The provision of adequate nutrients for young animals during the pre-weaning phase is a critical component in livestock production systems. In modern pig production, breeding for hyperprolificacy has resulted in sows that produce increasingly large litters, with a lower average birth weight(Reference Foxcroft, Dixon and Novak1), and more piglets than the sow can naturally rear(Reference Bruun, Amdi and Vinther2). This increases the need for alternative management systems to meet the nutritional requirements of the piglets(Reference Lay, Matteri and Carroll3). Such alternatives include milk replacer or liquid feed systems as a supplement to sow milk, given either manually or dispensed automatically in a cup system. Commercial milk replacer products typically contain skimmed milk powder as well as whey. Vegetable components are also added such as plant-based starch and protein from, for example, wheat and soya, that the small intestines (SI) are not yet adapted to digest(Reference Pluske, Hampson and Williams4).
Preparing the gut for the transitional period of weaning (i.e. going from a diet of milk solids to vegetable ingredients) is an increasingly pertinent issue, due to the need to produce robust healthy piglets that can be weaned without excessive use of zinc oxide or antibiotics. Early weaning of piglets is typically accompanied by a severe growth check and diarrhoea(Reference Lalles, Bosi and Smidt5). Typically, dry feed is offered to piglets before weaning to prepare the SI for an increasing percentage of vegetable ingredients as well as preparing the piglets to eat dry feed. However, a large percentage of piglets do not eat the feed offered (non-eaters)(Reference Bruininx, Binnendijk and van der Peet-Schwering6), and this management scheme does not allow the sow to rear more piglets than teats. The provision of milk replacer may thus offer an opportunity to modulate the piglets’ digestion and growth conditions, as the nutrient composition of the replacer will influence the development of the intestine, for instance, by affecting the enzyme activity and morphology(Reference Kelly, Smyth and McCracken7) and potentially increase feed intake in piglets before weaning, thus improving SI maturation. Wheat flour, which is a commonly used ingredient in milk replacers, leads to a shift in carbohydrates from lactose to a combination of lactose and starch. However, it is not known what effect this has on piglet function and health. Whilst there may be potential beneficial effects of starch in terms of gut adaption, the early exposure of the immature intestine to complex carbohydrates may also compromise growth and potentially cause dysregulated immune function and inflammation.
The aim of this study was therefore to investigate the effect of a milk replacer containing a high level of starch (wheat flour) on growth, gut enzyme activity and immune function of piglets compared with those fed a diet only containing milk. The hypothesis tested was that adding a starch component induces maturation of the mucosa as measured by higher digestive activity and improved SI integrity and immunity.
Methods
The experiment was carried out with respect to animal experimentation and with approval from the Danish Animal Experimentation Inspectorate, license number 2014−15−0201−00418. The study was performed at the experimental facilities at the University of Copenhagen, Frederiksberg campus.
Study design
A total of thirty-six 3-d-old piglets from six sows (parity 2–6, Danish Landrace × Danish Yorkshire DanBred), mixed females and males, were included in the study. The piglets were selected on a commercial farm on the day of birth and assigned to one of two treatment groups fed either: (a) a commercial bovine whole milk powder (MILK, n 18) or (b) a commercial bovine whole milk powder with added wheat (WHEAT, n 18). Selection was based on birth weight so each of the three weight categories (small: 0·8–0·9 kg; medium: 1·0–1·2 kg and large: 1·3–1·5 kg) was equally represented in each group. The piglets were kept with their dam from days 0 to 3 on a commercial farm after which they were transported to University of Copenhagen for further rearing. In addition, a reference group (n 18) was kept on farm (data not shown). Statistical power calculations were performed, and for example to detect ≥1·5 times differences in gene expression with a statistical power of 80 % at a significance level of 0·05, the power calculations indicate that a group size of 12 would allow us to detect these differences. Piglets were studied from birth to 24–25 d of age.
Diet
Until 11 d of age, all piglets received a milk replacer diet consisting of a mixture of whey protein (DI-9224; Arla Foods A/S) and bovine whole milk powder (26 % Milk fat; Arla Foods A/S) with 15 % DM (Table 1). Pigs in the MILK group remained on this diet during the entire experiment, while on day 11, WHEAT piglets were given a milk replacer diet consisting of a mixture of whey protein, bovine whole milk powder and wheat flour hydrothermally treated by extruder (Gronin R 300; Agilia A/S) consisting of 15 % DM. The amount of wheat in the diet increased from 10 % of DM on day 11 to 40 % of DM on day 26 (Fig. 1). The chemical composition of the milk replacer diet (MILK) and milk replacer with increasing wheat content (Wheat 20 and 40 %) is shown in Table 1. All piglets received 300 ml/kg body weight per d. Piglets’ daily ration of milk was dispersed eight times across 24 h. To reduce the risk of leftovers, this was increased to sixteen times across 24 h on day 14. The feeding system was fully automatic (Big Dutchman).
