Vitamin D deficiency has become epidemic for all age groups worldwide, and it has been estimated that about one billion people have either vitamin D deficiency or relative insufficiency. Vitamin D insufficiency is associated with metabolic bone disease and increases the risk for many common chronic diseases(Reference Holick1). Vitamin D is essential for the maintenance of Ca homeostasis and bone mineralisation. Vitamin D deficiency can result in osteomalacia and aggravate age-related bone loss, leading to osteoporosis(Reference Chapuy, Arlot and Duboeuf2). Restricted sunlight exposure, reduced cutaneous synthesis of vitamin D and inadequate dietary intake are considered to be the main factors responsible for inadequate vitamin D status(Reference Webb, Pilbeam and Hanafin3, Reference Salamone, Dallal and Zantos4). Serum 25-hydroxyvitamin D (S-25OHD) is the most commonly used index of vitamin D status because it gives information on the amount of vitamin D in the liver, the major tissue reserve of vitamin D(Reference Fraser5).
Seasonal variations in S-25OHD concentrations in northern latitudes have been reported previously. Due to the increased zenith angle of the sun, most solar UV-B photons are absorbed by the earth's ozone layer 4–6 months per year in winter (from October to March) and the skin makes little or no vitamin D3. Due to this high latitude and limited exposure to sunlight, populations living in Nordic countries are likely to be dependent on dietary sources of vitamin D to meet their biological needs during a considerable part of the year. The intake of fish, milk and milk products is very low in immigrant populations living in Europe(Reference Henriksen, Brunvand and Stoltenberg6). Milk and margarine have been the major food sources fortified with vitamin D in Finland and adequate consumption of these food sources is necessary to meet the requirement. Studies from the UK, Norway and The Netherlands have suggested that immigrants are at risk for serious vitamin D deficiency and low bone mass compared with native inhabitants(Reference Henriksen, Brunvand and Stoltenberg6–Reference Roy, Berry and Pye10). However, with no endogenous synthesis of vitamin D during winter together with inadequate dietary intake, immigrant women living in Finland may be at particular risk for the deleterious effects of vitamin D insufficiency, which could result in low bone mass. The increase in morbidity associated with osteoporosis and other vitamin D deficiency-related health problems among these subjects may involve future strain of the health care system of Finland. However, to the best of our knowledge, no study has reported vitamin D and bone mineral density (BMD) status in immigrant women in Finland. Thus, it is of great interest to compare vitamin D and BMD status between ethnic Finnish and immigrant women.
The study was conducted in late winter (late January to February), when S-25OHD concentrations are lowest. The objectives of the present study were to determine the prevalence of hypovitaminosis D and high serum intact parathyroid hormone (S-iPTH) concentration levels in immigrant subjects compared with ethnic Finnish women. Since vitamin D status and S-iPTH levels affect BMD in adults, we also investigated the ethnic differences in forearm bone parameters at the distal and diaphyseal radius, using peripheral quantitative computed tomography in two groups of immigrant women of South Asian and African descent and compared the findings with that of Finnish women.
Subjects and methods
Study setting and subjects
The study was carried out in the Helsinki area (60°N) during January–February 2008. A total of 143 subjects were recruited from two groups of immigrant women (Bangladeshi, n 34 and Somali, n 48) and a group of ethnic Finnish women (n 61; Caucasian). The eligibility criteria for inclusion of subjects comprised no history of serious medical conditions, no history of medication known to affect bone metabolism, no current pregnancies and no lactation within the previous 2 years. The immigrant participants were mainly pre-menopausal Muslim women who had immigrated to Helsinki at least 3 years earlier. The background, objectives and recruitment criteria of the present study were written in Bangla, Finnish and Somali languages. Our first step was to contact the immigrant subjects through personal communication, family connections and a community network to explain the purpose of the study, supply them all information and to request their cooperation. We had an advertisement at the Helsinki University webpage in Finnish. The ethnic Finnish subjects mainly showed their interest for participation with this approach and communicated with us by telephone and email connection. The subjects were asked to consent to participate in the study. In some cases in the immigrant groups, permission of the husband was sought for the subjects to participate. The study protocol was approved by the ethics committee of the Hospital of the District of Helsinki and Uusimaa, Finland.
