Fat is an important source of energy, facilitates the absorption of fat-soluble dietary components such as vitamins. Additionally, fats and oils are important sources of essential fatty acids (EFA). Most unsaturated fatty acids in the diet have the cis configuration, but trans fatty acids (TFA) are present as well. Fats also play an important role by enhancing the taste and acceptability of foods and fat components contribute to the texture, flavour and aroma of foods.
The approach to establish nutrient recommendations has changed over time. While the early sets of recommendations focused on ensuring adequate intakes of nutrients from foods to prevent deficiencies, a shift from this paradigm emerged in the middle of the 20th century.
Atwater published the USDA first dietary standards in 1894 and in 1916 the first USDA Food guide. In the UK an early report including dietary recommendations was published in 1938 by the Technical Commission on Nutrition of the League of Nations(Reference Gifford1).
Better socioeconomic, living and nutrition conditions for wider proportions of the population in many countries led to the epidemiological transition. Prevalence of cardiovascular diseases as well as mortality rates for this cause and also for cancer progressively increased, while death rates due to infectious diseases decreased.
The interest in dietary fat and its effects, especially with regard to its role in cardiovascular disease was stimulated by several papers published in the early 1950s. In the 70s, evidence from the Seven Countries’ study conducted by Ancel Keys and colleagues found a significant association between fat and saturated fat intake and heart disease mortality. Despite methodological limitations, the growing evidence linking diet and chronic diseases brought a new approach to dietary recommendations, aimed not only to prevent deficiencies, but also to encourage dietary changes in order to prevent chronic diseases(Reference Gifford1).
The first guidelines issued by the American Heart Association in 1957 already cited ‘Diet may play an important role in the pathogenesis of atherosclerosis’. In 1977, the Senate Select Committee on Nutrition and Human Needs published the report Dietary Goals for the United States. Three years later followed Dietary Guidelines for Americans(Reference Kritchevsky2).
In 1991 a document published in the UK introduced a new concept: Dietary Reference Values (DRVs)(3). In 1994 the Food and Nutrition Board of the Institute of Medicine (IOM) published the dietary reference intakes (DRIs) for the United States of America and Canada including many aspects of the conceptual framework from the report published in the United Kingdom(Reference Yates4). These DRIs represented a new conceptual framework in the way nutrient recommendations are established and used. Besides the prevention of deficiencies, DRIs were intended to help individuals optimize their health, prevent disease, and avoid consuming too much of a nutrient. The DRIs included four nutrient-based reference values: estimated average requirement (EAR), the recommended dietary allowance (RDA), the adequate intake (AI) and the tolerable upper intake level (UL). The EAR is defined as the average daily nutrient intake level that is estimated to meet the requirements of half of the healthy individuals in a particular life stage and gender group. The RDA represents the average daily dietary nutrient intake level that is sufficient to meet the nutrient requirements of nearly all (97–98 %) healthy individuals in a particular life stage and gender group. When an RDA cannot be determined, an AI is estimated which is the recommended average daily intake level based on observed or experimentally determined approximations or estimates of nutrient intake by a group (or groups) of apparently healthy people that are assumed to be adequate.
The Acceptable Macronutrient Distribution Ranges (AMDR) was introduced by the IOM in the 2002 report on energy and macronutrients(5). An AMDR is defined as a range of intakes for a particular energy source that is associated with reduced risk of chronic diseases while providing adequate intakes of essential nutrients. It is expressed as a percentage of total energy intake and has a lower and upper limit, determined mainly by the lowest or highest value judged to have an expected impact on health.
In 1978 and in 1994 expert groups convened by FAO/WHO published technical reports on dietary fats and health, including dietary recommendations(6). The expert group preparing the technical report on nutrition and the prevention of chronic diseases in 2002–2003 considered fat intake and fat quality as well(7). Dietary trends show that over the past decades, in line with the recommended dietary changes, fat consumption has decreased in many countries. However, the expected health outcomes have not paralleled the change and new problems emerged. Controversies raised by new evidence contribute to the debate with regards to total fat intake and fatty acids, and motivated an update to the FAO/WHO report in 2008(8). When preparing this report, the strength of the existing evidence for developing dietary guidelines was considered. The evidence was classified as convincing, probable, possible, and insufficient, according to directions in a background document prepared(Reference Smit, Mozaffarian and Willett9).
Many countries, or even different bodies and organizations within the same country, have formulated nutrient recommendations and dietary guidelines. However, different approaches have been used. The process followed, criteria used or the type of evidence are not always clearly specified.
To address this issue, we conducted a systematic review of the literature examining dietary reference intakes, nutritional goals and dietary guidelines for fat and fatty acids for healthy individuals to achieve optimal health and the approaches used to formulate them.
Methods
Search strategy and selection
We searched the following electronic literature databases: Medlars Online International Literature (MEDLINE) via PubMed (1966–present), EMBASE (1980–present), SCOPUS, OVID, Web of Knowledge, Institute for Scientific Information (ISI) (1981–present), The Cochrane Library Plus, International Pharmaceutical Abstracts (IPA), Psychology Information (PsycINFO) (1971–present), Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1981–present), Biological Abstracts, Food Science and Technology Abstracts (FSTA) (1969–present), Latin American and Caribbean Heath Sciences Literature (LILACS) (1982–present), Pan American Health Organization Library (PAHO) and World Health Organization Library Information System (WHOLIS). The last search was run on 10 April 2011. We also searched for relevant grey literature reports and documents following a snowball process and expert informants.