Table 1. Nutrient composition of experimental diets and analysed chemical composition (as is) in the control diet (milk) fed to the MILK group and the wheat diet with different percentages of increased wheat (20 and 40 % added wheat) fed to the WHEAT group
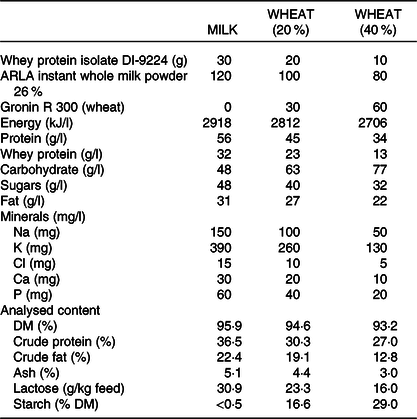
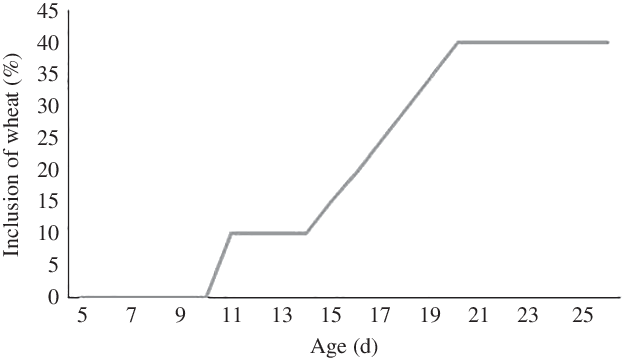
Fig. 1. Percentage of wheat in the diet during the experimental period for the WHEAT group.
Housing and management routines
At the day of arrival, the pigs were each placed in cages in groups of four (90 cm × 74 cm). They remained here the first 24 h to minimise stress and familiarise them with the feeding system and allowing them to learn from each other. After 24 h, each piglet was randomly assigned to individual cages. All thirty-six cages were placed in the same room. In the cages, floors were slatted and made of plastic. Heat-insulating mats were placed in the back of the cage occupying 50 % of the floor surface along with heating lamps placed above in the upper corner ensuring a temperature of approximately 30°C. Troughs were placed in the front of the cage with water nipples beside them. Each cage had its own feed valve, and under the cages, there were removable trays to collect urine and faecal matter. There was no additional heating besides the heating lamps, which ensured a room temperature of approximately 21–23°C at all times. Cages, troughs and trays were cleaned once per d. Feeding tanks were cleaned twice per d (morning and evening), and similarly, milk replacer was made twice per d (morning and evening) to ensure that it was fresh and a proper hygiene was kept. Piglets were given plastic balls for enrichment. All piglets received antibiotic treatment the first 3 d upon arrival which included an oral dose with 1 ml of Amoxicillin (50 mg/ml) (Scanvet), 1 ml of Gentocin Vet. (4·35 mg/ml) (Scanvet) and an electrolyte mixture (Revolyte Nutrition, 2–5 ml, Gunnar Kjems). In addition, piglets were given the same treatment if they experienced severe diarrhoea.
Recordings and measurements
All piglets were weighed at a maximum of 12 h after farrowing using a digital weight (UWE, Bjerringbro Vægte) and ear-marked according to the group. Piglets in the MILK and WHEAT groups were weighed once per d, and faecal matter was assessed visually (data not shown). From day 10 and onwards, leftovers in the troughs were collected and measured twice per d. On days 25 and 26 (half of the piglets were sampled each day), piglets were blood-sampled, anaesthetised (zoletil, ketamine, xylacin and butorphanol) and euthanised (sodium pentobarbitone).
Blood sampling
Blood samples were taken on the day of euthanasia. Piglets were held in a dorsal recumbancy, and 6 ml of blood were collected via jugular venepuncture using a 22-gauge needle and vacutainer tubes containing heparin (BD Vacutainer) for lipopolysaccharide (LPS) challenge, plain for serum for biochemistry and EDTA for haematology. Blood for biochemistry was then centrifuged at room temperature for 15 min at 1·20 g (CM-6MT; Elmi), and the resulting plasma was transferred to Eppendorf tubes (Sarstedt) and immediately frozen at −20°C.
