Proteins and peptides in human milk are important factors that affect infant growth and development. Most of the proteins and peptides have specific properties in addition to the newborn’s nutrition; these include disease protection and stimulation of the infant’s immune system. Neonates are vulnerable to infectious diseases during the first weeks of their life, and infections are a leading cause of neonatal morbidity and mortality. Thus, breast-feeding is the primary method of protecting a neonate against infections during this period. Additionally, breast milk also provides important immune factors for the development of an infant’s immune system(Reference Walker1). This is supported by studies indicating that early breast-feeding helps to reduce the neonatal mortality rate due to the protective components of breast milk(Reference Edmond2, Reference Turfkruyer and Verhasselt3). Maternal antibodies are transferred to the breast-fed infant by a polymeric immunoglobulin receptor through mammary epithelial cells; these are mainly represented by IgA and IgM(Reference Walker1–Reference Jennewein, Abu-Raya and Jiang4). The milk protein fraction contains bioactive peptides that were probably synthesised as a result of selective proteolysis; it has numerous functions, including anti-inflammatory activity, regulation of gastrointestinal function and antimicrobial activity(Reference Lönnerdal5–Reference Nongonierma and FitzGerald8).
Breast milk composition is influenced by several factors such as the age of the mother, prematurity, maternal diet, health status, lactation period, among others(Reference Calil, Leone and Ramos9–Reference Hila, Neamtu and Neamtu11). The influence of maternal age on milk composition raised interest in investigating lactation during adolescence, especially in countries where teenage pregnancy has been considered a public health problem. Previous studies have concluded that teenage pregnancy may have negative effects on both the mother and the infant, as the adolescent mother might not be physiologically mature to fully perform reproductively. These negative effects include low birth weight, preterm delivery and even maternal death(Reference Field10–Reference Kaplanoglu, Bülbü and Konca15). Furthermore, lactation during adolescence has been associated with reduced milk production and higher milk Na concentrations when compared with adult lactating women(Reference Motil, Kertz and Thotathuchery16, Reference Sipsma, Magriples and Divney17). Despite the large number of adolescent mothers worldwide, little is known about the physiological behaviour or the composition of their milk. This is the case even in Brazil.
Studies on the protein concentration of adolescents’ breast milk had controversial results, and recent data suggested that the protein concentrations in breast milk are influenced by the lactation period(Reference Pereira, Blondet De Azeredo and Barros Da Sileira18). The characterisation of the proteome of adolescent breast milk brings important information regarding both the mother’s and the infant’s health; nonetheless, it has not been investigated. Here, we aimed to investigate whether the proteome and peptidome of mature breast milk are influenced by the lactation period by comparing between two groups of adolescent mothers approximately 3 weeks (early mature milk) or approximately 5 weeks (advanced mature milk) postpartum. We hypothesised that early mature milk would have different protein and peptide composition than advanced mature milk, and this would be particularly relevant if proteins related to immune functions were also distinct. We used a combination of 1D-electrophoresis, nano-scale LC-quadrupole time-of-flight MS/MS (nLC-Q-TOF-MS/MS) analyses and bioinformatics tools to explore the proteome and peptidome of human skimmed milk expressed in these two groups of lactating adolescent mothers. Association maps showing interactions between peptides in breast milk were also used to determine the potential bioactivity of these peptides.
Subjects and methods
Collection of milk samples
Milk samples were obtained from twelve primiparous adolescent mothers (aged 14–19 years) with no diagnosed chronic diseases or complications during pregnancy. Pregnant adolescents who attended the Maternity School of the Federal University of Rio de Janeiro (UFRJ) in Brazil for prenatal care were invited to participate in the present study. The Maternity School of the UFRJ in Brazil is a national reference clinic for adolescent pregnancy prenatal care. The present study was approved by the UFRJ Maternity School ethics committee (protocol number CAAE: 0002.0361.000-09). Prior to the study enrolment, all the participants gave written informed consent signed by them (aged ≥ 18 years) or their parents or legal guardians (aged < 18 years). Participants were 21–51 d postpartum, exclusively breast-feeding and had given birth to healthy full-term infants. Samples were collected between September 2009 and June 2011.
Milk samples were divided into two groups according to postpartum period, approximately 3 weeks (early mature milk) and approximately 5 weeks (advanced mature milk), in order to evaluate the influence of the lactation period on the variables assessed. Breast milk sampling was carried out in the morning, prior to feeding (foremilk). The breasts and hands were thoroughly cleaned with deionised water prior to each sampling. Milk (at least 10 ml) was sampled by manual expression into disposable polypropylene tubes. Maternal and neonatal characteristics of the two groups are shown in Table 1. After collection, milk samples were aliquoted and stored at −80°C until further analysis. Samples were chosen randomly for microbiological analysis by standard methods to verify that the growth of micro-organisms was limited within acceptable levels for banked human milk. Immediately before analysis, milk samples were thawed in a 37°C water bath and then protease inhibitors, 1 mol/l phenylmethanesulfonyl fluoride, were added to decrease protein hydrolysis. The milk was centrifuged at 1500 g for 20 min at 4°C, to remove the cream layer(Reference Keller and Neville19), then the skimmed milk was collected and the pellet discarded (Fig. 1).
