The use of probiotic strains such as lactobacilli and bifidobacteria is increasingly expanding because of their beneficial effects( 1 ). Lactobacillus gasseri SBT2055 (LG2055) is a representative of probiotic lactic acid bacteria isolated from the gastrointestinal tract in Japan( Reference Seto, Kimura and Akai 2 , Reference Takahashi, Fujita and Suzuki 3 ), which has been shown to improve intestinal microflora and metabolism( Reference Fujiwara, Seto and Kimura 4 ). Previous studies have indicated that LG2055 decreases lymphatic TAG absorption and increases faecal fatty acid excretion in rats( Reference Hamad, Sato and Uzu 5 ), decreases postprandial TAG absorption in humans( Reference Ogawa, Kadooka and Kato 6 ) and exerts anti-obesity effect both in animals( Reference Hamad, Sato and Uzu 5 , Reference Sato, Uzu and Yoshida 7 – Reference Miyoshi, Ogawa and Higurashi 9 ) and in humans( Reference Kadooka, Sato and Imaizumi 10 , Reference Kadooka, Sato and Ogawa 11 ). However, the underlying mechanisms are not quite clear. Other probiotic bacteria have also been reported to improve metabolism and exert anti-obesity effects in rodents( Reference Lee, Park and Seok 12 – Reference Stenman, Waget and Garret 25 ). The key mechanisms include induction of lipolysis via production of trans-10,cis-12-conjugated linoleic acid( Reference Lee, Park and Seok 12 , Reference Lee, Paek and Lee 13 ), increase in sympathetic nerve activity( Reference Tanida, Shen and Maeda 14 ) and suppression of fat deposition via increased expression of angiopoietin-like 4, a circulating inhibitor of lipoprotein lipase( Reference Aronsson, Huang and Parini 15 , Reference Kondo, Xiao and Satoh 17 ). Furthermore, several Lactobacillus strains have been shown to induce transcriptional activation of fatty acid β-oxidation-related genes in the liver( Reference Fåk and Bäckhed 19 – Reference Kim, Park and Kim 21 , Reference Park, Ahn and Park 23 ) and muscle( Reference Kim, Park and Kim 21 ), while inhibiting the transcription of fatty acid synthase in the liver( Reference Yoo, Kim and Park 24 ), and to improve insulin sensitivity( Reference Kim, Park and Kim 21 , Reference Okubo, Takemura and Yoshida 22 ) and glucose tolerance( Reference Stenman, Waget and Garret 25 ). However, there is no direct evidence of the effects of probiotics on energy expenditure, which is closely related to the metabolism of macronutrients, especially carbohydrates and fats.
Obesity develops as a consequence of chronic deregulation in macronutrient oxidation levels( Reference Swinburn and Ravussin 26 , Reference Spiegelman and Flier 27 ). Therefore, to gain the mechanistic insights of anti-obesity effects exerted by LG2055, it is necessary to evaluate the relationship between energy and macronutrient metabolism. Several methods have been used to assess the balance between nutrient oxidation and energy generation, such as indirect calorimetry, which has been applied to estimate net rates of carbohydrate and fat oxidation in humans( Reference Swinburn and Ravussin 26 , Reference Ravussin, Lillioja and Anderson 28 ) and rodents( Reference Nagao, Wang and Inoue 29 , Reference Nagao, Jinnouchi and Kai 30 ) based on VO2 and carbon dioxide production. Another widely used convenient and reliable method is the oral glucose tolerance test (OGTT), which can help diagnose glucose intolerance and insulin resistance( 31 ).
Obesity is a part of the metabolic syndrome, which, according to previous reports, is associated with low-grade inflammation, shown to play a role in the pathogenesis of glucose disorders( Reference Calder, Ahluwalia and Brouns 32 , Reference de Rekeneire, Peila and Ding 33 ). Thus, insulin resistance and type 2 diabetes are characterised by higher levels of inflammatory markers including cytokines and acute-phase reactants( Reference Nilsson, Jovinge and Niemann 34 , Reference Fröhlich, Imhof and Berg 35 ). Previous studies have shown that LG2055 reduces serum levels of soluble intercellular adhesion molecule-1 induced by inflammatory cytokines in rats( Reference Kadooka, Ogawa and Ikuyama 8 ) and down-regulates mRNA expression of C-C motif chemokine ligand 2 (Ccl2), also known as monocyte chemoattractant protein 1, in adipose tissue of mice( Reference Miyoshi, Ogawa and Higurashi 9 ), indicating that LG2055 has anti-inflammatory activity. However, the effect of LG2055 on the level of acute-phase reactants has not been investigated.