Blood analysis
The haematology profile was analysed on an Advia 2120 Hematology System (Siemens Healthcare Diagnostics), and the serum analyses for biochemistry were assayed using an Advia 1800 Chemistry System (Siemens Healthcare Diagnostics). For cytokine concentration, the peripheral blood mononuclear cells were obtained from heparinised blood samples by differential centrifugation using histopaque 1·077 (Sigma-Aldrich). Cells were washed, counted and seeded at 5 × 106 cells/ml in RPMI 1640 media supplemented with 2 mm l-glutamine, 10 % heat-inactivated fetal calf serum, 100 U/ml penicillin and 100 µg/ml streptomycin. The peripheral blood mononuclear cells were then stimulated with either 1 µg/ml LPS (Escherichia coli O55:B5; Sigma-Aldrich) or mock-treated with PBS as controls. The peripheral blood mononuclear cells were then cultured for 24 h at 37°C and 5 % CO2, before the supernatant was harvested and stored at −20°C. Cytokine concentrations were then measured by ELISA using commercial antibody pairs according to the manufacturer’s instructions (IL-10 and TNFα – ThermoFisher; IL-6, and IL-1β – R and D Systems).
Dual-energy X-ray absorptiometry scan and post-mortem examination
The piglets were anaesthetised with an intramuscular injection of a Zoletil mix (Zoletil 50; Virbac) containing xylacin (Narcoxyl 20 mg/ml; MSD Animal Health), ketamine (Ketaminol 100 mg/ml; MSD Animal Health) and butorphanol (Torbugesic 10 mg/ml; ScanVet). When full anaesthesia was achieved, the pigs were weighed and dual-energy X-ray absorptiometry (DXA) scanned by placing them in ventral recumbency with the hind legs extended and the forelegs positioned caudally. The whole-body composition was analysed using the small animal mode (Lunar Prodigy Advance; GE Healthcare) providing readings of body fat and muscle mass. The DXA scanner was calibrated using a QC Phantom according to the manufacturer’s specifications.
Organ sampling
Afterwards, the piglets were euthanised with an intracardial injection of 2–3 ml pentobarbital (200 mg/ml). The liver, spleen, kidneys, heart, lungs, adrenal glands and brain were harvested and blotted dry before they were weighed on a precision scale (Radwag). Furthermore, the stomach was removed intact, rinsed out and weighed. The SI was measured in length and weighed along with the colon (full and empty), stomach (full and empty), kidneys, liver, lungs, spleen and heart. Furthermore, three samples of the SI were taken from three different places: proximal, middle and distal, and one sample was taken from the colon. Samples for enzyme activity, histology and gene expression were taken.
Gut morphology and histopathology
For further morphological and histological analysis, the tissue samples were prepared for embedding. The tissue sample was cut into slices, from the middle of the sample, at the size of 4 cm × 0·3 cm or less, and placed into cassettes. The tissue went through a fixation process of a minimum 24 h (Excelsior VIP processor). The processing procedure went from (10 %) formalin to alcohol (75–99 %) and hereafter to xylene and lastly paraffin. After the dehydration process, the plastic cassettes containing the slices of tissue were then taken to an embedding station. Paraffin blocks were stained with haematoxylin–eosin for intestinal morphology and histology analysis at the commercial laboratory ALAB Veterinaire (Poland). The general morphological and histopathological examination was done using a standard light microscope Olympus BX41 and CellSens software (Olympus Corporation). All evaluations at the microscope were done blinded to treatment, and the magnification was 10×, 40× and 100× (objective lens) and 10× (eyepiece). The mucosa, the submucosa, the villous height, the intestinal crypt depths and enterocyte height of the mid-jejunum were examined. The morphometric and histopathological analysis gave the following results: Mid-jejunum villous height (10× magnification), mid-jejunum crypt depth (10× magnification) and enterocyte height (40× magnification). Furthermore, the analysis gave two types of scoring: for intraepithelial lymphocytes infiltration and for stromal lymphocytes infiltration (data not shown).
RNA sequencing
Total RNA was extracted from about 30 mg intestinal tissue from a subset of piglets using a commercial kit (miRNeasy R Mini Kit) in accordance with the manufacturer’s guidelines. Briefly, tissue was homogenised in QIAzol lysis buffer using a gentleMACS™ dissociator (Miltenyi Biotec). Purity and concentration of total RNA were then measured using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies), and RNA integrity was measured using an Agilent 2100 Bioanalyzer (Agilent). The complementary DNA (cDNA) libraries were prepared and sequenced (paired-end reads of 100 bp) on a BGISEQ-500 sequencer (BGI), generating an average of 42·7 million reads per sample (sd 0·9 million; n 21). Clean reads were mapped to the Sus scrofa genome using Bowtie2 (version 2.2.5). Differentially expressed gene analysis was conducted using Deseq2 and NOIseq(Reference Tarazona, Garcia-Alcalde and Dopazo8). Gene-set enrichment analysis (Broad Institute, http://software.broadinstitute.org) was used for pathway analysis. RNA-seq data are available at GEO under accession number GSE1148110.