Table 1. Characteristics of Brazilian lactating adolescents and their newborns, according to lactation period (Mean values and standard deviations; numbers of infants)
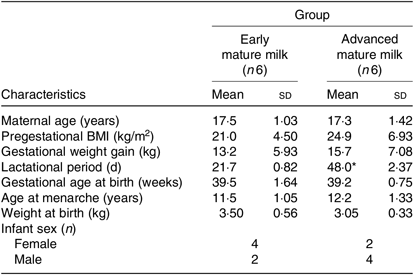
* Mean value was significantly different from that of the early mature milk group (P < 0·05; Student’s t test).
Measurement of protein concentration
The protein concentration was determined according to the Lowry(Reference Lowry, Rosebrough and Farr20) protein assay, using bovine serum albumin as a calibration standard.
Investigation of the general protein profile of milk by SDS-PAGE and digestion
The proteins were separated and analysed on a 12 % SDS-PAGE(Reference Laemmli21) to compare the protein profile between the milk of the two groups of volunteers, that is, early mature milk and advanced mature milk. Approximately 20 µg of protein from each milk sample was mixed 1:1 with Laemmli buffer. Each sample was run independently on a gel in parallel with the molecular weight standards, and blank lanes were filled with buffer; and each day one sample from early and one from advanced mature milk groups were randomly selected for SDS-PAGE analysis. Electrophoresis was carried out at 200 V for 2 h in 25 mmol/l Tris-HCl, 200 mmol/l glycine and 0·1 % (w/v) SDS, and Coomassie Blue Silver(Reference Candiano, Bruschi and Musante22) was used to stain the gels. The gels were analysed using ImageJ software (http://imagej.nih.gov/ij; National Institutes of Health) for densitometric quantification of protein bands. Gel pieces were excised into 1·0 mm bands based on the molecular weight (corresponding to appropriate standards run in parallel in each run) and washed three times using 25 mmol/l ammonium carbonate–acetonitrile (ACN) (1:1, v/v) for 30 min. After all the wash solution was removed, the samples were incubated with ACN for 20 min at 25°C. Following the removal of ACN, the bands were dried in a vacuum centrifuge. The bands from gels were reduced by incubation with 100 mmol/l dithiothreitol and 25 mmol/l NH4HCO3 for 1 h at 56°C and alkylated by the addition of 50 mmol/l iodoacetamide and 25 mmol/l NH4HCO3 for 30 min in the dark and dried again. The gel pieces were treated with 20 µg/ml trypsin (Promega, modified sequencing grade) prepared in 25 mmol/l NH4HCO3 and incubated overnight for 20 h at 37°C. Tryptic peptides were extracted twice with 50 μl of 0·1 % (v/v) trifluoroacetic acid (TFA) in 50 % (v/v) ACN for 20 min with ultrasonication. The supernatants were pooled, dried and then dissolved in 0·1 % (v/v) formic acid (FA) in ultrapure water for MS analysis.
Purification and concentration of tryptic peptides (or hydrolysis derivatives)
C18 ZipTip pipette tips (Millipore) were used for desalting and concentrating the peptides prior to electrospray ionisation quadropole time-of-flight MS (ESI-Q/TOF MS) analysis. The peptides were eluted with 50 % ACN/0·1 % TFA solution and concentrated in a centrifuge concentrator (Speed-vac; Savant Instruments).
Analysis of tryptic peptides by nano-LC-electrospray ionisation quadropole time-of-flight
An aliquot containing 7·5 µl of each sample was loaded on Waters CapLC™ system (Waters). The nLC separations were performed using a nano-Ease C18 150 mm × 75 μm column (Waters) and eluted using a linear gradient (10–50 %) of ACN containing 0·1 % FA, at 0·2 µl/min. Peptides were eluted using solutions A (0·1 % FA) and B (0·1 % FA in ACN) in a gradient (0–20 min, 3 % B; 20–70 min, 3–35 % B; 70–85 min, 35–50 % B; 85–95 min, 50 % B; 95–100 min, 50–10 % B). The exact mass from peptides (MS) and fragments (MS/MS) were automatically determined using the Q-TOF’s LockSpray™ (Waters). The MS/MS acquisitions were performed on precursors with charge states of 2, 3 or 4 over a range of 50–2000 m/z and within a 2-m/z window. The resulting MS/MS spectra were searched to identify peptides using MASCOT software (http://www.matrixscience.com; Matrix Science). The following parameters were used: carbamide methylation as a fixed modification; acetylation at the N-terminus; deamidation of asparagine and glutamine; di-oxidation of methionine; oxidation of methionine, histidine and tryptophan; phosphorylation of serine, threonine and tyrosine as variable modifications; one trypsin missed cleavage and a tolerance of 10 parts per million for precursor and 1 Da for the fragment ions.
Preparation of peptide ultrafiltrate and determination of peptide concentration
An aliquot of skimmed milk (1·5 ml) from each sample was ultrafiltered through Microcon concentrators (YM-10K; Millipore) to separate the peptide fractions (<10 kDa) from proteins (>10 kDa), and they were then centrifuged for 20 min at 14 000 g and 4°C. The peptide content in the filtrate (<10 kDa) was determined by the Qubit™ Quantitation Fluorometer.