In the present study, we addressed the mechanistic insights of LG2055 anti-obesity activity by evaluating energy and glucose metabolism, as well as measuring the levels of serum amyloid P component (SAP), an acute-phase reactant, in rats.
Methods
Preparation of milk fermented by Lactobacillus gasseri SBT2055
Milk fermented by LG2055 was prepared as described previously( Reference Hamad, Sato and Uzu 5 ). In brief, skimmed milk (SM) powder (Megmilk Snow Brand Co. Ltd) was dissolved in de-ionised water, supplemented with yeast extract and sterilised at 95°C for 30 min. After inoculation with LG2055, the mixture was incubated at 37°C for 16 h, freeze-dried and used for subsequent experiments. Non-fermented SM prepared by treating SM powder in the same conditions without LG2055 was used as control. Chemical compositions of control SM (34·7 % protein, 0·9 % fat, 52·6 % carbohydrate, 7·9 % ash and 3·9 % moisture) and fermented SM (35·4 % protein, 0·9 % fat, 52·6 % carbohydrate, 7·7 % ash and 3·4 % moisture) were similar, except that the latter also contained 11·8 g lactic acid/100 g. The final concentration of viable LG2055 in the fermented SM-containing diet was 6×107 colony-forming unit/g diet.
Animal experiments
For the present study, 4-week-old male Sprague–Dawley rats (Kud:SD) were obtained from Kyudo. The rats were housed individually in metal cages in a temperature-controlled room (24°C) under a 12 h light–12 h dark cycle. Experimental diets were prepared according to the AIN-76 formula( 36 ) with some modifications and contained (g/kg) 100 fat (90 lard and 10 maize oil), 200 non-fermented SM powder (control diet) or LG2055-fermented SM powder (LG2055 diet), 125 casein, 150 α-maize starch, 50 cellulose, 3 DL-methionine, 35 mineral mixture (AIN-76), 10 vitamin mixture (AIN-76) and sucrose to 1000 g. The protein concentration provided by fermented and non-fermented SM was 70·8 and 69·4 g/kg diet, respectively. Diets containing high sucrose and lard rich in SFA were used to induce obesity and glucose intolerance.
In the experiment performed at Saga University, rats were allowed free access to commercial chow for a week, and then divided into the control and LG2055 groups (n 6). There was no significant difference in the initial body weight between the two groups (control, 139 (sem 1) g; LG2055, 139 (sem 1) g). The experimental diets were pair-fed to the rats. After 1 week, the animals were subjected to respiratory gas analysis, then anaesthetised with Somnopentyl® (Kyoritsu Seiyaku Corporation) and killed by aortic exsanguination.
In the experiment performed at Kyushu University, rats were acclimatised as described above and divided into the same groups (n 6); the initial body weight was not significantly different between the two groups (control, 141 (sem 2) g; LG2055, 140 (sem 3) g). The experimental diets were pair-fed for 4 weeks. After 3 weeks, rats were analysed by OGTT. At the end of the feeding period, animals were subjected to a 9-h fasting period, anaesthetised with somnopentyl and killed by aortic exsanguination. The pancreas, liver, white adipose tissue (WAT; mesenteric, perirenal, retroperitoneal, epididymal and subcutaneous), quadriceps femoris, pancreas and caecum were immediately excised and weighed. EDTA-containing plasma was prepared by blood centrifugation at 1750 g for 15 min, and DPP-IV inhibitor (EMD Millipore Co.) was added to measure glucagon-like peptide-1 (GLP-1) levels. Serum was separated by incubating blood samples for 30 min at room temperature. The samples were maintained at −30°C until analysis.