Anthropometric measurements and other data
Standing height (m) was measured, using a wall-mounted scale to the nearest 0·5 cm. Body weight was measured without shoes and with light clothing on a weighing scale to the nearest 0·5 kg. We used the classifications of BMI (weight (kg)/height (m2)) recommended by the WHO(11). A complete questionnaire was developed regarding necessary information such as name, age, address, education level, parity, out-door clothing, frequency and duration of sunlight exposure, use of sunlight protection cream, medical history, use of vitamin D supplements within 3 months, etc. A FFQ was used to determine the dietary vitamin D intake from the last 1 month based on frequency of consumption of specific foods that are believed to contribute to the dietary intake of vitamin D. We have developed and validated a FFQ, which was used for the estimation of dietary intake of vitamin D in the Finnish population. For the present study, we further developed and validated the FFQ in a small group of subjects. Due to some limitations, estimation of dietary intake of vitamin D and collection of other background information was not possible in Bangladeshi subjects.
Laboratory measurements
Fasting blood samples were collected between 08.30 and 10.00 hours. The serum was preserved at − 20°C in the freezing room of the Division of Nutrition, Department of Food and Environmental Sciences, University of Helsinki until analysed.
S-25OHD was measured from all fasting samples with an OCTEIA immunoenzymometric assay (IDS, Boldon, UK). The intra- and inter-assay CV were 4·9 and 6·8 %, respectively. Reproducibility was ensured by adhering to the Vitamin D External Quality Assessment Scheme. Standardised concentrations of S-25OHD were provided. We defined vitamin D status as severely deficient when S-25OHD was < 25 nmol/l, deficient when S-25-OHD was < 50 nmol/l, insufficient when S-25-OHD was between 51 and 74 nmol/l and sufficient when S-25-OHD was >75 nmol/l, according to the reference values for an adult population(Reference Holick12, Reference Bischoff-Ferrari, Giovannucci and Willett13). The S-iPTH level was measured with a commercial two-site immunoenzymometric assay (OCTEIA, IDS), with 10–65 ng/l as a reference range. Intra- and inter-assay CV for iPTH were 3·8 and 5·7 %, respectively. We defined secondary hyperparathyroidism (SHPT) as S-iPTH levels >65 ng/l. The laboratory analysis was carried out at the Department of Food and Environmental Sciences.
Peripheral quantitative computed tomography
Peripheral quantitative computed tomography measurements were taken in the radius, using a Stratec XCT-2000 scanner (Stratec, Pforzheim, Germany), software version 5.50d. All measurements were taken in the non-dominant forearm at 4 and 66 % of the forearm length (measured with a tape measure), proximal to the distal radial joint surface. The total bone mass (g/cm), total area (mm2), cortical area (mm2), trabecular density (mg/mm3) were determined at the radius 4 % (distal) site. At the radius 66 % (proximal) site, total bone mass (g/cm), total area (mm2), cortical area (mm2), cortical density (mg/mm3), stress–strain index (a measure of bone torsional strength; mm3) and cortical area:total area ratio (mm2) were measured. Phantom scans were executed daily to maintain quality assurance. The long-term CV for the phantom BMD and bone mineral content were 1·9 and 1·1, 2·7 and 0·79 and 0·50 and 0·78 % in the total, cortical and trabecular bone, respectively. Short-term precision (CV%) was determined with duplicate measurements of eight subjects. CV for the total BMD and bone mineral content were 4·4 and 4·1, respectively.