The key words and search equation used were as follows: ((((‘Dietary Fats/administration and dosage’(Majr) OR ‘Dietary Fats/adverse effects’(Majr)) OR (‘Fatty Acids, Omega-3/administration and dosage’(Mesh) OR ‘Fatty Acids, Omega-3/adverse effects’(Mesh))) OR (‘Fatty Acids, Omega-6/administration and dosage’(Mesh) OR ‘Fatty Acids, Omega-6/adverse effects’(Mesh))) OR (‘Trans Fatty Acids/administration and dosage’(Mesh) OR ‘Trans Fatty Acids/adverse effects’(Mesh))) AND ‘Nutrition Policy’(Majr) AND (‘humans’(MeSH Terms) AND (English(lang) OR French(lang) OR German(lang) OR Italian(lang) OR Spanish(lang) OR Portuguese(lang))).
For this review we considered documents written either in English, French, German, Italian, Spanish, or Portuguese. Documents were included if they reported information on either recommended intake levels or dietary reference values or nutritional objectives or dietary guidelines regarding fat and/or fatty acids and/or cholesterol intake or if reported background information on the process followed to produce the recommendations. The reference values referred to healthy population or specific age and gender healthy population groups. When a series of dietary recommendations were updated at different points in time, we considered the most updated documents.
Following this process, 304 documents and reports were retrieved and screened by title and abstract, including 11 reports from grey literature. After the first screening process, 102 duplicated references plus 38 non relevant documents were excluded, thus yielding 164 potentially relevant documents and reports which were retrieved full text and analyzed in detail. According to the PRISMA statement(Reference Moher, Liberati, Tetzlaff and Altman10), we also scanned reference lists of articles retrieved full text. After reading full text, 18 more references were identified, searched and added. Some 72 documents satisfied the inclusion criteria and were considered for this review (Fig. 1). Methods of the search and inclusion criteria were specified in advance and documented in a protocol. Two independent reviewers screened the references retrieved and analyzed the documents for inclusion using EndNote and Systematic Reviews Collaborative Tool (SysCollab)(11), a software tool specifically designed to be used in this series of systematic reviews on omega-3.
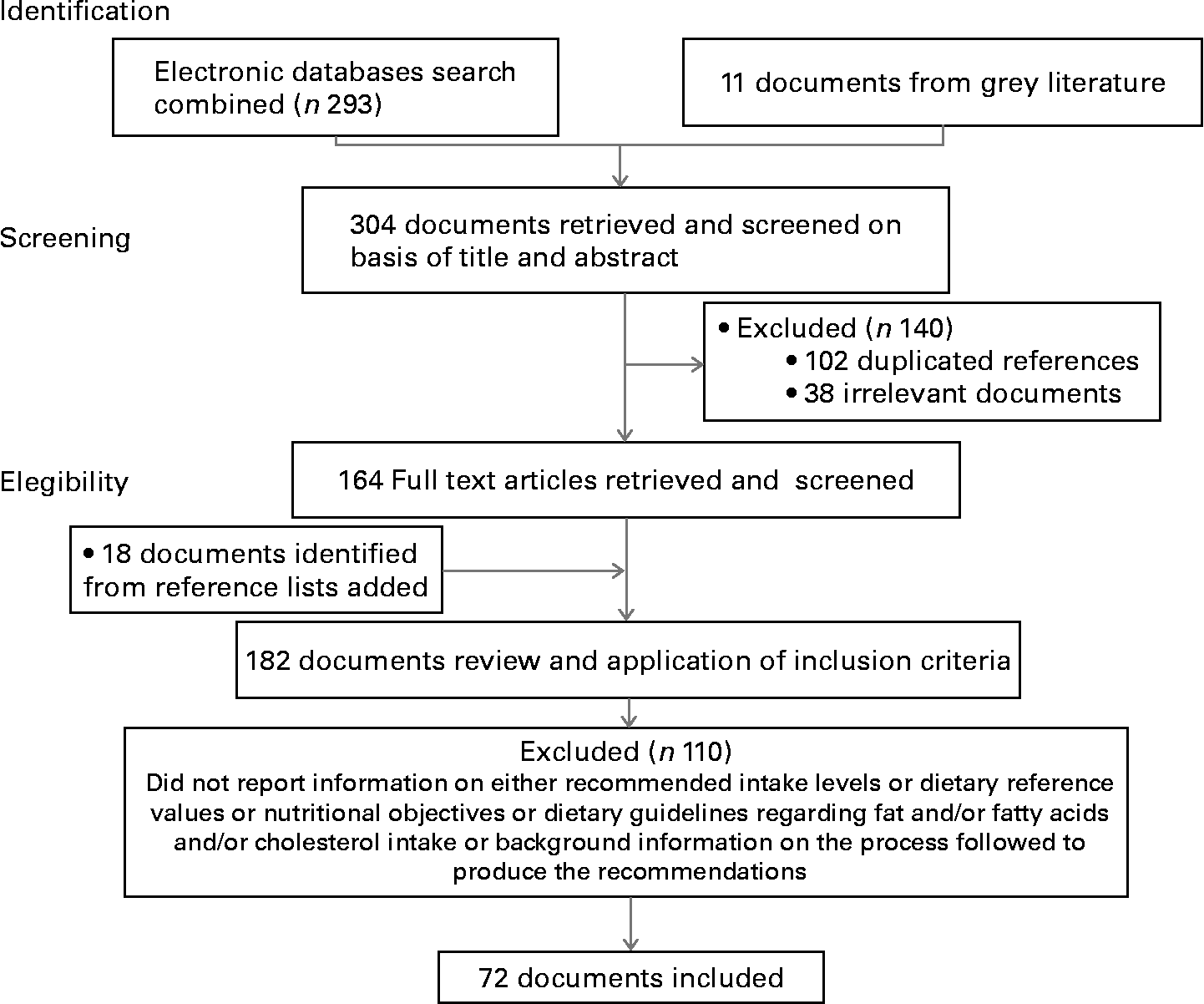
Fig. 1 Flow diagram of study identification, screening and selection for the systematic review.