All animal experiments were conducted according to the Guidelines for Animal Experiments of Saga University and Kyushu University, as well as the law (no. 105) and notification (no. 6) of the government of Japan. The animal protocols were approved by the review committees of Saga University (authorisation no. 19-002-1) and Kyushu University (authorisation no. A22-159-1).
Respiratory gas analysis
After 1 week of feeding the diets, each rat was placed into a metabolic chamber (210 cm2 in square, 11·5 cm in height) for 21 h to measure VO2 and the RQ. The system consisted of six acrylic metabolic chambers, a MS (model WSMR-1400), gas sampler (model WGSS-1000) and switching controller (model WMSC-2000); all instrumentation and software were obtained from Arco System. Room air was pumped through the chambers at a rate of 1·7 l/min; expired air was dried by passing through a thin cotton wool-containing column and directed to a MS. The air from each chamber was sampled for 1 min every 7 min, and the data were stored in a spreadsheet. The RQ, carbohydrate and fat oxidation, and energy production rates were calculated by the software using the following formulae:
where VCO2 is carbon dioxide exhaustion.
During the analysis, rats were pair-fed diets and had free access to water. The experiment was carried out in a separate room to avoid animal stress.
Oral glucose tolerance test
After 3 weeks of feeding, rats were subjected to 16 h of fasting and then orally administered a glucose bolus (2 g/kg body weight). Blood was drawn from the tail vein at 0, 15, 30, 60, 90 and 120 min after bolus administration, and glucose levels were measured using an Accu-Chek® Aviva Nano blood glucose meter (Roche Diagnostics). The AUC of blood glucose concentration (0–120 min) was calculated using the trapezoidal rule.
Analysis of metabolic parameters
The levels of blood HbA1c were measured using a commercial kit based on the latex-agglutination assay (RAPIDIA® Auto HbA1c-L; Fujirebio Inc.). Plasma TAG, cholesterol, free fatty acids and glucose levels were measured using enzyme assay kits (Triglyceride E-Test, Cholesterol E-Test, NEFA C-Test and Glucose CII-Test, respectively; Wako Pure Chemicals). Plasma levels of insulin, glucagon, glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 and serum levels of fatty acid-binding protein 4 (FABP4) and SAP were measured using commercial ELISA kits (rat insulin ELISA; Shibayagi Co. Ltd, rat/mouse GIP total ELISA; EMD Millipore Co., rat glucagon ELISA kit and rat GLP-1 ELISA kit; Wako Pure Chemicals, CircuLex Rat FABP4/A-FABP ELISA kit; CycLex Co. Ltd, Serum Amyloid P Rat ELISA kit; Abcam). Glycogen levels in the liver and muscle were determined according to the method of Lo et al. ( Reference Lo, Russell and Taylor 37 ).
Analysis of caecal lactic acid and SCFA
The caecal levels of lactic acid and SCFA were determined as described elsewhere( Reference Han, Tsuchihira and Nakamura 38 ) with some modifications. In brief, caecal content was homogenised in sterile water, de-proteinised with 60 % HClO4 and separated by centrifugation at 8500 g for 10 min at 4°C. The supernatant was filtered through a cellulose acetate membrane filter (0·45 μm; Advantec), and individual SCFA were determined by the bromothymol blue (BTB) post-column method using an HPLC system (SHIMADZU SCL-10A; Shimadzu). The analytical conditions were as follows: column, TSKgel OApak-A (7 μm, 7·8 mm i.d.×30 cm; Tosoh Co.); guard column, TSKgel OApak-P (10 μm, 6·0 mm i.d.×4 cm; Tosoh Co.); eluent and flow rate, 5 mm-HClO4 at 0·7 ml/min; column temperature, 60°C; reagents and flow rate, 0·1 mm-BTB and 15 mm-Na2HPO4 at 1·2 ml/min; and detector wavelength, 445 nm. The injection volume was 25 μl, and the total run time was 40 min. Total SCFA present the sum of acetate, propionate and butyrate levels.
Statistical analysis
All values are expressed as means with their standard errors. Differences between the two groups were determined by Student’s t test and were considered statistically significant at P<0·05.