Quantitative PCR
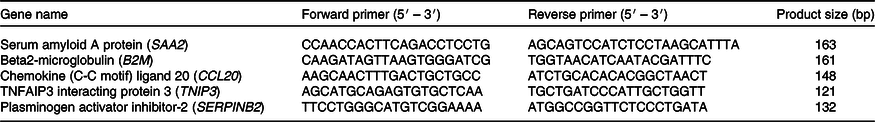
A quantity of 500 ng of RNA was used to synthesise cDNA using a Quantitect reverse transcription kit (Qiagen). Quantitative PCR was performed using the following cycling programme on an AriaMx PCR system (Agilent): 2 min at 95°C followed by forty cycles of 15 s at 95°C and 20 s at 60°C. Data were normalised to a reference gene (B2M), and relative expression and fold changes were calculated using the ΔΔCT method. Primers are listed in Table 2.
Table 2. Primers used to validate quantitative PCR
Enzyme analyses
Assays for mucosal activity of disaccharidases (maltase, sucrase and lactase) and peptidases (aminopeptidase N, aminopeptidase A and dipeptidyl peptidase IV) were performed on SI tissue homogenates (homogenised in 1 % Triton X-100) using specific substrates, as described previously(Reference Sangild, Siggers and Schmidt9).
Statistical analysis
Data were analysed in the statistical programme SAS (GLM procedure of SAS; SAS Inst. Inc.) according to the following model:
where Y ijk is the dependent variable measured (cells, blood characteristics, cytokines, blood parameters, DXA and morphology), μ denotes the overall mean, α i denotes the effect of treatment (i = milk and wheat), β j denotes the effect of birth weight class (j = small, medium and large), δ k denotes the effect of sex (k = male and female), θ is the random effect of sow (l = 1, 2, 3, 4, 5 and 6), (αβ) ij is the interaction between treatment × birth weight class and ϵ ij describes the random error term. The interaction between treatment × birth weight class was only included when significant. For analyses of weight, the model included treatment, time (days 0–24), sex, birth weight class and their interactions. Time was included in the repeated statement with pig ID as subject, and the sow was included as random effect. Some of the enzyme data had to be log-transformed for analysis (presented as back-transformed data). Means were separated using the PDIFF option and presented as least square means with their standard errors and considered significant when P < 0·05 and a tendency when P < 0·10.
Results
Growth, dual-energy X-ray absorptiometry and organ weights
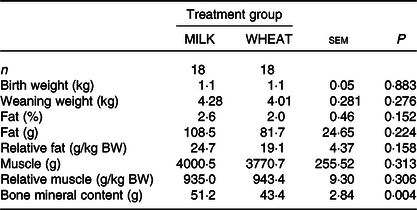
Overall, there was no difference in growth between the MILK and WHEAT groups (P > 0·10; Table 3). There was an effect of time (P < 0·001), where piglets became heavier over time and of birth weight class where specific growth rates were similar. There was no difference between the groups on most DXA measurements except for bone mineral content which was higher in the MILK group (Table 3). The relative organ and SI weights (weight per kg body weight) can be seen in Table 4. Relative to body weight, the full stomachs were heavier in the MILK group (P < 0·05) and there were tendencies for the middle part of the SI, the full colon and the liver to be heavier in the MILK group (P < 0·10).
Table 3. Effect of milk replacer diet on weaning weight and body composition (dual-energy X-ray absorptiometry)
(Least square mean values with their standard errors)
BW, body weight.
Table 4. Effect of milk replacer diet on the relative small intestine (SI) and organ weights
(Least square mean values with their standard errors)
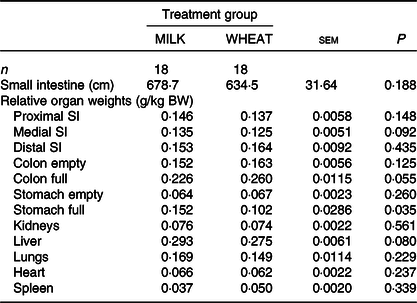
BW, body weight.