The following data were extracted from the selected documents: recommended intakes (type of Dietary Reference Intake (DRI) and amount) for total fat, saturated fatty acids (SFA); monounsaturated fatty acids (MUFA); polyunsaturated fatty acids (PUFA); trans fatty acids; linoleic acid (LA); n3 FA; eicosapentaenoic acid (EPA); docosahexaenoic acid (DHA); EPA+DHA; alpha-linolenic acid (ALA); n6:n3 ratio; cholesterol; year of publication; country and/or institution responsible; reported criteria and evidence used to establish the recommendations. Data extraction forms were documented on a protocol.
Recommended intakes for total fat and fatty acids
A number of papers reported information related to recommendations issued by the same organization. The documents retrieved that met the inclusion criteria have been produced by international organizations with a worldwide scope, such as expert committees convened by the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO)(8), the International Society for the Study of Fatty Acids and Lipids(Reference Smit, Mozaffarian and Willett9, 12) or the World Association of Perinatal Medicine, Early Nutrition Academy and Child Health Foundation(Reference Koletzko, Lien and Agostoni13). Other reports were produced by American organizations and institutions, such as the American Heart Association(Reference Lichtenstein, Appel and Brands14, Reference Harris, Mozaffarian and Rimm15); American Academy of Pediatrics(Reference Anderson and Zlotkin16, Reference Gidding, Dennison and Birch17); the United States Department of Agriculture (USDA)(18) or the Institutes of Medicine (IOM) of the National Academies of Science(5); European organizations and agencies, such as the European Agency for Food Safety (EFSA)(19) or the European Society of Cardiology(20) or reports produced by organizations from different European, Asian and Pacific countries. The oldest document was produced in 1991, while several documents were issued in 2010.
A number of countries have published their own recommended intakes and guidelines or have adopted those suggested either by international organizations or by other countries. There are disparities across countries in current dietary reference values and recommendations for total fat and fatty acids. So far, no standardized methodology to define these values has been adopted, although some initiatives have suggested methodological approaches, such as Eurreca, an EU funded Network of Excellence aimed to harmonize dietary reference values for micronutrients across Europe(Reference Doets, de Wit and Dhonukshe-Rutten21, Reference Pavlovic, Prentice and Thörsdottir22). Despite total fat and fatty acids was not the main focus of Eurreca, the tools, products and processes developed during the project can also be useful to harmonize and update recommended intakes for fats. It is also worth mentioning the framework for DRI Development produced as a background paper by Taylor in 2008 for the IOM(23) and the background document prepared for the FAO/WHO expert group 2008(Reference Smit, Mozaffarian and Willett9).
The reports and documents included in this review were produced at varying points in time, thus considering the different evidence available at the time. Additionally, different types of evidence, endpoints or even diverse assumptions may have been considered to establish the recommended values. In the documents reviewed the background information on the formulation process often is not sufficient to clarify these aspects.
Criteria used to establish recommendations
The types of criteria and types of evidence used to set specific guidelines was not always clearly specified in the reports. The aims used to define dietary requirements included to prevent clinical deficiencies; to provide optimal health or to reduce the risk of developing chronic disease.
Examples of chronic disease outcomes used as criterion for dietary recommendations for fatty acids include coronary heart disease (CHD), obesity, diabetes, and specific types of cancers. Examples of physiological measures used as criterion to set dietary recommendations for dietary fatty acids are serum cholesterol levels, triglyceride levels, and neural integrity. Deficiency symptoms are most often studied in the case series, animal experiments, or short-term controlled feeding studies.
When insufficient data are available on disease or physiological criteria for setting recommendations, average intakes in national surveys are used. That is the case for some fatty acids and age groups. However, such intakes may not be optimal for reducing disease risk.
Equilibrium maintenance describes the balance of nutrient intake and loss, as measured by factorial estimation, which involves estimating the determinant factors for the requirement, such as increased requirements for growth, pregnancy, and lactation, or losses via the urine or feces(23, Reference Iglesia, Doets and Bel-Serrat24).
Type and level of evidence used
Some reports specify the outcomes and related supportive evidence, although the kind and level of evidence is missing (ecological studies, prevalence studies; retrospective case-control studies of disease outcomes; randomized controlled trials (RCTs) of physiological measures; prospective cohort studies of disease outcomes; RCTs of disease outcomes).
DRIs used
EAR and RDA have not been traditionally used for fats and fatty acids. UL has been used for total fat, saturated fat, total polyunsaturated fat, ALA, EPA+DHA, and dietary cholesterol. AI has been used for total fat, LA, ALA, and EPA+DHA. Acceptable macronutrient distribution range (AMDR) has been used for total fat, LA, and ALA. However, some reports do not specify the DRI value used.
The framework for the DRI development background paper suggests that even if there is limited data, scientific judgment can be important. Nevertheless, it must be noted that reliance on scientific judgment in the absence of optimal data, can be misleading and result in even harmful health consequences(23).
Dietary recommended intakes
Total fat
Table 1 shows recommendations for total fat, SFA, TFA, cholesterol, MUFA and PUFA for adults in the different sets compared(5, 7, 8, 12–Reference Harris, Mozaffarian and Rimm15, 18, 19, Reference Kris-Etherton and Innis25–Reference Alasfoor, Rajab and Al-Rassas38) and Table 2 shows recommendations for infants and children. In most documents the recommended intake for total fats in adults is 20–35 %E, although the lower recommended value is 15 % in Asian countries (Oman, India, Korea), and also in the FAO/WHO report 2008(8). In some sets the upper level is up to 40 % for people with optimal body weight. Higher levels of total fat intake are recommended for infants 0–6 mo., 40–60 %E. The FAO/WHO 2008(8); IOM(5) and EFSA(19) reports use AMDR for total fat for adults and children.