Analysis of endogenous peptides by nano-LC-electrospray ionisation quadropole time-of-flight
The endogenous peptides were identified from MS/MS spectra using the Mascot Search (http://www.matrixscience.com) by correlation of tandem mass spectra to that of the spectra hosted on the National Center for Biotechnology Information database. The following search parameters were used: search type, MS/MS ion search; enzyme, no cleave; taxonomy, Homo sapiens; variable modifications, methionine oxidation, phosphorylation of serine and threonine, N-terminus acetylation, C-terminus amidation; mass values, monoisotopic; protein mass, unrestricted; mass tolerance of peptides, ±0·2 Da; fragment mass tolerance, ±0·2 Da; instrument type, ESI-QUAD-TOF. Protein Blast searches were used to confirm the sequences.
Bioinformatics
The functional classification of proteins was achieved using the database Protein Knowledgebase (UniProtKB) (http://www.uniprot.org/). In the enriched biological process, we used Gene Ontology (GO) analysis, and the canonical pathway enrichment analysis was based on Kyoto Encyclopedia of Genes and Genomes pathway database.
Statistical analysis
The results are shown as means and standard deviations. Student’s t test was performed to determine the differences between the two groups according to lactational period (early or advanced mature milk). Two-tailed P values < 0·05 were considered as statistically significant. Statistics was performed using Prism® (GraphPad Prism Software).
Results
Maternal and neonatal characteristics, and protein and peptide concentrations in breast milk according to lactational period
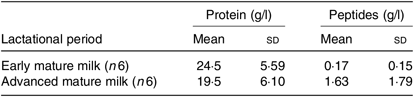
Maternal age, duration of gestation, age at menarche and neonatal characteristics were similar between adolescents with regard to early and advanced mature milk (Table 1). The only statistically significant difference between groups was the lactational period. Additionally, no statistical significant differences were observed in protein and peptide contents between adolescents’ early and advanced mature breast milk (Table 2).
Table 2. Total protein and peptide content in breast milk from adolescent mothers at early (approximately 3 weeks) and advanced mature milk (approximately 5 weeks) (Mean values and standard deviations)
Protein analysis
The protein profile of skimmed milk was analysed by 1D-SDS-PAGE and the proteins were visualised by Coomassie staining (Fig. 2). A total of twelve SDS-PAGE gels were run to analyse all samples; in each gel, protein standards were run in parallel for qualitative (as molecular weight standards) and quantitative purposes. The concentration of the proteins in the standard mixtures was used to normalise the amounts of proteins in the samples, based on the optical density determined by the Image J software. Gel slices were excised according to molecular weight, subjected to in-gel digestion and then the extracted peptides were analysed by LC-MS/MS. We identified a total of 1317 proteins, of which 424 were non-redundant proteins (online Supplementary Table S1) and 137 were found in both groups. Densitometric analysis revealed significant variation in the relative intensities of the bands in the following molecular weight ranges: 75–150 kDa, 50–75 kDa, 25–37 kDa, 20–25 kDa and <20 kDa (online Supplementary Fig. S1).
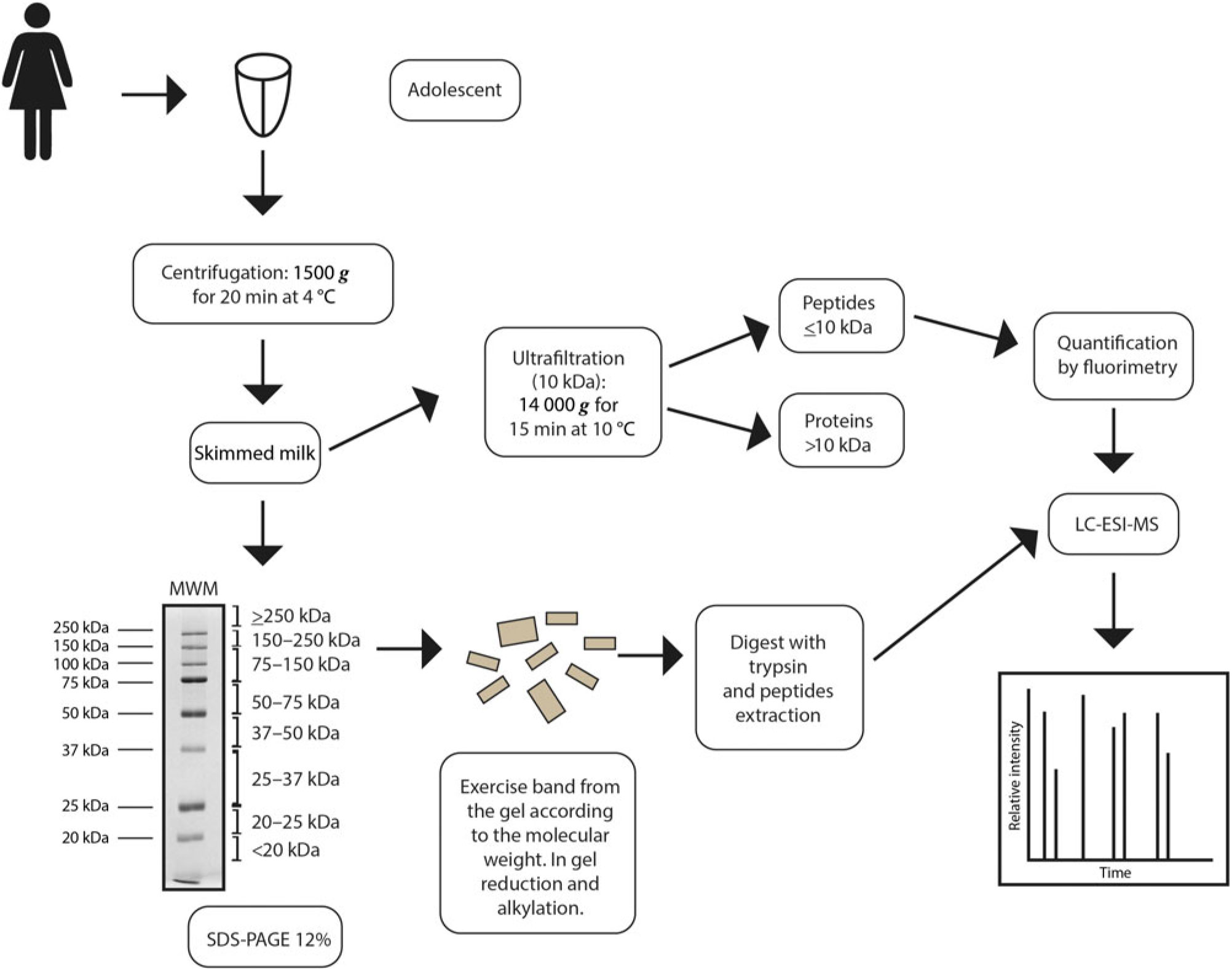
Fig. 1. Diagram showing mature human milk sample preparation, and proteome and peptidome analyses by nano-scale LC-quadrupole time-of-flight MS/MS (nLC-Q-TOF-MS/MS). MWM, molecular weight marker; ESI, electrospray ionisation.