Enzyme activity
The enzyme activity of sucrase, maltase, lactase and aminopeptidase N, aminopeptidase A and dipeptidyl peptidase IV (U/g tissue) across the proximal, middle and distal SI and the colon can be seen in Fig. 2. The activity levels of sucrase were higher in the proximal SI (11·1 v. 7·1 U/g; P < 0·001) and in the colon in the WHEAT group (12·2 v. 1·2 U/g; P < 0·012) compared with the MILK group, respectively. Maltase activity was higher in the proximal part of the SI in the WHEAT group compared with the MILK group (55·6 v. 32·7 U/g; P = 0·002). There was a tendency for higher lactase activity in the colon of the MILK group compared with the WHEAT group (P = 0·082). No differences between groups were found for aminopeptidase N or aminopeptidase A activity across the gut in the two groups. The activity of dipeptidyl peptidase IV was higher in the proximal part of the SI for the WHEAT group compared with the MILK group (2·5 v. 2·1 U/g; P < 0·016).
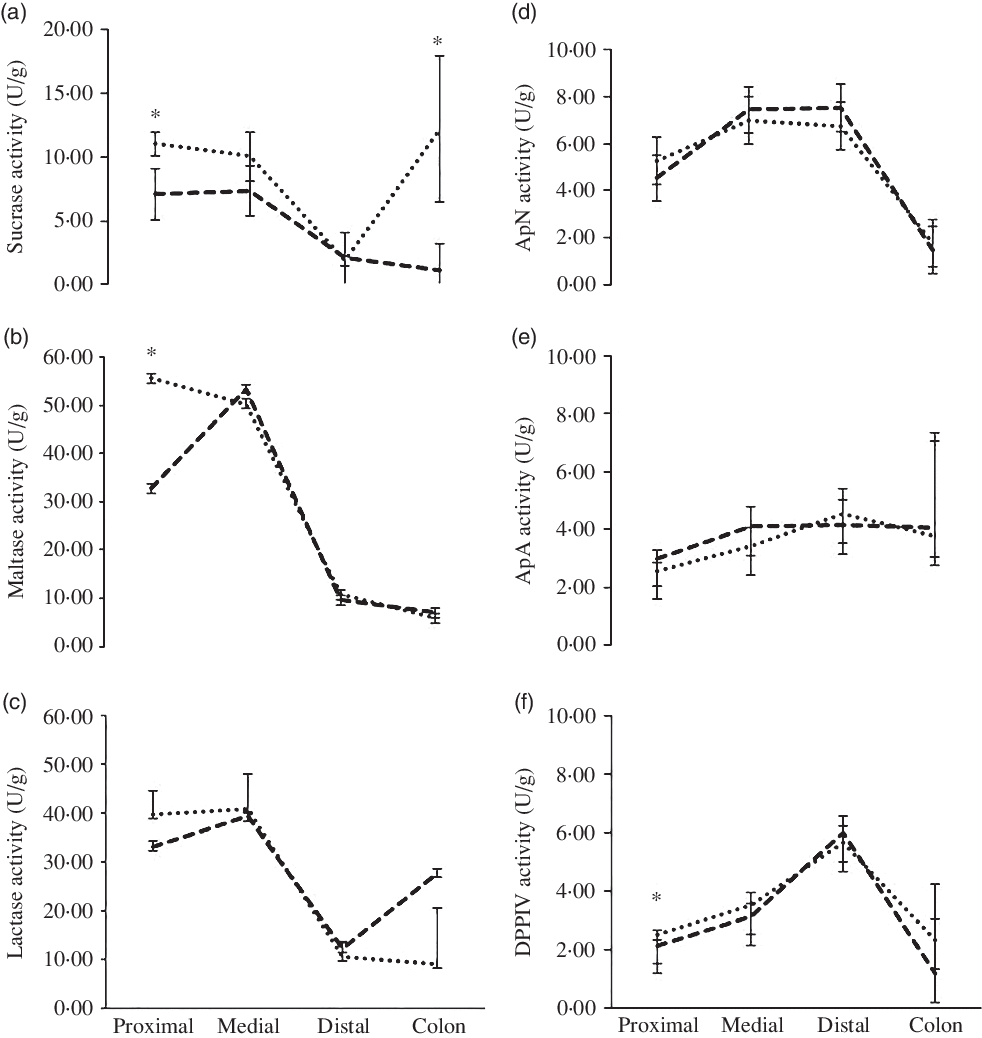
Fig. 2. Effect of diets on enzyme activity across the gut. Results are back-transformed data for (a) sucrase, (b) maltase, (c) lactase, (d) aminopeptidase N (ApN), (e) aminopeptidase A (ApA) and (f) dipeptidyl peptidase IV (DPPIV) activity across the proximal, medial and distal of the small intestine and the colon. * Significance at P < 0·05. ![]() , MILK group;
, MILK group; ![]() , WHEAT group.