Table 1 Recommendations for fat and fatty acid intakesa for adults according to different bodies

SFA: Saturated Fatty Acids; Trans: Trans Fatty Acids; MUFA: Monounsaturated Fatty Acids; PUFA: Polyunsaturated Fatty Acids %E: % Energy intake. M: males; F: Females; AI: Adequate Intake.
a Recommendations for total fat, saturated fat (SFA), trans fatty acids (Trans), monounsaturated fats (MUFA) and polyunsaturated fats (PUFA) are expressed as percent energy intake.
b Region: Continent or region where recommendations apply.
c Total fat [%E]–SFAs [%E]–PUFAs [%E]–TFAs [%E].
d Can amount up to 15–20 %E.
e The AHA supports an omega-6 PUFA intake of at least 5 % to 10 % of energy in the context of other AHA lifestyle and dietary recommendations.
Table 2 Recommendations for fat and fatty acid intakesa for infants and children according to different bodies

SFA: Saturated Fatty Acids; Trans: Trans Fatty Acids; MUFA: Monounsaturated Fatty Acids; PUFA: Polyunsaturated Fatty Acids; %E: % Energy intake.
a Recommendations for total fat, saturated fat (SFA), trans fatty acids (Trans), monounsaturated fats (MUFA) and polyunsaturated fats (PUFA) are expressed either as percent energy intake or g/day.
b Region: Continent or region where recommendations apply.
To set these values the documents refer to evidence in relation to total fat intake and obesity, weight gain, coronary heart disease (CHD) and cancer. Most recent documents report on the insufficient evidence for the association between total fat intake and body weight and probable evidence that increased SFA intake results in increased weight(Reference Bray and Popkin39–Reference Marantz, Bird and Alderman42); no association between total fat intake and cancer(43); no clear benefits of a low fat high carbohydrate diet in relation to blood lipids, glucose or blood pressure(Reference Nordmann, Nordmann and Briel44, Reference Howard, Van Horn and Hsia45). Evidence from cohort studies reported conflicting results on total fat intake and the positive association with type 2 diabetes(Reference Melanson, Astrup and Donahoo46).
Low fat diets < 20 %E reduce LDL-cholesterol but also HDL-cholesterol, increase triglycerides (TGC) and can increase the risk of inadequate intakes of essential fatty acids and fat-soluble vitamins. Some reports highlight that the lower limit of fat intake for adults is difficult to define because there is limited evidence(5, 6, 18).
The FAO/WHO 2008 recommendations for total fat for infants slightly decrease in the lower and upper values of the acceptable range compared to previous reports. This change is based on the need to control energy intake to prevent the progression of the obesity epidemic, in light of the physiologic standards for energy intake and the acceptable weight for children 0–5 years according to the new WHO growth reference standards(8).
SFA
Most documents include a recommended intake for SFA below 10 %E. The American Heart Association recommends an SFA intake below 7 %E(Reference Lichtenstein, Appel and Brands14). In some cases reports advise either to limit intake or to keep intake of SFA as low as possible. The same value is reported for infants and children, except in the Dutch recommendations which set this limit up to 15 % for infants up to 12 months. An upper AMRD is reported for adults and children in the FAO/WHO 2008 report.
To set this value, reports refer to convincing evidence, about LDL-cholesterol and risk of cardiovascular disease(Reference Krauss, Eckel and Howard47–Reference German54). Most recent evidence shows a positive effect when SFA are reduced by replacing with PUFA, fruit and vegetables(Reference Mann55, Reference Mensink, Zock and Kester56), but not when replaced by easily digested carbohydrates(Reference Sacks and Katan57). Replacement of SFA with carbohydrates may have little benefit. In line with this, the Dietary Guidelines for Americans 2010 recommend SFA intake < 10 % by replacing them with monounsaturated and polyunsaturated fatty acids.
It is noted that individual SFA lauric (C12 : 0), myristic (C14 : 0), palmitic acids (C16 : 0), and stearic acid (C18 : 0) have different effects on plasma cholesterol levels.
MUFA
The majority of the documents included in this review do not set a recommended intake for MUFA. The FAO/WHO 2008 report(8) does not include a recommended level but states that an AMDR for MUFA intake should be calculated by difference, subtracting from total fat intake levels those for SFAs, PUFAs and trans fatty acids. In Spain, a country with high olive oil consumption, MUFA intake can account up to 20 %E(34).
Evidence reported to support the recommendations refer to the potential benefits of MUFA intake on blood lipid profile and CVD risk factors. There is convincing evidence that substituting carbohydrates with MUFAs raises HDL-cholesterol levels; possible evidence that substituting carbohydrates with MUFAs improves insulin sensitivity; convincing evidence that substituting SFAs with MUFAs lowers LDL-cholesterol levels and the TC/HDL-cholesterol ratio(Reference Kris-Etherton, Daniels and Eckel48, Reference Mensink, Zock and Kester56, Reference Jakobsen, O'Reilly and Heitmann58).
It is unclear whether sufficient new evidence exists to re-evaluate the lack of specific dietary recommendations for monounsaturated fat intake.
PUFA
Many reports do not include a value for all PUFAs, but specify recommended intake values for n-3 PUFAs and for n-6-PUFAs. For those reports including a value for PUFAs, there are discrepancies in the recommended intake levels. While some reports recommend intakes between 6–10 %E for PUFAS, other sets of recommendations consider lower values, 6–6·5 %E. For infants between 6–12 mo., an upper AMRD of 15 % is included in the FAO/WHO 2008 report, 11 % for children aged 2–18 yr.