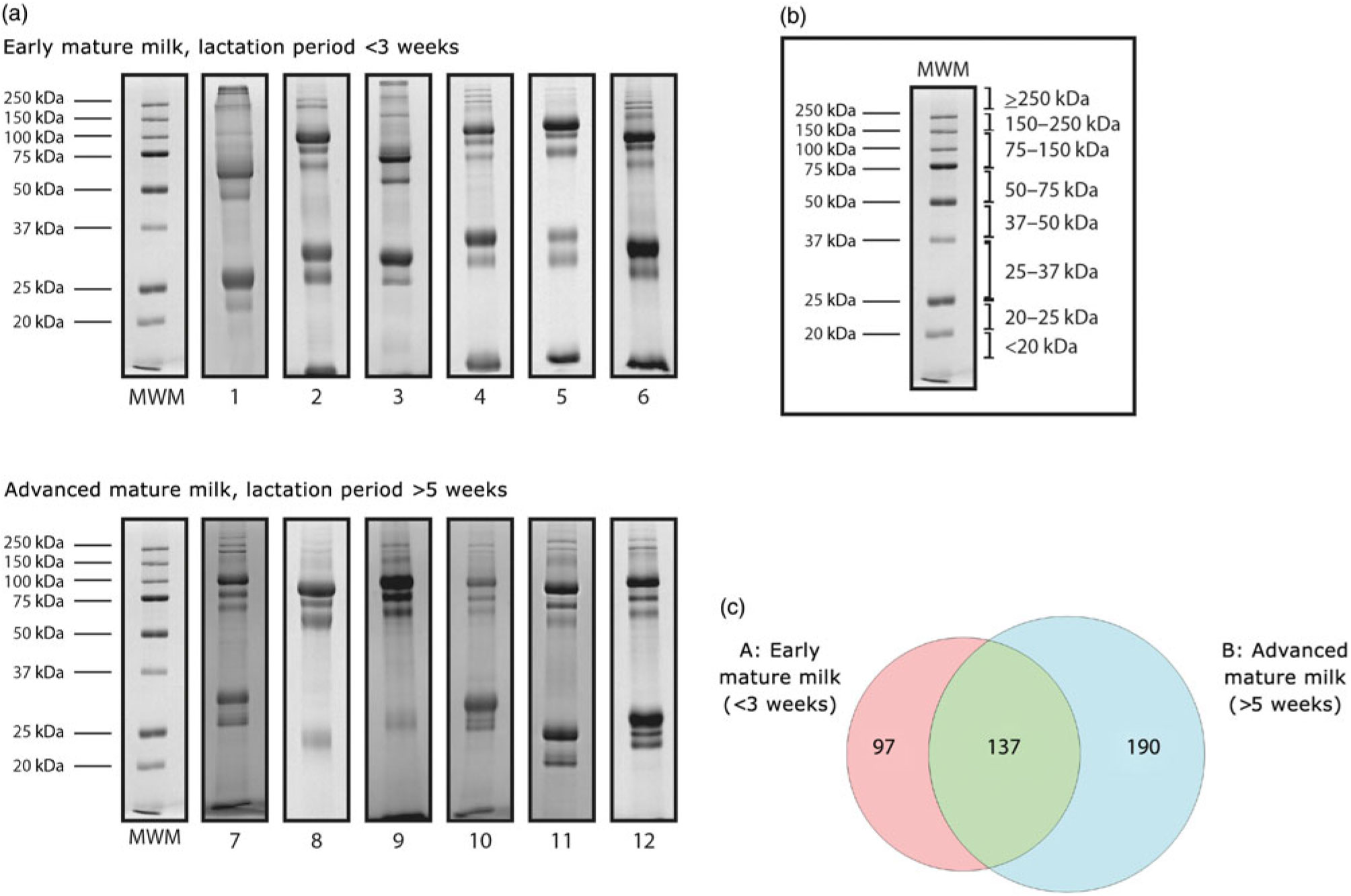
Fig. 2. (a) SDS-PAGE electrophoretograms of twelve mature human skimmed milk samples from adolescent mothers giving birth at term. A quantity of 20 μg of the total proteins was run through a 12 % SDS-PAGE, and the gel was stained with Coomassie Blue. (b) Gel pieces were excised according to molecular weight for MS analyses. MWM, molecular weight marker. (c) Venn diagram of proteins identified by nano-scale LC-quadrupole time-of-flight MS/MS (nLC-Q-TOF-MS/MS) in the two groups: early mature milk (approximately 3 weeks) and advanced mature milk (approximately 5 weeks).
