Conventional solvent-extracted soyabean meal (SBM) disturbs intestinal homeostasis in salmonids and initiates enteropathy in the distal part of the intestine, commonly referred to as SBM-induced enteritis(Reference Baeverfjord and Krogdahl1). The first signs of enteropathy are noticeable after a few days on diets containing SBM, thus restraining the use of SBM for salmonids. The severity of enteropathy is dependent on both the inclusion level of SBM(Reference Krogdahl, Bakke-McKellep and Baeverfjord2) and the origin of the soyabeans(Reference Uran, Schrama and Jaafari3). Inflammation is associated with enhanced intestinal permeability(Reference Nordrum, Bakke-McKellep and Krogdahl4), reduced endocytotic uptake of macromolecules(Reference Uran, Aydin and Schrama5) and increased proliferation of intestinal epithelial cells attributed to alterations of epithelial cell differentiation and maturation(Reference Romarheim, Øverland and Mydland6, Reference Bakke-McKellep, Press and Baeverfjord7). Also, the activities of trypsin in the intestinal digesta and a trypsin-like protease in the intestinal mucosa increase(Reference Lilleeng, Froystad and Ostby8), whereas the reactivities of several brush-border enzymes decrease(Reference Bakke-McKellep, Press and Baeverfjord7).
Saponins interacting with other alcohol-soluble components are the most likely feed components that either directly or indirectly disrupt the intestinal barrier(Reference Knudsen, Jutfelt and Sundh9, Reference van den Ingh, Olli and Krogdahl10). An increase in intestinal epithelium permeability might expose inflammation-inducing receptors on the otherwise shielded basolateral surfaces of epithelial cells to luminal antigens, which in turn initiate a T-cell-mediated inflammation(Reference Lilleeng, Penn and Haugland11, Reference Bakke-McKellep, Frøystad and Lilleeng12). Augmented levels of cell populations reacting for MHC II antibodies and the recently established monoclonal antibodies to detect cluster of differentiation 8 α (CD8α)(Reference Hetland, Jørgensen and Skjødt13) are therefore expected in response to SBM-induced enteritis.
Inclusion of microbial protein sources and extracts from microbes or yeast rich in nucleotides, phospholipids, cell wall oligo- and polysaccharides, and other bioactive components in fish diets might affect gut microflora, disease resistance and growth performance(Reference Merrifield, Dimitroglou and Foey14–Reference Li and Gatlin16). A recent study by Romarheim et al. (Reference Romarheim, Øverland and Mydland6) revealed that bacterial meal (BM) containing mainly Methylococcus capsulatus grown on natural gas prevents the development of SBM-induced enteritis in Atlantic salmon. BM has proved to be a suitable protein source for fish as well as other monogastric animals, and there are no restrictions on its use in diets for farmed animals within the European Union(Reference Øverland, Tauson and Shearer17). Dietary inclusion of BM has also been shown to increase the intestinal weight (g/kg whole body) in salmonids(Reference Romarheim, Øverland and Mydland6, Reference Storebakken, Baeverfjord and Skrede18), which indicates a profound effect of BM on the intestinal wall.
The present experiment was designed to study intestinal changes associated with enteropathy in Atlantic salmon fed diets with a high content of SBM in combination with graded levels of BM. We hypothesised that (1) the protective effect of BM against SBM-induced enteritis is dose dependent, (2) the inflammatory response will be linked to the enhanced density of inflammation markers in salmon such as MHC II and CD8α positive (CD8α+) T lymphocytes and (3) dietary BM will normalise the expression of these inflammation markers.
Materials and methods
Diets and fish experiment
For the present experiment, eight diets were formulated: a fish meal (FM)-based control diet, and seven diets with the FM replaced by 200 g/kg SBM combined with BM levels ranging from 0 to 300 g/kg BM (Table 1). The diets were formulated to have similar ratios among crude protein, crude lipid and starch based on analyses of the feed ingredients. Feed was produced at the Center for Feed Technology at the Norwegian University of Life Sciences, Ås, Norway, using extrusion technology and vacuum coating with fish oil after drying. The finished feed pellets were 3·5 mm in diameter.