, WHEAT group.
Blood lipopolysaccharide challenge and haematology
The concentrations of cytokines released from LPS-stimulated and mock-treated cells are shown in online Supplementary Table S1. There were no differences for any of the cytokines between the two groups. The haematology profile of the pigs is shown in online Supplementary Table S2. Piglets in the WHEAT group had higher levels of leucocytes (7·57 v. 5·77 billion/l; sem 0·686; P < 0·009), neutrophils (2·56 v. 1·52 billion/l; sem 0·379; P < 0·007) and percentage of neutrophils (32·45 v. 25·03 %; sem 3·409; P < 0·027).
Blood biochemistry
The blood biochemistry levels can be seen in online Supplementary Table S3. Piglets in the MILK group had higher levels of albumin (29·9 v. 25·5 g/l; sem 1·73; P < 0·013), cholesterol (2·7 v. 2·0 mmol/l; sem 0·16; P < 0·001), inorganic phosphate (2·2 v. 1·7 mmol/l; sem 0·19; P < 0·010), blood urea nitrogen (6·6 v. 3·5 mmol/l; sem 0·64; P < 0·001) and TAG (1·2 v. 0·7 mmol/l; sem 0·15; P < 0·001) compared with the WHEAT group.
Transcriptomic analysis
Differential gene expression analysis showed only minor differences between the MILK and WHEAT groups, indicating that the intestinal transcriptome was largely unaffected by the modified milk replacer. After correction for multiple testing, DEseq2 analysis did not detect any significantly altered genes. NOIseq analysis also did not detect any differentially regulated genes at the standard false discovery rate cut-off of q ≥ 0·8. However, inspection of genes ranked by fold change between groups showed that a number of the most down-regulated genes in the WHEAT group compared with the MILK group were related to inflammatory processes (e.g. serum amyloid A protein (SAA2) and chemokine (C-C motif) ligand 20 (CCL20)) (online Supplementary Table S4). Quantitative PCR analysis of four down-regulated genes with q values > 0·6 (SAA2, CCL20, plasminogen activator inhibitor-2 (SERPINB2) and TNFAIP3 interacting protein 3 (TNIP3)) confirmed the negative fold changes and tended towards significance (P < 0·15) but again was not statistically different between groups (Fig. 3(a)). Of the top ten up-regulated genes identified by NOIseq analysis, seven were involved with B-cell function (e.g. CD19) (online Supplementary Table S4). Consistent with this, gene-set enrichment analysis identified four pathways, all involved with B-cell signalling, to be significantly enriched in the WHEAT group compared with the MILK group (P < 0·005; q < 0·2; Fig. 3(b)). Thus, wheat inclusion did not induce significant transcriptional changes in the intestine, apart from minor indications of an enhancement of humoral immunity-related gene pathways.
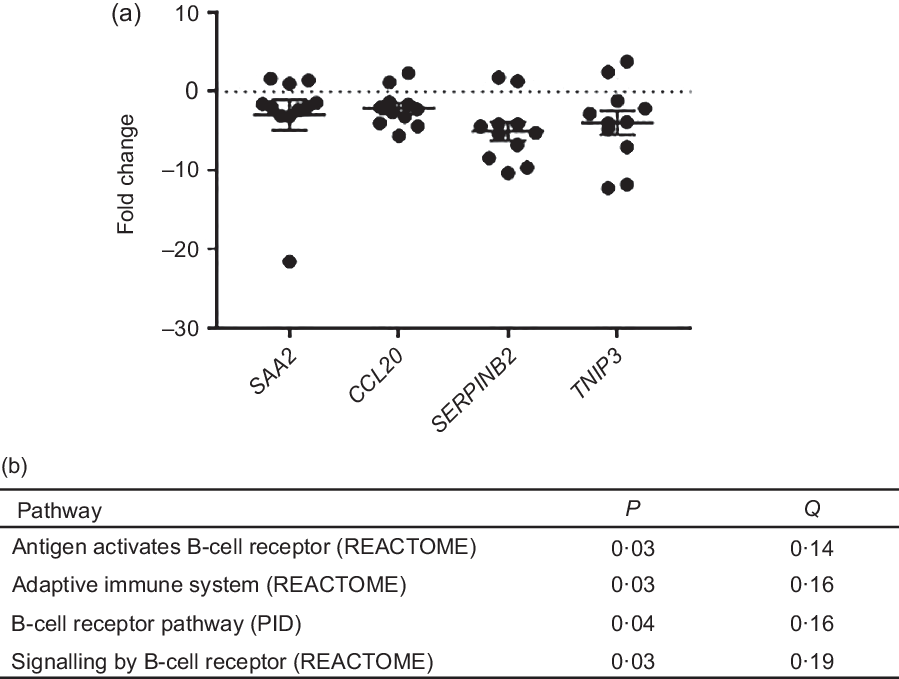
Fig. 3. (a) Fold changes of gene expression of serum amyloid A protein (SAA2), chemokine (C-C motif) ligand 20 (CCL20), plasminogen activator inhibitor-2 (SERPINB2) and TNFAIP3 interacting protein 3 (TNIP3) induced by WHEAT treatment as measured by quantitative PCR. Fold change is relative to the MILK group. Values are means with their standard errors. (b) Significantly up-regulated gene pathways in the WHEAT group compared with the MILK group (P < 0·05; Q < 0·2) identified by gene-set enrichment analysis from REACTOME and PID databases.