Results
Effects of the LG2055 diet on nutrient oxidation and energy expenditure
The effects of the LG2055 diet on energy metabolism were assessed on the basis of respiratory gas analysis in rats fed the same amount of diets (control group, 10·7 (sem 0·1) g; LG2055 group, 10·8 (sem 0·1) g). The LG2055 group demonstrated significantly higher carbohydrate oxidation in the dark cycle (active phase for rats) compared with the control group, which resulted in a significant increase in energy expenditure (Fig. 1(a) and (c)). Moreover, the LG2055 diet tended to induce fat oxidation in the light cycle (resting phase for rats) (Fig. 1(b)), although the effect was not statistically significant.

Fig. 1 Time course of the changes in carbohydrate oxidation (a), fat oxidation (b) and energy expenditure (c) in rats fed the control (![]() ) diet or the LG2055 (
) diet or the LG2055 (![]() ) diet for 1 week. Values are means (n 6), with their standard errors. * Mean values were significantly different from those of the control group (P<0·05). BW, body weight.
) diet for 1 week. Values are means (n 6), with their standard errors. * Mean values were significantly different from those of the control group (P<0·05). BW, body weight.
Effects of the LG2055 diet on morphometric and metabolic parameters
The two groups of rats did not differ in their food intake after the 4-week feeding period. Body weight gain was significantly lower in the LG2055 diet-fed rats (Table 1). However, there were no differences between the two groups in terms of organ weights (liver, quadriceps femoris, pancreas, caecum and WAT), plasma parameters (TAG, cholesterol, free fatty acid, glucose, insulin, glucagon, GIP and GLP-1), blood HbA1c, serum FABP4 and glycogen content of the liver and muscle (Table 1). At the same time, SAP levels were significantly reduced in the LG2055 diet-fed rats. Table 2 shows that the contents of lactic acid, the ratios of acetate:total SCFA and propionate:total SCFA in the caecum did not differ between the two groups, but the propionate+butyrate:total SCFA ratio tended to be higher (P=0·085) and the butyrate:total SCFA ratio was significantly higher (P<0·05) in the LG2055 group. Total butyrate content in the caecum also tended to increase (7·02 (sem 1·08) μmol/g in the control group v. 8·58 (sem 0·46) μmol/g in the LG2055 group), but the difference was not statistically significant.
Table 1 Effects of diets containing skimmed milk (control) and fermented milk (LG2055) on morphometric and metabolic parameters of rats (Mean values with their standard errors)

HbA1c, glycated Hb; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; FABP4, fatty acid-binding protein 4.
* Mean values were significantly different from those of the control group (P<0·05).
Table 2 Effects of diets containing skimmed milk (control) and fermented milk (LG2055) on caecal lactic acid contents, caecal SCFA contents and molar SCFA ratios in ratsFootnote † (Mean values with their standard errors)
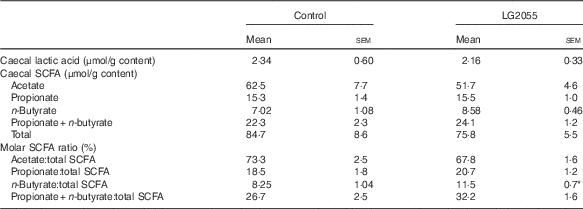
* Mean values were significantly different from those of the control group (P<0·05).
† Total SCFA (acetate, propionate and n-butyrate).
Effects of the LG2055 diet on postprandial glucose responses
OGTT analysis indicated that the LG2055 diet tended to lower postprandial glucose levels in rats starting 15 min after bolus challenge compared with the control diet, resulting in a significant decrease in cumulative glucose levels (AUC, P<0·05; Fig. 2 inset).

Fig. 2 Glucose tolerance in rats fed the control (![]() ) diet or the LG2055 (
) diet or the LG2055 (![]() ) diet for 3 weeks. Blood glucose was determined after 16 h of fasting. Following oral glucose administration, blood glucose levels were measured at the indicated intervals. The inset shows the AUC for each group. Values are means (n 6), with their standard errors. * Mean values were significantly different from those of the control group (P<0·05).
) diet for 3 weeks. Blood glucose was determined after 16 h of fasting. Following oral glucose administration, blood glucose levels were measured at the indicated intervals. The inset shows the AUC for each group. Values are means (n 6), with their standard errors. * Mean values were significantly different from those of the control group (P<0·05).