Table 1 Formulation and analysed chemical composition of the experimental diets
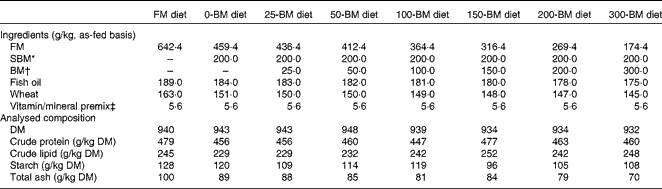
FM, fish meal; BM, bacterial meal; SBM, soyabean meal.
* Deno-Soy F, solvent-extracted and toasted SBM (Denofa).
† BioProtein (Norferm AS).
‡ Supplied/kg of diet: all-trans-retinyl acetate, 860 μg; cholecalciferol, 37·5 μg; d, l-α-tocopherol acetate, 200 mg; menadione, 10 mg; thiamin, 15 mg; riboflavin, 25 mg; nicotinic acid, 75 mg; pantothenic acid, 30 mg; pyridoxine, 15 mg; folic acid, 5 mg; cyanocobalamin, 20 μg; ascorbyl monophosphate, 125 mg; biotin, 0·25 mg; Ca, 1·1 g; ZnSO4, 296 mg; MnSO4, 41 mg; CuSO4, 13 mg; CoSO4, 2·6 mg; CaI2, 3·5 mg; astaxanthin, 175 mg; Y2O3, 100 mg.
The fish experiment was carried out at the Nofima research station at Sunndalsøra, Norway. Non-vaccinated Atlantic salmon (Salmo salar) with an average initial weight of 273 g were randomly distributed into sixteen tanks (fifty fish/tank). The tanks contained 460-litre seawater each (10·3°C; 32·5 g NaCl/l) and were supplied with 18 litres water/min and light 24 h/d. All fish were fed the FM diet during the last 3 weeks before the feeding experiment to avoid interactions with the commercial diet used at the research station. Thereafter, each of the eight experimental diets were fed to two replicate fish tanks every 32 min by electrically driven disc feeders for 47 d. Feed intake was measured according to the method of Helland et al. (Reference Helland, Grisdale-Helland and Nerland19). The experiment was conducted in accordance with the regulations given by the National Animal Research Authority in Norway (Animal Protection Ordinance concerning experiments with animals of 15 January 1996).
Sampling and chemical analysis
Fish were weighed at the start and end of the experiment. At the termination of the experiment, all fish were anaesthetised with tricaine methanesulfonate (60 mg/l water) and killed by a sharp blow to the head. Tissue from the distal intestine of six fish/tank was fixed in 10 % neutral phosphate-buffered formalin for 2 d before being dehydrated in ethanol, equilibrated in xylene and embedded in paraffin according to standardised routines. Faeces were stripped from the remaining fish in each tank according to the procedure described by Austreng(Reference Austreng20), pooled by tank, frozen and freeze-dried before the analysis. The gastrointestinal tract from ten fish/tank was emptied for digestive chyme and weighed. Thereafter, the gall bladder and fat from the mid- and distal intestine were removed, and the weight of the liver, stomach, pyloric region, mid-intestine and distal intestine was measured.
Diets and faeces were analysed for DM (105°C overnight), ash (550°C overnight), crude protein (Kjeldahl, N × 6·25), crude lipid after hydrolysis with petroleum diethyl ether on an accelerated solvent extractor (Dionex ASE200; Dionex), and starch as total glucose after hydrolysis with α-amylase and amyloglucosidase after lipid removal by acetone. Yttrium as a marker for nutrient digestibility was determined by inductively coupled plasma (PerkinElmer Optima 5300 DV; Perkin Elmer).
Histology, immunohistochemistry and morphometry
Routine histological examination was performed on formalin-fixed and paraffin-embedded sections stained by haematoxylin and eosin. Scoring of the sections was performed according to the following criteria:
(1) Lamina propria – accumulation of leucocytes such as lymphocytes, granulocytes and eosinophilic granular cells in the lamina propria.
(2) Changes in the epithelium – reduced supranuclear vacuolisation, reduced cellular height and increased cytoplasmic basophilia.
(3) Atrophy – reduced height of the intestinal folds.
(4) Oedema – inflammatory oedema comprising accumulation of protein-rich fluid in the lamina propria.