Peptide analysis
Endogenous peptides originating from skimmed milk obtained from twelve adolescent mothers were concentrated by ultrafiltration (cut-off 10 kDa) and identified using MS/MS. The most abundant milk proteins such as casein, lactoferrin and α-lactalbumin were not represented in the peptide fragments. A total of 162 peptide sequences were obtained, and redundant proteins were excluded from the list (online Supplementary Table S2).
A parallel experiment was carried out to confirm that the protocol used was more appropriate for the analysis of less abundant endogenous peptides. Milk proteins were precipitated by TCA and, after centrifugation, the peptides in the supernatant were analysed by LC-MS/MS, by following the same protocol as the one used for peptides isolated by ultrafiltration. In this acid-precipitation experiment, peptides originating from β-casein were the most abundantly detected (data not shown), thus suppressing the detection of endogenous peptides derived from proteins with lower abundance.
Analysis of protein–protein interaction network
In order to better understand the molecular mechanisms underlying the biological potential of peptides and proteins present in skimmed breast milk from adolescents, we performed a functional enrichment analysis in our networks using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) algorithm(Reference Szklarczyk, Franceschini and Wyder23), to build protein–protein interaction networks. Comparisons were made by overlaying the generated networks with the overrepresented biological functions found in the GO analysis. Abbreviations used for protein names in the networks are presented in online Supplementary Table S3. Fig. 3(a) represents protein–protein interactions in the early mature milk group (gestational age up to 3 weeks), while Fig. 3(b) presents protein–protein interactions in the advanced mature milk group (gestational age over 5 weeks of lactation). Protein functions have been summarised in Fig. 3(c) and (d). Fig. 4(a) depicts the interaction networks of peptides identified in both groups, and Fig. 4(b) presents their respective functions.
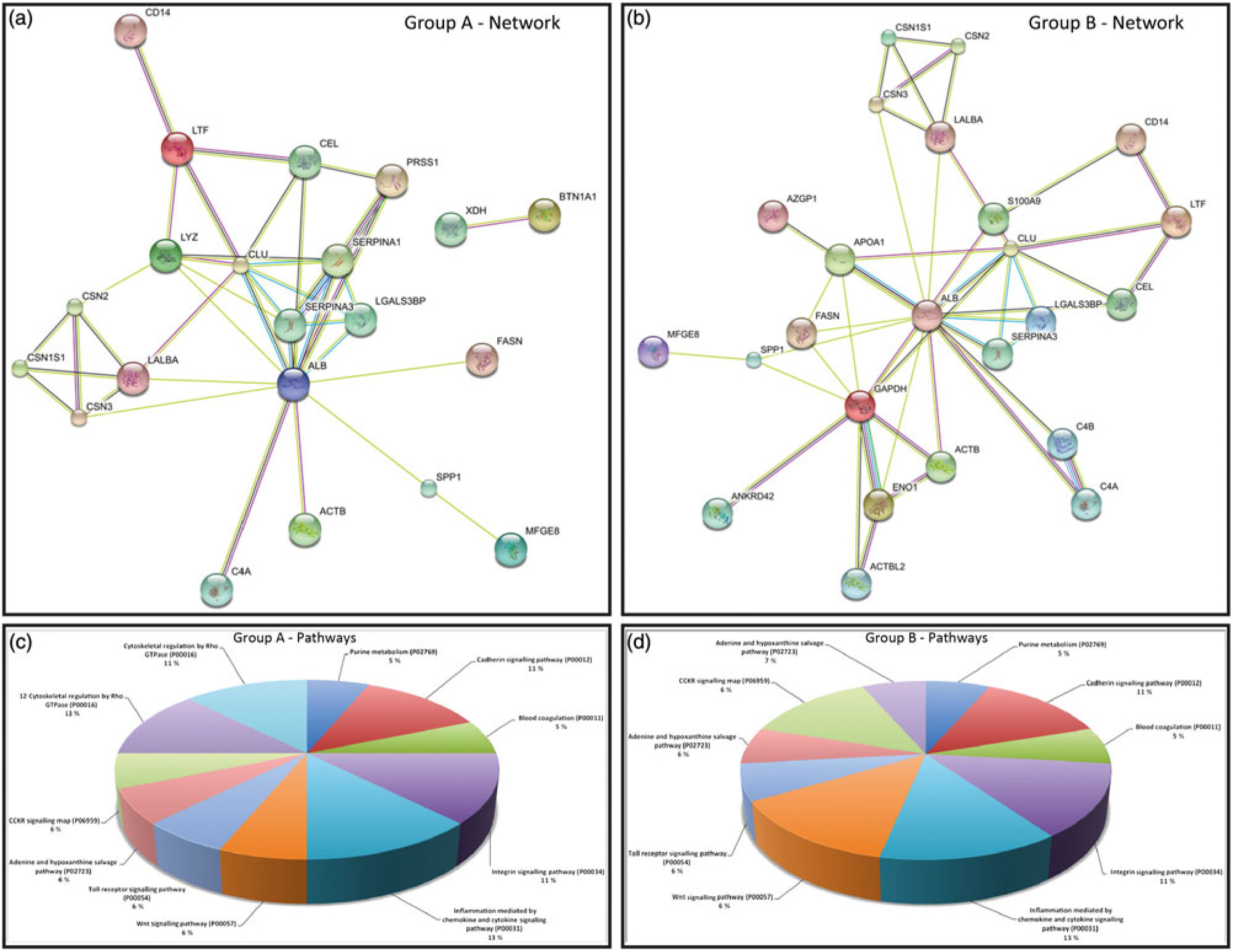
Fig. 3. (a) and (b) Protein–protein interaction network analysed by STRING software. Group A: early mature milk (approximately 3 weeks) and group B: advanced mature milk (approximately 5 weeks). (c) and (d) In the Enriched biological process (Gene Ontology (GO)) for protein data wound healing was the most reliable GO term (8·04 × 10-5 false discovery rate (FDR)). Enriched Canonical pathway based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. In this situation, the complement and coagulation cascades were the most enriched (0·00471 FDR). Abbreviations of the names of proteins are described in online Supplementary Table S3.