PUFAs include n-6 and n-3 fatty acids. Linoleic acid (LA), an n-6 fatty acid, and alfa-linolenic acid (ALA), an n-3 fatty acid, are both essential fatty acids that cannot be synthesized by humans and must be supplied with the diet. The minimum intake levels for essential fatty acids are 2·5 %E LA plus 0·5 %E ALA to prevent deficiency symptoms in adults(5, 8).
Evidence considered to establish these recommendations is related to the favourable effects of LA on blood lipid profile, and its association with a lower risk of CHD events and reduced risk of type 2 diabetes(Reference Skeaff and Miller59). Although clinical benefits have not been observed across all studies, several new experimental and prospective observational studies support that ALA consumption reduces the incidence of CHD(Reference Mozaffarian60). Consumption of n-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have demonstrated physiological benefits on blood pressure, heart rate, triglycerides, and likely inflammation, endothelial function, and cardiac diastolic function(Reference Gebauer, Psota and Harris51, Reference Mozaffarian and Rimm61–Reference Simopoulos68). Aggregate data from randomized trials, case-control and cohort studies, and long-term animal feeding experiments indicate n-6 PUFAs reduce the risk of CHD relative to lower intakes. Outcomes from epidemiologic studies and controlled clinical trials have shown that replacing SFAs with unsaturated fat (cis MUFA and PUFA) is more successful in reducing the risk of CHD than simply reducing total fat consumption. Evidence is consistent for a reduced risk of fatal CHD and sudden cardiac death at consumption of around 250 mg/day of EPA plus DHA(Reference Mozaffarian and Rimm61, 69).
DHA also plays a major role in development of the brain and retina during foetal development and the first 2 years of life(Reference Koletzko, Lien and Agostoni13, Reference Simopoulos68, Reference Brenna70–Reference Gil-Campos78). These findings support the need for recommendations for adequate intakes of preformed DHA in pregnant women, lactating women, and young children. DHA should be considered conditionally essential during early development, since formation of DHA from ALA is limited, highly variable (1–5 %)(Reference Uauy and Dangour77).
The FAO/WHO 2008 report(8) defines 11 %E as upper-AMRD for total PUFAs in adults, 15 % for infants 6–12 mo., due to a decrease in bioavailability of vitamin E leading to a higher risk of lipid peroxidations, especially when tocopherol supply is not increased accordingly. EFSA report does not provide DRIs for total PUFA, but for specific fatty acids.
n-6 PUFAs: LA
Table 3 shows recommendations for n-6 PUFA, LA, n-3 PUFA, EPA and DHA for adults in the different sets compared and Table 4 shows recommendations for infants and children(5, 7, 8, 12–Reference Harris, Mozaffarian and Rimm15, Reference Gidding, Dennison and Birch17–19, Reference Kris-Etherton and Innis25–Reference Alasfoor, Rajab and Al-Rassas38, Reference Koletzko, Cetin and Brenna71, Reference Wolfram79).
Table 3 Recommendations for Polyunsaturated Fatty Acid intakesa in adults according to different bodies

LA: linoleic acid; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid; ALA: alpha-linolenic acid. %E: % Energy intake.
AI: Adequate Intake; AMDR: Acceptable Macronutrient Distribution Ranges.
M: males; F: Females.
a Recommendations for n-6, n-3 and ALA are expressed either as percent energy intake or g/day.
b Region: Continent or region where recommendations apply.
c WHO supports recommended intake levels of EPA+DHA of 400–1000 mg/day. This intake levels can be achieved by regular consumption of fish (1–2 servings of fish/week).
d AHA recommendation of EPA+DHA intake for primary prevention is 500 mg/day. This intake levels can be achieved by regular consumption of fish (2 portions of fish/week). For secondary prevention recommended intake level is 1 g/d.
e According to these documents, recommended intake levels of EPA+DHA (450 mg/day) should be achieved by regular fish consumption, as two servings of fish weekly.
Table 4 Recommendations for polyunsaturated fatty acid intakesa in infants and children according to different bodies

U-AMDR: Upper limit of Acceptable Macronutrient Distribution Ranges.
LA: linoleic acid; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid; ALA: alpha-linolenic acid. %E: % Energy intake; HM: human milk.
M: males; F: Females.
a Recommendations for n-6, n-3 and ALA are expressed either as percent energy intake or g/day.
Not all the documents include recommended intakes for LA. There is a wide variation in the recommendations for LA intake for adults between the documents including this value. The lowest limit varies from 2 %E to 6 %E, while for the upper limit varies from 5 to 10 %E. Some documents provide a single value of adequate intake, established between 2 %E and 4 %E. The limited information available on LA intake shows a wide variation between population groups.
The FAO/WHO 2008 report(8) and IOM report(5) include AMDR, EAR and AI values for n-6 PUFAs. AI for LA is based on median intakes in the USA or lowest mean intakes in Europe.
According to the FAO/WHO 2008 report, the resulting acceptable range for n-6 fatty acids (LA) is between 2·5 and 9 %E, considering the maximum intake levels of PUFAs (11 %E) and n-3 fatty acids (2 %E). The lower value (2·5 %E) is established taking into account the prevention of deficiency symptoms, whereas the higher value can be part of a healthy heart diet by lowering LDL and TC levels, and therefore the risk for CHD(8).
The American Heart Association (AHA)(Reference Harris, Mozaffarian and Rimm15) recommended intakes of at least 5–10 %E from n-6 PUFA in order to reduce CHD risk, based on evidence from intervention studies with increased LA intake. Controversy raised based on concerns about lipid peroxidation or animal models suggesting that high intakes of LA could promote some cancers and increased likelihood of inflammation and thrombosis. In 2010 a systematic review of the effects of n-6 PUFA and LA in particular reported that n-6 PUFAs lower total and LDL concentrations, and do not adversely affect blood pressure, platelet aggregation, or inflammation and concluded that an n-6 PUFA intake above 5 % of energy and ideally about 10 % of energy should be consumed(Reference Czernichow, Thomas and Bruckert80).