The sections were given an individual score for each of the criteria above ranging from 0 to 2, where 0 indicated no signs of abnormal changes, 1 weak changes, and 2 marked changes. The score 0·5 was given if slight changes appeared, although such changes were likely to be within the normal range. A score of at least 1 should be obtained for parameters 1–3 in order to claim typical SBM-induced enteritis.
Paraffin sections from the distal intestine were used for immunohistochemistry. The procedures of antigen retrieval and immunostaining including details of antibodies used have been reported previously; for proliferating cell nuclear antigen (PCNA), the procedure was as described by Romarheim et al. (Reference Romarheim, Øverland and Mydland6), and for CD8α and MHC II, the procedure followed was according to Hetland et al. (Reference Hetland, Jørgensen and Skjødt13).
CD8α+ cells and stretches of epithelial cells stained for PCNA were measured using ImageJ software (1.42q version; ImageJ). The quantities of CD8α+ cells were expressed as the density of stained cells at the base of the intestinal epithelium in simple folds. The length of stretches with PCNA-reactive epithelial cells was expressed in percentage of a baseline drawn through the base of the folds.
Calculations and statistical analysis
Feed intake during the experiment was expressed as DM in feed (DMfeed)/initial body weight (BW0). Weight gain was calculated as:
The feed conversion was calculated as:
Thermal growth coefficient (TGC) was calculated as:
Apparent digestibility of nutrients was calculated as:
where I D and I F represent the concentration of the inert marker in diets and faeces, and N F and N D represent the concentration of nutrients in faeces and diets, respectively.
The data were analysed by one-way ANOVA to determine the effects of diets, or Welch's test if the variances differed significantly among the treatments. Comparisons among treatment means were ranked by the least significant difference multiple-range test given a significant model. Quadratic regression analysis was applied to determine the effects of graded inclusion of BM in diets with the SBM. Non-parametric data from the histological evaluation were analysed by Kruskal–Wallis ANOVA by ranks. Statistical analysis was performed using SAS procedures (SAS Institute, Inc.). P< 0·05 was considered as statistically significant.
Results
Histological and immunohistochemical evaluation
All sampled fish fed the 0-BM diet developed typical SBM-induced enteritis with accumulation of leucocytes in the lamina propria, reduced supranuclear vacuolisation and cellular height of epithelial cells, increased epithelial cytoplasmic basophilia, atrophy with shortening of the simple and complex intestinal folds, and widening of the lamina propria and submucosa due to cellular infiltration and oedema (Figs. S1 and S2, available online), as previously described in details by Baeverfjord & Krogdahl(Reference Baeverfjord and Krogdahl1). The morphological changes of the intestinal tissue gradually disappeared as the inclusion of the BM increased, and all features mentioned above were evaluated to concur with minor changes (scores between 0·5 and 1·0) when 50–100 g/kg BM were added to the diets (Fig. 1). Fish fed diets with 150 g/kg BM and more had no or only weak signs of intestinal change, not significantly different from fish fed the FM diet. Occasionally (two of twelve fish on the 200-BM diet and one of twelve fish on the 300-BM diet), single and isolated leucocyte aggregates of mainly lymphocytes were found in an otherwise normal intestine. These aggregates were located in the lamina propria/submucosa above the stratum compactum. Large pale mononuclear cells were found in the centre of the aggregates in the individual fed the 300-BM diet, indicative of granuloma formation (results not shown).

Fig. 1 Histological changes in the distal intestine of Atlantic salmon fed diets with 200 g/kg soyabean meal in combination with increasing inclusion of bacterial meal (BM, ■). The individual histological sections were evaluated with respect to changes in (A) leucocyte infiltrates in the lamina propria/submucosa, (B) epithelium, (C) atrophy of the simple and complex folds and (D) oedema, i.e. accumulation of fluid in the lamina propria, and were given a grading of 0 (no signs of intestinal changes), 0·5 (traces of changes), 1 (weak changes) or 2 (marked changes). All sampled fish fed the FM diet (▲) had a normal distal intestine as evaluated by the studied criteria. Values are means (n 12), with standard deviations represented by vertical bars. a,b,c,d,eMean values with unlike letters were significantly different (P< 0·05).
