D was lower for women than for men (women 17·4 ng/ml, men 20·3 ng/ml). The proportion of graduates with college education or higher, that of smokers, drinkers and those engaged in manual jobs and the proportion of subjects engaged in regular exercise were higher among men than among women. Women had higher proportions of disease history and dietary supplement intake compared with men.
Table 1 Age-adjusted characteristics of the study subjects (Mean values with their standard errors; percentages)

ASMM, appendicular skeletal muscle mass; ASMMI, ASMM index; 25(OH)D, 25-hydroxyvitamin D (ng/ml).
* ASMM: lean body mass (kg) of arms and legs.
† Percentage of ASMM: lean body mass (kg) of arms and legs/total weight of arms and legs×100.
‡ ASMMI (kg/m2): ASMM (kg)/height (m)2.
§ Hypovitaminosis D; 20 ng/ml<serum 25-(OH)D.
║ History of diabetes, osteoarthritis, rheumatoid arthritis, myocardial infarction, angina, chronic obstructive pulmonary disease, tuberculosis, stroke and cancer.
¶ Taking dietary supplements for 2 weeks or more during the past 1 year.
** 20 min or more/session of vigorous-intensity activity 3 d or more/week, or 30 min or more/session of moderate-intensity physical activity 5 d or more/week.
The factors related to 25(OH)D status, according to sex and age groups, are presented in Table 2. Most of the participants who had a low 25(OH)D (<20 ng/ml) were, in higher proportions, commonly from the groups who had non-manual jobs, who were physically inactive, and whose participation season was spring or winter; their PTH values were also higher, regardless of sex or age group, when compared with those with a high 25(OH)D level (≥20 ng/ml). In men with a low 25(OH)D level, the mean value of ASMMI was lower and the education level was higher compared with men who had a high 25(OH)D level. The average intakes of energy in subjects with a low 25(OH)D were lower in men aged 65 years and above and in women aged 40–64 years when compared with those with a high 25(OH)D. There was a higher proportion of current smokers among participants aged 65 years and older who had a low 25(OH)D level compared with those with a high 25(OH)D.
Table 2 Age-adjusted mean values of factors related to 25-hydroxyvitamin D (25(OH)D) status by sex and age group (Mean values with their standard errors; percentages)

ASMM, appendicular skeletal muscle mass; ASMMI, ASMM index; PTH, parathyroid hormone; Q, quintile; RE, retinol equivalents.
* P value was calculated using the Student’s t test or the χ 2 test.
† ASMMI (kg/m2)=weight of ASMM (kg)/height (m)2.
‡ Physical activity=20 min or more/session of vigorous-intensity activity 3 d or more/week, or 30 min or more/session of moderate-intensity physical activity 5 d or more/week.
After adjusting for confounding factors, a positive relationship between 25(OH)D and ASMMI was seen in men but not in women (Table 3). There was a significant difference in ASMMI based on 25(OH)D status in men regardless of age, showing a lower mean value of ASSMI in those with hypovitaminosis (8·09 for <20 ng/ml of 25(OH)D and 8·24 for ≥20 ng/ml among those aged 40–64 years, P value 0·0037; and 7·55 for <20 ng/ml of 25(OH)D and 7·70 for ≥20 ng/ml among those aged 65 years or more, P value 0·0175). However, there was no difference among women in both the younger and older age groups.
Table 3 The difference in appendicular skeletal muscle mass index between 25-hydroxyvitamin D status (<20 and ≥20 ng/ml) by sex and age group (Mean values with their standard errors)
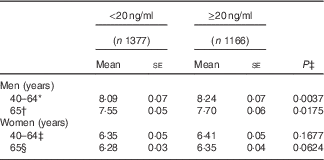
* The model was adjusted for age, parathyroid hormone (PTH), educational level, occupation, current drinking status, physical activity and survey season.
† The model was adjusted for age, PTH, educational level, occupation, disease history, current smoking status, current drinking status, taking dietary supplements, survey season, energy, carbohydrate and fat.
‡ The model was adjusted for age, PTH, occupation, current drinking status, taking dietary supplements, survey season, postmenopausal status, energy and carbohydrate.
§ The model was adjusted for age, BMI, PTH, occupation, current smoking status and survey season.
Discussion
Given the inconsistent findings for the relationship between 25(OH)D and skeletal muscle mass, we aimed to study the relationship between 25(OH)D and skeletal muscle mass by sex and age group in the Korean adult population. We found that there was a positive relationship between 25(OH)D level and skeletal muscle mass in both young and old age groups among men.
Several studies have addressed the mechanism of how vitamin D may relate to the skeletal muscle. Evidence has indicated that the receptor for 1,25-dihydroxyvitamin D (1,25(OH)2D), vitamin D receptor (VDR), is expressed in skeletal muscle and is a crucial mediator of 1,25(OH)2D, affecting muscle contractility( Reference Roth, Zmuda and Cauley 31 ). However, the result is still inconsistent as shown by another study in which the VDR was undetectable in skeletal, cardiac and smooth muscle( Reference Wang and Deluca 32 ). The vitamin D deficiency might affect muscle protein turnover by inducing hypocalcaemia and decreasing insulin secretion( Reference Wassner, Li and Sperduto 33 ). Vitamin D metabolites have been found to affect muscle metabolism in three ways: (1) by mediating gene transcription; (2) through rapid pathways not involving DNA synthesis; and (3) by the allelic variant of the VDR( Reference Visser, Deeg and Lips 20 ).