Gut morphology
The gut morphology results can be seen in online Supplementary Table S5. No differences were found in villous height, crypt depths, enterocyte heights or villous:crypt depth ratio between the two groups (P > 0·05).
Discussion
The purpose of this trial was to investigate the effect of a milk replacer with an increasing amount of wheat on gut function and health of piglets, relative to pure milk. The hypothesis was that adding wheat induces maturation of the mucosa as measured by higher digestive activity and improved integrity and immunity.
The WHEAT group had markedly higher levels of maltase and sucrase in the proximal part of the SI compared with the MILK group. As carbohydrate digestion and absorption begin in the proximal part of the SI, this suggests that the WHEAT group had a higher digestive capacity towards vegetable feed products. The gut contains enzymes that can be altered dependent on diet as early as 2 d of age. For example, Jensen et al. (Reference Jensen, Elnif and Burrin10) found in bottle-fed newborn piglets that pigs fed milk replacer showed elevated sucrase activity and lowered maltase, lactase and aminopeptidase N activities relative to colostrum-fed controls. Correspondingly, the enzyme activity levels in the WHEAT group showed an increase in sucrase and maltase in the proximal SI. In addition, higher levels of sucrase were found in the colon of the WHEAT group compared with the MILK group and numerically higher levels of lactase in the MILK group compared with the WHEAT group. This is surprising as there is no normally lactase activity in the colon as lactase activity is only found in the brush border of the SI in humans(Reference Forsgård11) and pigs(Reference Drochner12). Kim and colleagues found nearly twice the amount of lactase activity in the caecal and colon content of pigs given a diet high in lactose and showed that this was associated with more volatile fatty acids than the control group(Reference Kim, Benevenga and Grummer13). As this suggests microbial carbohydrase activity, the observed high colon lactase activity in the present study could indeed be due to microbial activity. In addition, it was observed (but not quantified) that the WHEAT piglets did have more sticky faecal matter and especially the colon tissue was difficult to clean for faecal matter without disrupting the tissue for both groups. Therefore, we speculate that our colon samples were in fact contaminated with faecal matter.
The piglets in the present study were restricted in their access to the milk replacer to minimise the risk of diarrhoea. Consequently, a slower growth pattern was observed compared with the on-farm reference group, mainly due to a low energy content in the diet which was also apparent at the end of the trial where the two groups of pigs only had 2 % fat, where the on-farm reference group had 14 % fat (data not shown). The milk contained less energy than sow milk as it was designed to mimic a supplementary situation where the milk would be given as a supplement to sow milk. The price of milk replacer is a limiting factor for pork producers, and replacing expensive milk components with cheaper complex carbohydrates components such as wheat can significantly reduce costs. However, it is not known what effect this replacement has on growth performance and gut health of the piglet.
The blood biochemistry generally corresponded to the diets; the WHEAT group was given less protein and therefore had less serum albumin and BUN compared with the MILK group. In addition, inorganic phosphate was higher in the milk product and correspondingly higher in the MILK group. The MILK group received more fat through the diet, which was reflected by the higher TAG and cholesterol level compared with the WHEAT group.