Staining for CD8α identified a population of CD8α+ lymphocytes at the base of the intestinal epithelium (Fig. 2), in location corresponding to that earlier reported for CD3 in salmon(Reference Bakke-McKellep, Frøystad and Lilleeng12). CD8α+ lymphocytes in the epithelium were more prevalent on the 0-BM diet than on the FM diet. An increasing inclusion of BM resulted in a gradual decline in the density of CD8α+ intraepithelial lymphocytes, and inclusion of 200 g/kg BM gave values not significantly different from that of fish fed the FM diet (Fig. 3). The lamina propria and submucosa also contained a considerable proportion of CD8α+ lymphocytes at low dietary BM levels (Fig. 2).

Fig. 2 Immunohistochemistry for CD8α. (A) Fish meal (FM) diet, (B) soyabean meal bacterial meal (BM) diet at 0 g/kg (0-BM), (C) soyabean meal 50-BM diet and (D) soyabean meal 300-BM diet. Positive lymphocytes were largely confined to the basal part of the epithelium, although a few were found in the lamina propria/submucosa. On the 0-BM and 50-BM diets, a marked increase in the intensity of reaction was seen, whereas numbers were similar to those of the FM diet. Magnification is the same in (A)–(D); scale bar, 200 μm.

Fig. 3 Density of CD8α+ lymphocytes in the lamina propria and submucosa of the simple folds in the distal intestine of Atlantic salmon fed diets with different dietary combinations of soyabean meal (SBM) and bacterial meal (BM), or neither for 47 d. The quantities of CD8α+ cells were expressed as the density of stained cells. Values are means (n 12), with standard deviations represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P< 0·05). Quadratic equation: y= 9·45 − 0·026x+0·000035x 2, R 2 0·33, P< 0·001. ■, Fish meal diet; ▲, diets with SBM.
Staining for MHC II gave a strong reaction in the brush border and the supranuclear cytoplasm of the epithelium, in leucocytes at the base of the epithelium, and in scattered cells of the lamina propria and submucosa of fish fed the FM diet (Fig. 4). The reactive leucocytes often showed distinct reactive cytoplasmic extensions or dendrites, corresponding to a reaction in dendritic cells or macrophages. Such reactive leucocytes were numerous in fish fed the 0-BM diet, whereas the epithelial reaction was mostly conspicuously absent or weak. With increasing inclusion of BM, this reaction approached that of fish fed the FM diet.

Fig. 4 Immunohistochemistry for MHC II. (A) Fish meal (FM) diet, (B) soyabean meal bacterial meal (BM) diet at 0 g/kg (0-BM), (C) soyabean meal 50-BM diet and (D) soyabean meal 300-BM diet. On the FM diet and the 300-BM diet, the staining pattern was quite similar: There was a strong reaction in the epithelium and particularly close to the luminal border. Reactive dendritic cells were seen in the basal part of the epithelium and scarcely in the lamina propria/submucosa. On the 0-BM and 50-BM diets, a marked decrease in the intensity of epithelial reaction was apparent. Scattered reactive dendritic cells were seen in the epithelium and lamina propria/submucosa. Magnification is the same in (A)–(D); scale bar, 200 μm.
The epithelium of fish fed the 0-BM diet had the longest stretches of PCNA-stained cells, and the reactivity was reduced with increasing inclusion of BM (Fig. 5). Fish fed the diets with 150 g/kg BM or more had PCNA-stained stretches not significantly different from those fed the FM diet.

Fig. 5 Stretches of proliferating cell nuclear antigen (PCNA)-reactive epithelial cells in the distal intestine of Atlantic salmon fed diets with different dietary combinations of soyabean meal (SBM) and bacterial meal (BM), or neither for 47 d. The length of PCNA stretches was measured and expressed in relation to a baseline drawn through the base of the folds. Values are means (n 12), with standard deviations represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P< 0·05). Quadratic equation: y= 289 − 1·32x+0·0028x 2, R 2 0·59, P< 0·001. ■, Fish meal diet; ▲, diets with SBM.