The skeletal muscle can experience functional improvement over time; the effect of 25(OH)D on the skeletal muscle mass could be important for improving muscle-related outcomes. However, most of the studies that examined the effects of 25(OH)D on muscle-related outcomes were focused on muscle function and performance( Reference Annweiler, Beauchet and Berrut 15 , Reference Bischoff-Ferrari, Dawson-Hughes and Staehelin 34 ). Moreover, the effects of 25(OH)D on the skeletal muscle mass were mostly evaluated on the elderly population( Reference Iannuzzi-Sucich, Prestwood and Kenny 18 – Reference Visser, Deeg and Lips 20 , Reference Rejnmark 35 , Reference Jacques, Felson and Tucker 36 ), indicating a lack of data on the effects of 25(OH)D on outcome measures in younger adults. Even for the elderly population, the findings on the effect of 25(OH)D on the skeletal muscle mass were inconsistent, showing either a positive( Reference Iannuzzi-Sucich, Prestwood and Kenny 18 , Reference Scott, Blizzard and Fell 19 , Reference Visser, Deeg and Lips 20 , Reference Jacques, Felson and Tucker 36 ) effect or no effect( Reference Iannuzzi-Sucich, Prestwood and Kenny 18 , Reference Marantes, Achenbach and Atkinson 37 ). In terms of inconsistent findings, several studies explained that the reason for the absence of effects of 25(OH)D on the muscle mass could be that 25(OH)D might affect the coordinative muscle function more than it does muscle mass or strength. A systematic review or a meta-analysis of randomised controlled trials showed that supplemental vitamin D improved either the physical functioning or reduced the risk of falls( Reference Annweiler, Beauchet and Berrut 15 , Reference Bischoff-Ferrari, Dawson-Hughes and Staehelin 34 ).
Although this study indicated that 25(OH)D was positively related to skeletal muscle mass in men regardless of age group, there were no consistent results by sex. Shantavasinkul et al.( Reference Shantavasinkul, Phanachet and Puchaiwattananon 38 ) found that 25(OH)D levels have a positive relationship with a marker of muscle mass in older men. In contrast, a recent study on the effect of 25(OH)D on the muscle mass reported that only older women had lower skeletal muscle mass associated with lower 25(OH)D levels( Reference Iannuzzi-Sucich, Prestwood and Kenny 18 ).
A possible explanation for the difference due to sex could be the effect of the genotype of VDR (Fok1) on the muscle mass( Reference Roth, Zmuda and Cauley 31 ). The risk allele (F allele) that would be associated with reduced muscle mass was differently distributed in each sex and ethnicity. Women had a lower proportion of the risk allele compared with men and, among ethnic groups, Asian groups, such as the Japanese, had the lowest proportion of the risk allele than did UK, French and North Indians( Reference Bahanushali, Lajpal and Kulkarni 39 ). In this study on the Korean population, a positive relationship between 25(OH)D and skeletal muscle mass in men might be explained by the characteristic of men having a higher proportion of the risk allele. Given that men have a relatively more rapid decreased rate of skeletal muscle mass after 40 years, it could be important to maintain their serum 25(OH)D levels in the normal range. Further investigation on the ethnicity- and sex-specific effects of serum 25(OH)D on muscle mass in additional diverse populations of adults is needed to better understand the mechanism of these findings.
Several limitations should be considered while interpreting the findings of our study. First, the cross-sectional nature of this study did not allow for the assessment of the causal relationship between 25(OH)D levels and lean body mass. Second, this study was conducted year round. As season may affect the 25(OH)D levels and muscle mass at the same time, season was adjusted in all analyses to minimise the effect from seasonal variation. Third, vitamin D measured at tissue levels might be more sensitive to muscle mass than that measured at serum levels; these findings had some discrepancies( Reference Wang and Deluca 32 ). Despite the above-mentioned limitations, this study included a representative population-based sample with a large number of adults, including younger adults, and studied the relationship between serum 25(OH)D and skeletal muscle mass, considering age and sex.
In conclusion, in this representative population-based sample of Korean adults of varying ages, there might be a positive relationship between serum 25(OH)D concentration and skeletal muscle mass among men regardless of age, indicating that the interventions to improve 25(OH)D levels aimed at increasing muscle mass could be beneficial for men with more rapid decreased rate of skeletal muscle mass.
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (no. 2013R1A1A1060704). This work was supported by the National Evidence-based Healthcare Collaborating Agency.
M. J. K. designed the study, contributed to data interpretation and wrote the manuscript. S. Y. analysed the data. K. O. contributed to data interpretation and assisted with statistical analyses. K. K. designed the study, contributed to data interpretation and supervised all aspects of its implementation. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest.