Ramsden et al.(Reference Ramsden, Hibbeln and Majchrzak81), re-evaluated randomized controlled dietary interventions that increased LA intake and pointed out that quite a number of the trials of n-6 PUFAs actually also increased the intake of the plant n-3 PUFA ALA acid or of marine n-3 PUFAs and restricted the intake of trans-fatty acids. Results of the analysis of four combined data sets from three trials of enhanced LA intake showed a 13 % increase in relative risk of nonfatal myocardial infarction (MI) and coronary heart disease mortality and a 17 % increase in relative risk of coronary heart disease mortality. The authors concluded that the advice to maintain or increase intake of n-6 PUFAs should be reconsidered and analyze the evidence in depth.
Calder and Deckelbaum further discussed this issue and highlighted the need for fatty acid-specific advice based upon the fatty acid-specific evidence base and the need to conduct appropriate studies to fill in the gap(Reference Calder and Deckelbaum82, Reference Deckelbaum and Calder83).
n-3 Fatty acids: ALA, DHA, EPA
EFSA as well as other organizations do not establish recommended intakes for n3-PUFAs but for specific n-3 fatty acids, namely ALA, EPA and DHA(Reference Gidding, Dennison and Birch17), or even for docosapentaenoic acid DPA(35). EFSA sets and adequate intake value (AI) of 0·5 %E for ALA for all population groups, adults, children, pregnancy and lactation. The AI for the sum EPA+DHA according to this set of recommendations is 250 mg/day for adults; 100 mg/day DHA for children aged 7–24 mo. And for pregnancy and lactation, the recommended intake for adults (EPA+DHA = 250 mg/d) should be supplemented by 100–200 mg/d DHA. Governments (France, Belgium, UK, The Netherlands, New Zealand and Australia) and health organizations (FAO/WHO, American Dietetic Association, American Heart Association) now recommend dietary intakes for total n-3 PUFA of 1·4 to 2·5 g/d, with EPA and DHA ranging from 140 to 600 mg/d depending on the authority issuing guidelines(Reference Molendi-Coste, Legry and Leclercq84).
Recommendations by FAO/WHO 2008 advice and AMDR value for n-3 PUFA (ALA+long chain n-3 FA) of 0·5–2 %E for adults and 0·250–2 g/d for EPA+DHA(8).
According to the IOM, AMDR for ALA is 0·6–1·2 %E for adults, ensuring that the lower limit meets the AI for ALA, while the upper limit corresponds to the highest ALA intake from food consumed by individuals in the USA and Canada(Reference Koletzko, Uauy and Palou72). It is advised that approximately 10 % of the AMDR for ALA can be consumed as EPA and 7 % DHA. The upper AMRD for n-3 PUFA is by 3 g/day, because high supplement intakes of n-3 PUFAs have been demonstrated to reduce cytokine production and increase lipid peroxidation(Reference Elmadfa and Kornsteiner40).
The American Heart Association distinguishes between people without disease (500 mg/day EPA and DHA), with documented coronary artery diseases (1 g/day), and hypertriglyceridemic patients, who benefit from a high dosage with 3–4 g per day of EPA and DHA from fish oil(Reference Harris, Mozaffarian and Rimm15).
The Dietary Guidelines for Americans advise to increase consumption of n-3 fatty acids by consuming two servings of seafood per week which provide an average of 250 mg/day of omega-3 fatty acids from marine sources. These reports note there is limited evidence suggesting an association between consumption of fatty acids in fish and reduced risk of mortality from cardiovascular disease for the general population, and other sources of EPA and DHA may provide similar benefits, although more research is needed(18).
Recommendations from different organizations, for primary prevention of coronary disease, advice consumption of at least two servings of fish per week. Recommendations for long chain n-3 FA (EPA+DHA) vary between 250 and 667 mg/d.
In older infants, DHA intakes at levels of 50 to 100 mg per day have been found effective for visual function in the complementary feeding period and are considered to be adequate for that period. An AI of 100 mg DHA for older infants (>6 mo.) and young children below the age of 24 months. The currently available evidence does not permit the definition of an age specific quantitative estimate of an adequate dietary intake for EPA and DHA for children aged 2 to 18 years. However, dietary advice for children should be consistent with advice for the adult population(Reference Koletzko, Cetin and Brenna71, Reference Koletzko, Uauy and Palou72, Reference Lapillonne and Jensen73).
Evidence supporting recommendations for adults is mostly related to primary and secondary prevention of CVD. ALA consumption is suggested to probably reduce CHD risk. Although clinical benefits are not consistent in all studies, new experimental and prospective observational studies support that ALA consumption reduces the incidence of CHD. Physiological benefits of EPA and DHA consumption have been demonstrated on blood pressure, heart rate, triglycerides, and likely inflammation, endothelial function, and cardiac diastolic function, and consistent evidence for a reduced risk of fatal CHD and sudden cardiac death at consumption of around 250 mg/day of EPA plus DHA. A minimum intake of 250 mg/day of EPA and DHA, obtained from seafood consumption, has been suggested for primary prevention of CHD death(Reference Mozaffarian and Rimm61).
In 2002, the IOM concluded that insufficient data were available to set an RDA or an AI for EPA and/or DHA. An estimated average requirement was not established because of the lack of data in support of an (n-3) PUFA requirement for healthy individuals and for adequate growth and neural development(Reference Brenna70, Reference Kris-Etherton, Grieger and Etherton85).