Growth and nutrient digestibility
Only one fish died 2 d after the experiment started; otherwise no mortality was observed. The ANOVA and regression analyses did not show any effects of diets on feed intake, weight gain, feed conversion ratio or TGC (P>0·05; Table 2). An effect of diet was found for crude protein digestibility (P= 0·037), i.e. slightly lower protein digestibility in fish fed the 300-BM diet (82·2 %) than in those fed the FM diet (83·7 %) and diets with 0–150 g/kg BM (83·8–84·9 %). This effect was confirmed by a regression line (R 2 0·60, P= 0·006), indicating reduced protein digestibility with a high dietary inclusion of BM, whereas moderate inclusion levels had a minor effect. Lipid digestibility ranged from 95 to 96 %, and starch digestibility from 63 to 69 %, but no significant effects of the diets were found.
Table 2 Feed intake, growth, feed conversion and apparent digestibility of macronutrients in fish fed a fish meal (FM) diet or diets with 200 g/kg soyabean meal in combination with increasing inclusion of bacterial meal (BM) for 47 d (Least square mean values with their pooled standard errors, n 3 tanks/diet)
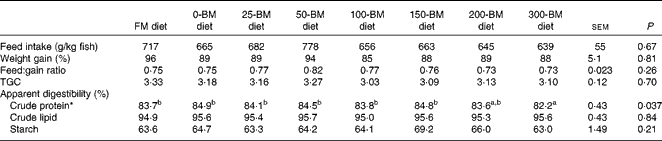
TGC, thermal growth coefficient.
a,bMean values with unlike superscript letters were significantly different (P< 0·05).
* Quadratic equation: y= − 0·00003x 2+0·002x+84·5, R 2 0·60, P= 0·006. The FM diet was not included in the regression analysis.
Liver and gut weights
The weight of the distal intestine relative to the whole body weight was affected by the diet (P= 0·033), whereas no significant differences were found for total gut, liver, stomach, pyloric region or mid-intestine (Table 3). Fish fed the 25-BM diet had the lowest distal intestine weight (5·2 g/kg body weight) and fish fed the 300-BM diet had the highest weight (7·0 g/kg body weight). The regression analysis indicated that increasing dietary BM levels increased distal intestinal weights throughout the whole inclusion range.
Table 3 Relative weights of the liver and gut in fish fed a fish meal (FM) diet or diets with 200 g/kg soyabean meal in combination with increasing inclusion of bacterial meal (BM) for 47 d (Least square mean values with their pooled standard errors, n 3 tanks/diet)
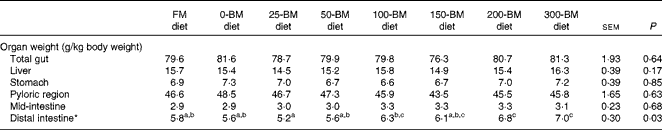
a,b,cMean values with unlike superscript letters were significantly different (P< 0·05).
* Quadratic equation: y= − 0·000008x 2+0·008x+5·3, R 2 0·68, P= 0·002. The FM diet was not included in the regression analysis.
Discussion
Healthy individuals maintain a fine balance between tolerance to the commensal intestinal microbiota and feed antigens on the one hand, and an appropriate immune response against both bona fide pathogens and potential pathogens, on the other hand. This homeostasis seems to be interrupted in salmonids fed SBM, but may be restored by sufficient dietary addition of BM produced on natural gas, as shown in the present experiment. The mobilisation of CD8α+ T cells in the distal intestine of salmon with SBM-induced enteritis supports the interpretation that this is a T-cell-mediated inflammation(Reference Lilleeng, Penn and Haugland11, Reference Bakke-McKellep, Frøystad and Lilleeng12). It seems likely from our immunohistochemical studies that a large percentage of the intraepithelial lymphocyte population is CD8α+, as observed in mammals(Reference Cheroutre, Lambolez and Mucida21). It is thus possible that CD8α+ lymphocytes perform the same surveyor role in the salmon epithelium as in mammals, and that stress induced by SBM challenges CD8α+ lymphocytes to eliminate affected epithelial cells whether this is appropriate or not. Thus, SBM-induced enteropathy may resemble chronic intestinal conditions such as inflammatory bowel disease in humans, where the gut microbiota(Reference Cerf-Bensussan and Gaboriau-Routhiau22) and the activation of Toll-like receptors at the basolateral surface of epithelial cells(Reference Abreu23) seem to play a central role. Dietary pro- and prebiotics may affect fish health through alteration of the intestinal microbiota(Reference Merrifield, Dimitroglou and Foey14, Reference Nayak24), and the substitution of dietary FM by SBM has shown to alter the microbiota of salmonids(Reference Krogdahl, Bakke-McKellep and Baeverfjord2–Reference Bakke-McKellep, Penn and Salas27). Although Bakke-McKellep et al. (Reference Bakke-McKellep, Penn and Salas27) reduced the population of bacteria adherent to the intestinal wall and in the digesta by adding 3 g/kg of the broad-spectrum antibiotic oxytetracycline to a diet with SBM, no effect was observed on the severity of SBM-induced enteropathy.