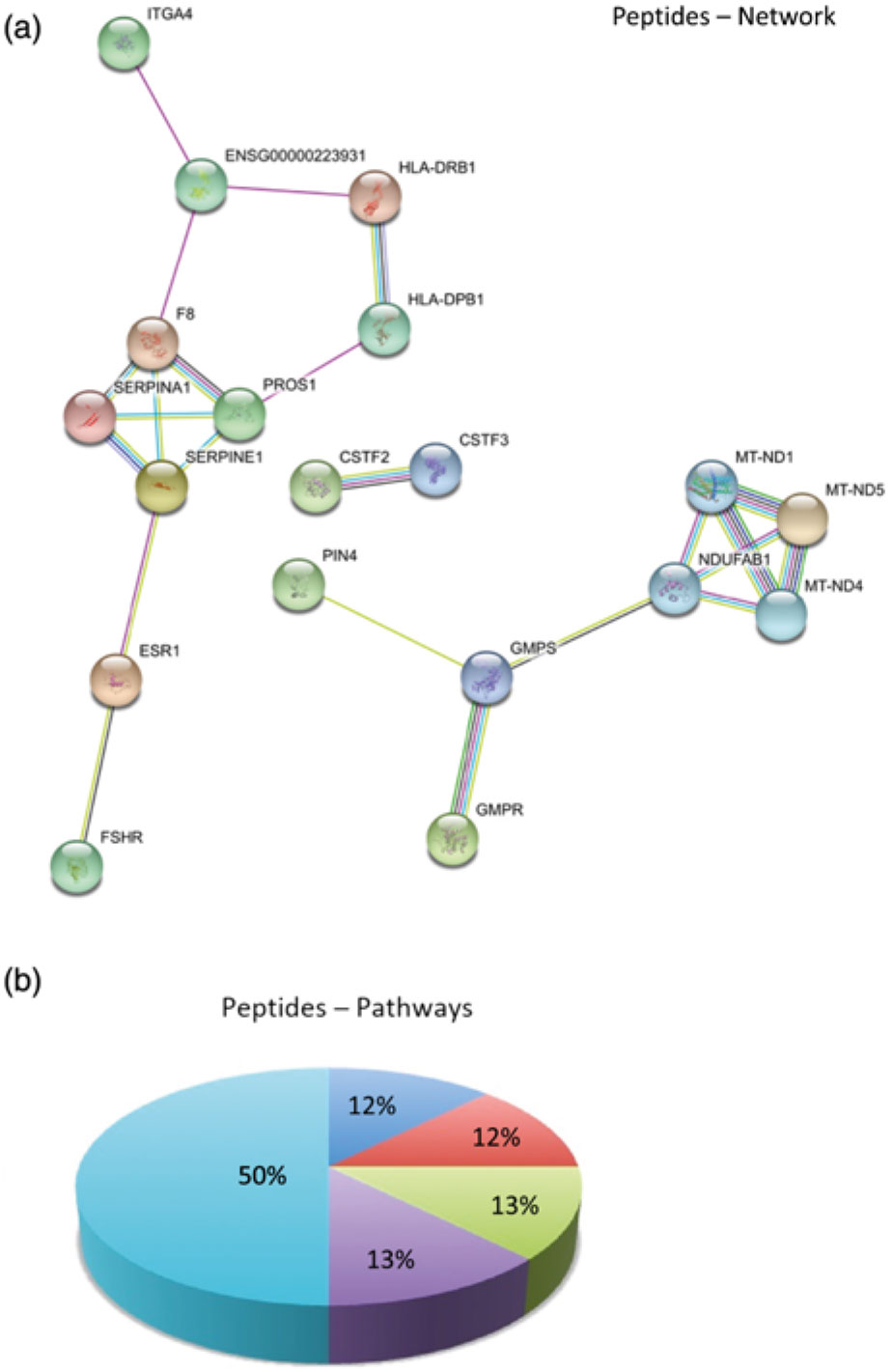
Fig. 4. (a) Protein–protein interaction network analysed by STRING software. Peptides identified in low-molecular-weight fraction (<10 kDa) in samples of skimmed milk. (b) The enriched biological process (Gene Ontology (GO)) for protein data wound healing was the most reliable GO term (8·04 × 10-5 false discovery rate (FDR)). Enriched Canonical pathway based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. In this situation, the complement and coagulation cascades were the most enriched (0·00471 FDR). Abbreviations of the names of proteins are described in online Supplementary Table S3. ![]() , CCKR signalling map (P06959);
, CCKR signalling map (P06959); ![]() , De novo purine biosynthesis (P02738);
, De novo purine biosynthesis (P02738); ![]() , plasminogen activating cascade (P00050);
, plasminogen activating cascade (P00050); ![]() , p53 pathway (P00059);
, p53 pathway (P00059); ![]() , blood coagulation (P00011).