The data from the secondary and primary prevention studies support the theory that the intake of n-3 PUFAs reduces all-cause mortality, cardiac and sudden death, and stroke(Reference Wang and Harris86). n-3 PUFAs appear to confer cardiovascular health benefits mainly through EPA and DHA enrichment of membrane phospholipids. The observational evidence that a strong inverse relationship is present between n-3 PUFAs or fish intake and risk of CHD is convincing; three well-designed randomized controlled trials (GISSI(69), DART I(Reference Ness, Hughes and Elwood87) and JELIS(Reference Yokoyama, Origasa and Matsuzaki88) reported significant benefits for n-3 PUFAs in patients with established CHD. The conclusions of some recent meta-analysis support these findings(Reference Skeaff and Miller59, Reference Marik and Varon89).
However, other studies such as DART-2(Reference Burr, Dunstan and George90); OMEGA trial(Reference Rauch, Schiele and Schneider91), the Alpha Omega trial(Reference Kromhout, Giltay and Geleijnse92) and meta-analysis including them have not reported a beneficial effect(Reference Hooper, Thompson and Harrison65, Reference Filion, El Khoury and Bielinski93). Some of these studies have been criticized for ignoring baseline n-3 PUFA dietary intake, n-6 PUFA or antioxidant intake, among other reasons. Unfolding evidence contributes to the debate with new trials supporting that n-3 PUFAs may exert beneficial effects in the prevention of atrial fibrillation(Reference Nodari, Triggiani and Campia94), but others yielded conflicting results(Reference Liu, Korantzopoulos and Shehata95). The benefits of n-3 PUFAs on neurological functions are recognized, although more research is needed in this area(Reference Crawford, Bazinet and Sinclair96).
In most populations, current intakes of PUFA and especially n-3 PUFA are insufficient for optimal health(Reference Elmadfa and Kornsteiner40). Overall, the evidence about seafood-derived n-3 PUFA is convincing that modest consumption of fish or fish oil reduces CHD death, and may favourably affect other clinical outcomes. Regarding plant-derived n-3 PUFA, there is currently only possible evidence that ALA consumption prevents CVD.
The evidence supporting recommendations for pregnant and lactating women is based on optimal pregnancy outcomes and on possible beneficial effects on foetal and infant development. Currently there is a lack of sufficient evidence to link levels of dietary intake of DHA and/or EPA to improved physical, mental or other functional benefits in children. Quantitative dietary intake recommendations for children cannot be established at this time(Reference Brenna70).
One of the main considerations when trying to meet current recommendations for n-3 fatty acids is that current intake of PUFAs consists primarily of n-6 fatty acids. The competition for desaturases and elongases in n-3 and n-6 PUFA metabolism results in inverse effects on tissue concentrations of these fatty acids. This is of even greater concern in vegetarians and vegans, who have relatively high intakes of LA combined with low intakes of EPA and DHA(Reference Kornsteiner, Singer and Elmadfa97). Another consideration for meeting recommendations for marine-derived n-3 fatty acids is that some populations do not consume fish because of concerns about environmental toxins, taste preferences, or ethical reasons. Studies have shown that certain fish contain high levels of environmental toxins, such as mercury and polychlorinated biphenyls (PCBs), a challenge faced when increasing dietary fish intake. Of particular importance is the neurologic damage to developing foetuses and young children caused by toxic levels of methylmercury(Reference Gebauer, Psota and Harris51).
In this sense the European Food Safety Authority and the joint FAO/WHO expert consultation concluded that women of childbearing age consuming two fish servings per week should generally not exceed the provisional tolerable weekly intake of environmental pollutants(98). The US Food and Drug Administration advise following recommendations about consumption of certain fish high in mercury because of potential harmful effects to the foetus or infant(99).
n-6 PUFA:n-3 PUFA ratio
A lower ratio of n-6 to n-3 fatty acid consumption has been recommended under the assumption that higher intakes of n-6 fatty acids may reduce the formation of anti-inflammatory mediators from n-3 fatty acids(Reference Simopoulos67). However, this hypothesis is not supported by studies in humans(Reference Simopoulos68). At moderate intakes of 5 %–8 % E, increasing LA intake does not result in increased arachidonic acid in plasma or platelet lipids, and does not increase formation of proinflammatory mediators(Reference Wijendran and Hayes100). The advisory published by the American Heart Association (AHA) on n-6 fatty acids and cardiovascular risk(Reference Harris, Mozaffarian and Rimm15) in 2009 stating that ‘consumption of at least 5 % to 10 % of energy from n-6 PUFAs reduces risk of CHD relative to lower intakes’ raised the controversy.
A focus on dietary ratios suggests that lowering n-6 fatty acid intake would have the same health effects as increasing n-3 fatty acid intake. Based on both evidence and conceptual limitations, there is no scientific rationale for the continued recommendation of a specific ratio of n-6 to n-3 fatty acids or LA to ALA. The focus on ratios, rather than on levels of intake of each type of PUFA, has many conceptual and biological limitations(Reference Calder and Deckelbaum82).
The use of the n-6 to n-3 fatty acid ratio ignores the contribution of each class of fatty acid and even individual fatty acids. This approach assumes that all n-6 fatty acids and all n-3 fatty acids are biologically equivalent to one another which is not correct(Reference Akabas and Deckelbaum62, Reference Ramsden, Hibbeln and Majchrzak81, Reference Burdge and Calder101), and supports that the actions of n-6 and n-3 fatty acids always oppose one another(Reference Calder and Deckelbaum82). The bulk of current evidence suggests that it is the absolute intakes of specific n-6 and n-3 PUFA that are associated with many different endpoints(Reference Hibbeln and Davis102–Reference Calder104). Thus, considerations about adequate intakes for both n-3 and n-6 fatty acids are relevant. The use of a ratio can disguise extremely low or very high intakes of n-6 and/or n-3 fatty acids(Reference Deckelbaum and Calder83).