The present experiment confirms that MHC II molecules are normal constituents of the salmon distal intestinal epithelium, as reported previously by Bakke-McKellep et al. (Reference Bakke-McKellep, Koppang and Gunnes28). An earlier study of rainbow trout lymphocytes derived from the intestinal epithelium showed that they have a polyclonal, differentiated profile and are probably quite responsive(Reference Bernard, Six and Rigottier-Gois29), compatible with an active role of the epithelium in uptake, processing and presentation of luminal antigens. In the human ileum, MHC II reactivity has been demonstrated in multivesicular late endosomes(Reference Hundorfean, Zimmer and Strobel30), and the findings in mice suggest that ileal epithelial MHC II reactivity might signify an antigen presentation by MHC II carried by exosomes(Reference Büning, von Smolinski and Tafazzoli31). Secretion of MHC IIβ-containing exosomes has also been found in head kidney leucocytes from salmon(Reference Iliev, Jørgensen and Rode32). Multivesicular endosomes are also present in the distal intestinal epithelium of rainbow trout(Reference Ezeasor and Stokoe33), although it is not known whether these multivesicular bodies are involved in the production of exosomes. Many teleosts, including salmonids, are also equipped for the uptake of intact or partially fragmented proteins through the distal intestine(Reference Sire and Vernier34), and this function is impaired in fish with SBM-induced enteritis(Reference Uran, Aydin and Schrama5). The MHC II reaction in epithelial cells in the distal part of the salmon intestine might therefore be associated with endocytic and/or exocytic activity.
The role of MHC II in the epithelium is not fully elucidated, but its constituent character suggests that it is not necessarily involved in the pro-inflammatory scenario that signifies fully developed SBM-induced enteritis. A role in exosome production or T-cell development/reactivity could be more likely, catering to the intraepithelial lymphocytes or dendritic cells as part of a role in maintaining intestinal homeostasis or tolerance. In such a perspective, the decline of MHC II during SBM-induced enteritis could also imply the lack of maturity associated with increased turnover of epithelial cells. The lack of epithelial differentiation and maturity in fish with SBM-induced enteritis is also supported by the enlarged stretches of PCNA-stained cells, and the lack of carbonic anhydrase isoenzyme 12 in the brush border found in a previous study(Reference Romarheim, Øverland and Mydland6). The role of MHC II on dendritic cells or macrophages is more obvious. Although present in the normal intestinal mucosa, MHC II-bearing dendritic cells clearly represent a constituent of the inflammatory infiltrate, such as the increased presence of CD8α+ T cells. The lack of suitable antibodies in fish prevented a further dissection of pathogenesis by immunohistochemistry. However, the mRNA expression of CD4, CD8α and CD8β was up-regulated about two-fold in the distal intestine of Atlantic salmon with SBM-induced enteritis(Reference Bakke-McKellep, Frøystad and Lilleeng12), which suggests active CD4+ populations.