Discussion
In the present study, we addressed the mechanistic insights of anti-obesity effects exerted by the probiotic lactic acid bacteria strain L. gasseri SBT2055. The results indicated that LG2055 enhanced energy expenditure and improved glucose tolerance, which correlates with previously shown reduction in postprandial TAG absorption( Reference Hamad, Sato and Uzu 5 , Reference Ogawa, Kadooka and Kato 6 ). We believe that the enhancement of energy expenditure via increase in carbohydrate oxidation (Fig. 1(a) and (c)) may have contributed to the LG2055-elicited anti-obesity effect (Table 1) and improvement in glucose tolerance (Fig. 2). Mammals utilise not only glucose, long-chain fatty acids and amino acids as energy sources, but also SCFA derived from colonic fermentation of dietary fibre by the gut microbiota. The association of microbial activity in the gastrointestinal tract with host energy homoeostasis and obesity pathogenesis is increasingly recognised( Reference Ley, Bäckhed and Turnbaugh 39 , Reference Turnbaugh, Ley and Mahowald 40 ). Recently, a series of orphan G protein-coupled receptors were shown to bind free fatty acids; among them, free fatty acid receptor 3 (FFA3/GPR41) is expressed in the intestine and sympathetic nervous system, and is activated by SCFA( Reference Hara, Kashihara and Ichimura 41 ) including propionate (C3) and butyrate (C4), which showed 9·6-fold and 11·9-fold higher receptor activation compared with acetate (C2)( Reference Brown, Goldsworthy and Barnes 42 ). GPR41 knockout mice exhibited lower energy expenditure and glucose tolerance compared with wild-type mice( Reference Bellahcene, O’Dowd and Wargent 43 ). Kimura et al. ( Reference Kimura, Inoue and Maeda 44 ) reported that SCFA directly up-regulate the activity of the sympathetic nervous system via GPR41, and thereby enhance body energy expenditure in mice. In addition, Gao et al. ( Reference Gao, Yin and Zhang 45 ) reported that dietary supplementation of sodium butyrate improves insulin sensitivity and increases energy expenditure in mice. In the present study, the LG2055 diet tended to increase the content of caecal butyrate and decrease that of acetate, which is less potent in GPR41 activation, resulting in a significantly elevated (39·4 %) ratio of butyrate:total SCFA (Table 2). These results suggest that the increase in butyrate production in the caecum by LG2055 may enhance energy expenditure and improve glucose tolerance via GPR41 activation. Further controlled studies are needed to determine whether and how changes in SCFA profile of the caecum by LG2055 are reflected in SCFA levels of portal and aortic sera, and whether GPR41 is activated by LG2055. Considering that other probiotic bacteria also inhibit obesity development in rodents( Reference Lee, Park and Seok 12 – Reference Stenman, Waget and Garret 25 ), it should be interesting to investigate whether their anti-obesity effects are associated with the enhancement of butyrate production. Furthermore, it is known that incretin hormones such as GIP and GLP-1 control energy metabolism( Reference Baggio and Drucker 46 ). Tolhurst et al. ( Reference Tolhurst, Heffron and Lam 47 ) reported that SCFA stimulate GLP-1 secretion via GPR41 in primary mouse colonic cells. Moreover, incretin hormones are released from the intestine in response to food ingestion( Reference Baggio and Drucker 46 ). However, in the present study, plasma levels of GIP and GLP-1 after 9 h of fasting did not differ between the groups (Table 1). These results may reflect that unlike prebiotics the change of SCFA profile by probiotics is milder.
Probiotics and prebiotics affect fermentation processes in the host caecum and regulate SCFA synthesis. Several studies have demonstrated that consumption of a diet high in inulin( Reference Cani, Dewever and Delzenne 48 ), oligofructose( Reference Cani, Neyrinck and Maton 49 ) or their combination( Reference Parnell and Reimer 50 ) reduced body weight by lowering energy intake in rats. Dietary prebiotic fibres containing inulin and oligofructose in a 1:1 ratio altered energy intake by increasing satiety hormones but not energy expenditure; however, as caecal SCFA levels were not measured in that study( Reference Parnell and Reimer 50 ), the contribution of SCFA to the reduction of body weight by prebiotics remains unclear. Together, these findings indicate that the reduction of body weight by dietary fibres depends on energy intake rather than energy expenditure, suggesting that prebiotics and probiotics may prevent obesity through distinct mechanisms. Therefore, diet supplementation with a mixture of probiotics and prebiotics (synbiotics) may exert more powerful anti-obesity effects due to synergism in SCFA production with the change in intestinal flora and should be a focus of future studies.