Nutrient-dense complex milk replacer supplementation might, therefore, help piglets through the transition period at weaning by increased body weight and increased capacity for uptake of nutrients(Reference de Greeff, Resink and van Hees14). In a study by de Greeff et al. (Reference de Greeff, Resink and van Hees14), morphometric analysis demonstrated that villous height and numbers of goblet cells did not differ between a group fed nutrient-dense complex milk replacer compared with a control group that did not receive milk supplementation. However, nutrient-dense complex milk replacer-fed piglets had deeper crypts (P < 0·001) and an increased expression of the cell proliferation marker proliferating cell nuclear antigen in crypts (P < 0·05), suggesting higher cell proliferation rates compared with the control group(Reference de Greeff, Resink and van Hees14). In the present study, no differences were found in gut morphology or the histopathological state of the SI in the two groups. There was a tendency towards a higher CV ratio in the WHEAT group, suggesting that some alternations had either taken place and had been compensated for or that the wheat has caused some differences. However, there was no difference in histopathological results (data not shown) suggesting that the gut had either recovered or was not as affected as expected. Gut villi have a quick recovery rate(Reference Yamauchi15), and it is plausible that the pigs had indeed regenerated gut villi.
Transcriptomic analysis was in agreement with the histopathology data, in that there was overall very little difference between groups. This indicates that the intestinal transcriptome was largely unperturbed by the inclusion of wheat in the diet. Some minor changes were observed in gene pathways associated with B-cell signalling and humoral immune responses. This perhaps suggests an accelerated shift from innate responses to humoral immune function in these piglets. In addition, more peripheral neutrophils were found in the WHEAT group, suggesting another potential modulation of immune function. Neutrophils are normally associated with inflammation and production of reactive oxygen species; however, they also contribute to regulation of both innate and adaptive immune responses, including B-cell function(Reference Cerutti, Puga and Magri16). To further assess the immune status of the piglets, we performed LPS stimulation of peripheral mononuclear cells, which showed no differences in cytokine production between groups, suggesting that no systemic inflammation was induced by the different dietary components. Taken together, these data indicate that immune function and intestinal homoeostasis were not substantially altered by the addition of wheat to the diet and that there are no apparent detrimental effects on piglet health.
One question left unanswered by this study is: how do the pigs perform after weaning when fed higher levels of starch? The pig industry weans piglets at approximately 21–28 d of age. This requires a mature gut that it is ready for the transition from liquid feed to solid feed, stress and change of environment. Creep feed is usually offered in piggeries from day 14 onwards mostly to train the pigs to eat but also to gradually allow them to adapt to a more vegetable diet. Using dry feed in the farrowing unit increased gut absorption after weaning, and creep feeding could be a useful tool in the prevention of post-weaning diarrhoea(Reference Kuller, van Beers-Schreurs and Soede17). However, the main problem with creep feed is that there is a large percentage of pigs that do not eat the creep feed (non-eaters)(Reference Bruininx, Binnendijk and van der Peet-Schwering6). Increasing feed intake by providing milk replacer with starch could potentially increase feed intake in piglets before weaning and also mature the SI prior to weaning.
In conclusion, the piglets given a milk replacer with wheat underwent changes suggesting adaptation to a vegetable diet. The increased enzyme activity in the WHEAT group compared with the MILK group, together with the unchanged gut morphology, histopathology and gene expression, suggests that the piglets given a milk replacer with wheat do not experience detrimental health effects and instead may have higher activity of carbohydrases that are key to digestion of starch and other carbohydrates of vegetable origin. We speculate that this adaptational response to inclusion of wheat in milk diets may improve their response to subsequent weaning process, yet further studies on the longer-term implications of these results are highly warranted.
Acknowledgements
The authors thank technician Mimi Eriksen and statistician Mai Britt Nielsen from SEGES and students and colleagues who helped with sampling: Kristina Vesterager Riddersholm, Anne-Sofie Hougesen, Sascha Coes, Line Enemark, Christina Larsen, Julie Lynegaard, Flemming Thorup, Camilla Højgaard and Anja Hahn Madsen. The authors also specially thank Kristian Volshøj from Agilia and Sønke Møller from Arla for helpful discussions before and during the trial.
The research project was funded by the Danish pig levy foundation (Svineafgiftsfonden).
The authors’ contributions are as follows: C. A., M. L. M. P. and T. T. contributed to the study design and data analyses; C. A. was the principal investigator and contributed to the study design and interpretation of the findings and wrote the manuscript; C. A., M. L. M. P., J. K., M. N. E., A. R. W., L. J. M. and T. T. contributed to the study design, subject briefings and data collection; J. K., M. N. E., A. R. W. and L. J. M. contributed to the study design and carried out data analyses. All authors read and approved the final version of the manuscript.
The authors have no financial or personal conflicts of interest to declare.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520004225