What could be the mechanism for the ameliorating effect of BM on SBM-induced enteritis? Most probably the effect is related to mechanisms ensuring tolerance to feed antigens and the commensal intestinal microbiota. Regulatory CD4+ and CD8+ T cells (Treg) expressing the forkhead box p3 (Foxp3) transcription factor are important for keeping an inflammatory action against food antigens and commensal bacteria at bay in humans and mice, and a reduction of Treg cells may interrupt the fine-tuned homeostasis and lead to inflammatory bowel disease(Reference Fleissner, Frede and Knott35, Reference Hardenberg, Steiner and Levings36). Rainbow trout express both a Foxp3a and Foxp3b gene with high similarities to mammals in the C-terminal region but with less homology in the N-terminal region(Reference Wang, Monte and Huang37). A CD4-2+CD25-like+Foxp3-like+ phenotype has been found in pufferfish, resembling CD4+CD25+Foxp3+ Treg cells in mammals, and depletion of these cells lead to intestinal inflammation, indicating a similar Treg immunological system in fish and mammals(Reference Wen, Fang and Xiang38). A stimulatory effect on Treg would thus be one of the most attractive candidate pathways for the homeostasis-supporting effect of BM. It is also possible that the pathway includes a microbial pattern recognition receptor in the brush border(Reference Abreu23). Such receptors might detect the known massive presence of microbial products on a BM diet. Receptors residing in the luminal brush border have been shown to be involved in subduing inflammation. Thus, deleterious host pro-inflammatory responses can be modulated or prevented by microbiota-derived factors(Reference Cerf-Bensussan and Gaboriau-Routhiau22). BM might thus have a protective effect on the epithelial cells resembling that of intestinal microbial commensals.
Feed intake, growth and nutrient utilisation are dependent on the characteristics of fish, experimental conditions and diets, and an evaluation of these parameters is important to unveil possible artifacts in the experiment. Feed intake in the present experiment was similar or slightly lower than recent table values for commercial farming of Atlantic salmon provided by Skretting AS(Reference Skretting39), whereas the feed conversion ratio was similar or slightly better. The slightly reduced crude protein digestibility with increasing BM inclusion is similar to previous results for salmonids(Reference Romarheim, Øverland and Mydland6, Reference Øverland, Tauson and Shearer17, Reference Aas, Grisdale-Helland and Terjesen40). This effect is likely to be caused by cell-wall components in BM, as seen with yeast products fed to salmonids(Reference Rumsey, Hughes and Smith41), and by the extensive intracytoplasmic membranes in M. capsulatus grown on natural gas, as suggested by Skrede et al. (Reference Skrede, Mydland and Øverland42).
The weights of the distal intestine were almost identical to those found by Romarheim et al. (Reference Romarheim, Øverland and Mydland6) when feeding similar diets. Fish with SBM-induced enteritis suffer from an excessive loss of epithelial cells in the distal intestine that lowers the weight of this segment(Reference Refstie, Bakke-McKellep and Penn43, Reference Romarheim, Skrede and Gao44), and the increased proliferation indicated by the long stretches of PCNA-stained cells cannot compensate for this loss. The finding that the relative weight of the distal intestine continued to increase with BM inclusion above the level necessary to prevent enteritis suggests that BM stimulates intestinal growth. This might be due to the high content of purine and pyrimidine bases in BM that can serve as growth substrates for intestinal epithelial cells(Reference Li and Gatlin16).
Soya is a suitable protein source for farmed fish with its relatively constant and well-balanced amino acid composition, but the morphological changes found with inclusion levels of a SBM as low as 7·6 %(Reference Krogdahl, Bakke-McKellep and Baeverfjord2) have been a major constrain for its use in diets for salmonids. The present findings open up for higher dietary inclusions of a SBM, if combined with BM.
It is concluded that the protective effect of BM against SBM-induced enteritis in salmon is dose dependent. The inflammatory response is linked to the enhanced density of CD8α+ T lymphocytes at the base of the epithelial cells and MHC II-reactive cells in the lamina propria and submucosa. The level of MHC II was, however, reduced in the epithelial cells and brush border in fish with an inflamed intestine. The level of these inflammatory regulators was normalised by adequate dietary inclusion of BM, but the underlying regulatory mechanisms remain unknown.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114512002899
Acknowledgements
This study was supported by the Research Council of Norway (grant no. 182543). O. H. R. had primary responsibility for the experimental design, feed production, fish experiment, statistically analyses and the writing of the manuscript. D. L. H. performed the immunohistochemistry, analysed the data and contributed to the writing of the manuscript. A. S., M. Ø. and L. T. M. were involved in the design of the experiment and manuscript drafting. T. L. was involved in the design of the experiment, performed the histology evaluation and contributed to the writing of the manuscript. All authors read and approved the final manuscript. The authors have no conflicts of interest to declare.