It has been proposed that low-grade inflammation is a feature of the metabolic syndrome, which may be promoted by the release of various inflammatory mediators from adipose tissue( Reference Calder, Ahluwalia and Brouns 32 ). Previous cross-sectional studies have shown that insulin resistance and type 2 diabetes are associated with higher levels of pro-inflammatory cytokines and acute-phase reactants such as C-reactive protein (CRP)( Reference Nilsson, Jovinge and Niemann 34 , Reference Fröhlich, Imhof and Berg 35 ), which is consistent with the observation that lipopolysaccharide (LPS) presence results in the amplification of inflammatory cascades via CRP production( Reference Michel, Nagy and Schroeven 51 ). Therefore, circulating CRP levels are used as a biomarker to assess inflammatory status( Reference Du Clos 52 ). However, in rats, serum CRP concentration is much higher compared with other species, exceeding even its maximal acute phase levels in humans( Reference de Beer, Baltz and Munn 53 ), which precludes the use of rat models in studies of CRP-associated inflammatory mechanisms. SAP is an acute-phase protein, which shares approximately 60 % homology with CRP( Reference de Beer, Baltz and Munn 53 , Reference Koenig 54 ) and has comparable serum levels in rats and humans. Besides, rat SAP does not function as an acute-phase reactant in response to exogenous inflammatory stimuli such as casein or croton-oil injections( Reference de Beer, Baltz and Munn 53 ), which is similar to human but different from mouse SAP( Reference de Beer, Baltz and Munn 53 ), suggesting that the findings in rats can be extrapolated to humans. SAP binds to gram-negative bacteria expressing LPS( Reference de Haas, van der Tol and Van Kessel 55 , Reference de Haas, van Leeuwen and van Bommel 56 ), indicating that SAP levels are indices for infection of gram-negative bacteria and LPS influx into the body. Several studies have shown that low-grade inflammation is defined as a 2- to 4-fold increases in circulating inflammatory acute-phase proteins( Reference Brüünsgaard and Pedersen 57 , Reference Petersen and Pedersen 58 ). The normal range of SAP in rat and human sera is 10–50 μg/ml( Reference de Beer, Baltz and Munn 53 , Reference Koenig 54 ). In the present study, we found that the LG2055 diet significantly reduced serum levels of SAP (192 (sem 6) μg/ml in the control group v. 165 (sem 8) μg/ml in the LG2055 group), suggesting that LG2055 can prevent low-grade inflammation associated with obesity and glucose metabolism disorders.
In conclusion, we found that, in addition to the inhibition of dietary TAG absorption reported previously, probiotic LG2055 enhanced energy expenditure via stimulation of carbohydrate oxidation, improved glucose tolerance and reduced the levels of pro-inflammatory mediator SAP in rats. Considering that these effects correlate with the reduction of body weight gain, our findings suggest multiple actions activated by LG2055 that may work in synergy to prevent obesity.
Acknowledgements
The authors thank Editage (www.editage.jp) for English language editing.
The study was supported by Megmilk Snow Brand Co. Ltd (Saitama, Japan). The cost of English language editing was supported by a Research Grant for Young Investigators of Faculty of Agriculture, Kyushu University.
B. S. wrote the manuscript. B. S., K. N., M. U., A. S., Y. M. and S. K. carried out the experimental work and collected and analysed the data. B. S., K. N., T. Y., A. O., Y. K. and M. S. contributed to the study design, supervised the study and commented on the manuscript. All authors have read and approved the final version of the manuscript.
A. O. and Y. K. are employees of Megmilk Snow Brand Co. Ltd. There are no other patents, products in development or marketed products to declare. All other authors have no conflicts of interest to declare.