Trans
Overall, recommendations for trans fatty acids intake advice to limit intake below 1 %E or to keep trans FA intake as low as possible.
Evidence supporting these recommendations is based on the effects of trans fatty acids on plasma lipids and cardiovascular disease. Trans fatty acid consumption has adverse effects on serum lipids, including increasing LDLC, lowering HDL-C, increasing TGs and lipoprotein(a), increasing ApoB levels, and decreasing ApoA1 levels. Moreover, they adversely influence LA and ALA metabolism and prostaglandin balance by inhibiting the enzyme delta 6-desaturase(Reference Mozaffarian and Willett105–Reference Eckel108).
In the USA average adult's daily ruminant trans fatty acid intake of both men and women is about 1·2 g, which correspond to 0·5 %E. If similar average intake values from industrially hydrogenated fat could be anticipated, then the trans fatty acid intake from all sources should be limited to 1 %E.
The Food and Drug Administration and EFSA have required that trans-fat be listed on the nutrition labelling. However, manufacturers are allowed to list foods with trans-fat content less than 0·5 g as 0 g of trans-fat. People may see a few products that list 0 g trans-fat on the label, while the ingredient list will have ‘shortening,’ ‘partially hydrogenated vegetable oil,’ or ‘hydrogenated vegetable oil’ on it. Nutrition education on label reading and portion control is essential in keeping trans-fat intake as low as possible(Reference Marcanson109).
Cholesterol
Most documents do not include a recommended intake for cholesterol. When advised, the recommended daily intake level is below 300 mg/day. The FAO/WHO 2008 report do not include this recommendation. The EFSA report and many countries, such as Canada, Korea, New Zealand and India do not set an upper limit for dietary cholesterol, focusing instead on controlling the intake of saturated fat and trans fat, which are the major determinants of blood cholesterol concentrations. Epidemiologic studies and clinical trial results suggest that compelling evidence is lacking for limiting cholesterol intake to 300 mg/d(Reference Fernandez and Calle110).
Dietary guidelines
Most reports advice to control total calorie intake to manage body weight; to choose lean meat cuts, low fat dairy products and increase consumption of fruit and vegetables, whole-grain cereals and fish.
Recommendations tend to focus on general dietary patterns and increased physical activity rather than on nutrients. During past decades, advice to decrease fat intake may have induced unwanted dietary changes that may have contributed to the increase in overweight and obesity. It is the sum of all dietary and lifestyle changes that produces the intervention's net health effects. To avoid this possibility in the future, specific and transparent classification of the quality of the evidence should form the basis for guidelines development. Dietary guidelines need explicit standards of evidence. The strengths and limitations of evidence should be provided in easy-to-understand language.
An adaptation of evidence ratings has been suggested which consider both the quality of the evidence and the net benefit of an intervention. The net benefit recognizes the potential for harm. The process includes a broader category for which no recommendation should be made, intended to provide the appropriate level of caution in the process of issuing guidelines(Reference Marantz, Bird and Alderman42).
Conclusions
Recommendations regarding fat intake share similar figures regarding total fat intake, SFA and TFA. Asian recommendations considering prevailing food and health patterns in the region, advice lower total fat intake. Many sets do not include a recommendation on cholesterol intake. Most recent documents provide advice regarding n-3 PUFA, ALA, EPA and DHA. Despite efforts to develop evidence based nutrient recommendations and dietary guidelines that may contribute to enhance health, there are still many gaps in research that need to be adequately addressed. In particular, further assessment of specific effects of DHA and EPA during pregnancy, lactation and infancy should be conducted on neuropsychological function, immune response, and rates of infection in the infant. There is limited information on dose-response effects of EPA and DHA on CHD, inflammation and immune response and even a large intervention trial on n-3 PUFA and primary prevention of CHD and metabolic syndrome.
The World Cancer Research Found (WCRF) and the American Institute for Cancer Research (AICR) published in 1997 a comprehensive analysis of the literature on diet, physical activity and cancer and second expert report on 2007(43). Since then a continuous update project has been established(111).
In line with this, as part of implementing the outcomes and recommendations of the WHO Nutrition Programme Review undertaken in 2008, the Department of Nutrition for Health and Development (NHD) established the WHO Nutrition Guidance Expert Advisory Group (NUGAG) guided by the WHO Steering Committee for Nutrition Guidelines Development. NUGAG started in 2010 and includes four working groups: 1) micronutrients, 2) diet and health, and 3) nutrition in life course and undernutrition and 4) monitoring and evaluation. The topics to be addressed by second subgroup, diet and health, include the effects of the level of total fat intake on obesity and other related noncommunicable diseases (NCDs) and the effects of sugars intake on health(112). The group held a third meeting in Geneva in March 2011.
In the near future, it would be desirable that all documents including nutrient recommendations and dietary guidelines follow standardized protocols that can contribute to provide solid evidence background to the recommendations. Additionally, it would be desirable that responsible bodies and organizations could plan timelines for regularly updating recommendations on light of the emerging evidence.
Acknowledgements and disclosures
The preparatory meetings for this series of reviews on fat and health were funded by Puleva Food. Neither Javier Aranceta nor Carmen Pérez-Rodrigo have conflicts of interest to disclosure.
Javier Aranceta and Carmen Pérez-Rodrigo contributed to the design of the strategy for the literature search, double screened and selected the retrieved documents. Javier Aranceta prepared the main outline of the manuscript. Javier Aranceta and Carmen Pérez-Rodrigo contributed to the preparation of the manuscript.