, blood coagulation (P00011).
Discussion
Adolescent pregnancy has been considered a public health problem in some countries, due to the occurrence of negative consequences that can affect both the mother and the newborn infant. Despite the large number of pregnant adolescents worldwide, little is known about the composition of their breast milk. In Latin America, this group represents approximately 18 % of total births(24). Previous studies have reported differences in milk volume throughout lactation and in breast milk Na levels between adult and adolescent mothers(Reference Motil, Kertz and Thotathuchery16– Reference Baldeón, Mennella and Flores26). However, the profiles of protein and peptides of adolescent breast milk are largely unknown, which limits comparisons with published data on adult breast milk. In the present study, we used proteomic tools to investigate the profiles of protein and peptide of breast milk samples, while simultaneously highlighting the differences between two distinct periods of lactation, early (21·7 (sd 0·82) d) and advanced (48·0 (sd 2·37) d) mature milk, of adolescent mothers with age ranging from 14 to 19 years.
SDS-PAGE analysis (Fig. 2) revealed differences among the protein band patterns in each sample. The milk protein profile and the contents of each protein are known to change throughout lactation in adults(Reference Liao, Alvarado and Phinney27, Reference Gao, McMahon and Woo28). Previous studies have shown that the milk of adult mothers tend to have more homogeneous protein composition when the nursing mothers are in the same stage of lactation and are in the same age group(Reference Hettinga, Reina and Boeren29, Reference Zhang, De Waard and Verheijen30). The present study calls attention to the peptidome of adolescent mothers’ breast milk because even among mothers with similar characteristics (primiparous, healthy, same lactational period, age and menarche), different profiles were observed. Future studies, with higher number of samples, are necessary to determine the extent of biological variability of the proteome and peptidome in adolescent mothers’ breast milk and possibly to demonstrate what genetic or epigenetic factors determine this apparently high biological variability.
The major proteins in skimmed milk obtained from adolescents are the same as those predominant in breast milk from adults, for example, caseins, α-lactalbumin, lactoferrin, secretory IgA, lysozyme and serum albumin(Reference Lönnerdal5– Reference Ballard and Morrow33). In the present study, 424 proteins were identified in skimmed milk from adolescent mothers, of which 137 were common to early and advanced mature milk groups. It has been shown that breast milk composition varies during the course of lactation, resulting in differences between the lactation periods(Reference Field10).
In the present study, we analysed the peptidome (<10 kDa) of skimmed breast milk obtained from adolescent mothers. Although the composition of peptides differed between early and advanced mature milk groups, no significant differences were observed in the total peptide content between the two groups, and no identifiable pattern of variation was observed between the two lactation periods investigated, suggesting that the inter-individual variability might have hampered the identification of any longitudinal pattern in the milk peptidome of adolescents.
The 162 peptide sequences detected in adolescents’ breast milk originated from 155 proteins, and most of these peptides did not come from precursor proteins in non-filtered skimmed milk. This suggests that most of the peptides found in adolescents’ breast milk did not originate from proteolysis of skimmed milk proteins.
Previous studies have shown that most breast milk peptides originate from casein hydrolysis or posttranslational processing(Reference Dallas, Guerrero and Khaldi34, Reference Dallas, Smink and Robinson35). However, in these studies, milk proteins were precipitated by TCA and the peptides in the supernatant were analysed. In a control experiment in the present study, we used this same protocol for protein precipitation (with TCA) and found that peptides originating from β-casein were the most abundant. Thus, the current protocol using ultrafiltration was more appropriate for sample preparation, because it allowed the determination of the physiological peptidome secreted in milk. In the present study, the absence of the artefactual casein-derived peptides added to the sensitivity of the analysis, allowing the detection of a more representative endogenous peptidome that is composed of less abundant peptides. Additionally, we showed that the characterised peptidome in breast milk obtained from adolescents is directly secreted in the form of preformed peptides.
Several of the peptides identified herein have not been previously described in breast milk nor have known function to human lactation. The analytical approach adopted in this work may contribute to the discovery either of new peptides or new biological functions in lactation. In order to understand the interactions between proteins and peptides in adolescents’ milk, we used STRING database (http://string-db.org/). The functions of the identified proteins were classified according to the UniProt database (http://www.uniprot.org/). Interactome networks revealed that albumin was a core of highly connected proteins in the two groups of lactating adolescents, and we noted interactions common to both networks (Fig. 3(a) and (b)). Although these interaction networks connected with albumin were present in breast milk from early and advanced mature milk, it seems that in the latter group the network was more connected, for instance, lysozyme, α-lactalbumin, lactotransferrin and casein, which have important functions in lactation (Fig. 3(c) and (d)).
We also identified protease inhibitors in skimmed milk (SERPINA1 and SERPINA3) and in skimmed milk fractions (SERPINE1 and SERPINA1). Serpins are responsible for the regulation of several physiological processes such as coagulation, fibrinolysis, complement activation, apoptosis, inflammation, among others. In our network, they interact with LGALS3BP and PRSS1 proteins. These proteins play important roles in the immune system, especially the innate immune system. In particular, SERPINA1 protects the proteins related to the immune system from degradation during digestion(Reference Janciauskiene36– Reference Zhang, van Dijk and Hetting38).
Analysis of the early mature milk group showed the presence of CD14 peptides that are related to the immune system. Lipopolysaccharide binding protein binds to monomeric lipopolysaccharide and delivers it to the LY96/TLR4 complex, thereby mediating the immune response to bacterial lipopolysaccharide(Reference Zhang, van Dijk and Hetting38). These results show that milk components, especially those of skimmed early mature breast milk, participate in the innate and adaptive immunity, thereby conferring immune defence to the newborn.
In the advanced mature milk group, we observed interactions among milk fat globule-epidermal growth factor 8 (MFGE8), secreted phosphoprotein 1 (SPP1) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) proteins. These proteins are involved in the neonatal immune system. MFGE8 plays an important role in intestinal epithelial homeostasis and the promotion of mucosal healing. Another important function of MFGE8 is to bind to rotaviruses and inhibit their replication(Reference Newburg, Peterson and Ruiz-Palacios39). SPP1 has also been associated with inflammatory processes(Reference Zhang, van Dijk and Hetting38). The GAPDH protein is a key regulator of energy metabolism and has additionally been reported as being essential for the maintenance of normal secretory function; it is a multifunctional protein in mammalian cells, and it may influence the activity of other proteins(Reference Sirover40, Reference Tisdale41).
Both networks processed by STRING analysis are related to the immune system, offering key information regarding the well-being and survival of newborns, especially for those who present an immature immune system; no major functional differences were found between early and advanced mature milk. These results highlight the intricate role of breast milk proteins as a major part of the defence system of newborns.
Analysis of the skimmed breast milk low-molecular-weight fraction (<10 kDa) revealed the presence of peptides identified in several samples (peptide sequences are listed in online Supplementary Table S2). In peptide interaction networks (Fig. 4(a)), we observed few interactions, possibly because these peptides have very diversified specific functions.
Interestingly, in case of both peptide and protein analyses, we found that proteins involved in the complement and coagulation cascades were similar between the two groups. The peptides identified were small, processed fragments derived from major proteins (e.g. serpin proteins). Because processing of protein milk into smaller peptides is well known, we used a proteomic approach in order to investigate the bioactive peptides. Here we show how breast milk is at the forefront of newborn health – especially as newborns do not have a mature immune system – as evidenced by the identification of peptides belonging to MHC in breast milk (Fig. 4). Although we showed that the peptidome of adolescents’ breast milk varies according to the stage of lactation, it was not possible to show that this was a biologically robust pattern due to the presence of huge inter-individual variation. In all samples, the major proteins present in skimmed milk were identified, for instance, caseins, α-lactalbumin, lactoferrin, secretory IgA, lysozyme and serum albumin. Comparative analysis of the human milk proteome using MS was shown to be a promising strategy to monitor differences in the protein profile of adolescent mothers at two distinct lactation periods. A suggestion for future analysis would be to perform a longitudinal study of milk samples. However, long-term follow-up of the volunteers is difficult due to the subject drop-off effects.
We also identified peptides that have not been previously reported in breast milk, to the best of our knowledge. The limitations of this work lie principally in the sample size, and in the fact that we did not have samples from the same lactating adolescent mothers in distinct lactation phases. However, we showed methodological improvements to the analysis of breast milk peptidome, through the use of ultrafiltration, which seems to be the most advisable method for sample preparation, and we showed a vast amount of data related to previously unidentified peptides in milk, with broad functionality. It would be valuable in future studies to further determine the role of bioactive peptides in milk, the range of their biological variation and their dependence on the maternal genome and epigenetics. Moreover, research into active peptides that mediate immune defences might help future technological developments in the field of infant formulae that would help prevent neonatal infections.
Acknowledgements
We are grateful to the Unidade de Espectrometria de Massas (UEMP-UFRJ) for the mass spectrometry studies.
This work is part of I. B. Campanhon’s PhD Thesis, and the authors would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance code 001) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) for financial support. The authors thank Ana Lucia O. Carvalho and Augusto Vieira (IBqM-UFRJ), and Dr Eduardo Mere Del Aguila (IQ-UFRJ) for their technical assistance.
A. G. T., F. F. B., I. B. C. and M. R. S. S. conceived and designed the study; I. B. C. performed the experiments; I. B. C. and M. T. Q. M. conducted the database searching, bioinformatics and statistical analysis; R. B. Z. provided technical support; I. B. C. wrote the first draft of the manuscript; and A. G. T., F. F. B., I. B. C., M. R. S. S., M. T. Q. M. and R. B. Z. contributed with data interpretation and critical input revising the manuscript. All authors read and approved the final manuscript.
The authors state that there are no competing interests involved in the present study.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114519002447