Statistical analysis
Statistical analyses were carried out with SPSS version 15.0 for Windows (SPSS, Inc., Chicago, IL, USA). Univariate analyses were performed for selected variables. The normal distribution of variables was assessed with a Kolmogorov–Smirnov test. If the data were not normally distributed, they were logarithmically transformed before further analysis. Ethnicity was used as a categorical variable as well as a dummy variable (Caucasian origin = 0, non-Caucasian origin = 1). Post hoc tests were performed with Tukey's honestly significant different test and Dunnett's test. Bone data and other selected variables were tested with both ANOVA and ANCOVA to show the effect of confounding factors. To find a statistically significant differences, post hoc tests for ANCOVA were done by comparing if the 95 % CI overlap. For each analysis, the covariates are mentioned in the tables. All data are reported as means and standard deviations; P < 0·05 was considered significant.
Results
Basic characteristics of the subjects
There were statistically significant differences in anthropometric characteristics in the groups of immigrant women and ethnic Finnish women (Table 1). In Bangladeshi subjects, short stature was highly prevalent and the mean BMI value was slightly above the upper limit of the desired range. Somali subjects had a significantly higher BMI value (P < 0·001) than Finnish subjects. We identified three levels of education (category 1 = primary level, category 2 = secondary level and category 3 = above the secondary level) among the subjects. About 61 % of the subjects in the Somali group had category 1 level of education. Less than 17 % of the subjects in this group had category 3 level of education, whereas more than 98 % of the subjects in both the Finnish and Bangladeshi groups had category 3 level of education. A significant group difference was apparent with respect to physical activity (P < 0·001). No significant difference was observed in the dietary intake of vitamin D between Somali immigrants and ethnic Finnish women (Table 1).
Table 1 Characteristics of the randomly assigned groups
(Mean values and standard deviations)

S-25OHD, serum 25-hydroxyvitamin D; S-iPTH, serum intact parathyroid hormone.
* Finnish v. Bangladeshi.
† Finnish v. Somali.
‡ Somali v. Bangladeshi.
§ P value adjusted for ethnicity.
∥ P value adjusted for S-25OHD and ethnicity.
Serum 25-hydroxyvitamin D and intact parathyroid hormone concentration
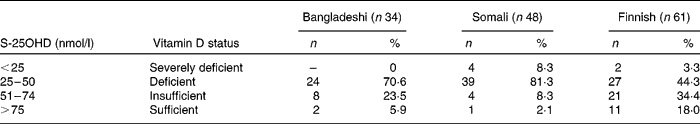
Of the 143 subjects studied, 117 (81·8 %) had S-25OHD < 50 nmol/l, which is generally considered the lower limit of the desired range. The mean concentrations of S-25OHD were significantly higher in Finnish women than in Bangladeshi (P = 0·005) and Somali women (P < 0·001). The proportion of subjects with vitamin D insufficiency was predominantly higher in those of Somali descent than in those of Finnish and Bangladeshi descent (Table 2).
Table 2 Distribution of vitamin D status in subjects: the percentage of subjects in different groups with serum 25-hydroxyvitamin D (S-25OHD) concentrations below and above predefined cut-offs
(Numbers and percentages)
The Somali subjects showed significantly higher S-iPTH concentrations than the Bangladeshi and Finnish subjects (P < 0·001). In comparison with subjects with a Finnish background, the prevalence of serious vitamin D deficiency defined as SHPT (S-iPTH levels >65 ng/l) was about five times higher (79·1 %) in subjects with a Somali background and two times higher (31·5 %) in subjects with a Bangladeshi background. There was a strong inverse linear correlation between ln S-25OHD and ln S-iPTH (r = − 0·49, P < 0·001). In linear regression analysis, one unit increase in S-25OHD on the ln scale decreased S-iPTH on the ln scale by 0·59 units. The model (ln S-iPTH = 6·286 − 0·593 × ln S − 25OHD) explained 24 % of the variation in S-iPTH. Calculated from the equation, the mean S-iPTH concentration (64 ng/l) was reached at S-25OHD concentrations above 36 nmol/l (Fig. 1). In multivariate regression analysis, S-25OHD and ethnicity together explained 30 % of the variation in S-iPTH. In the whole study, 37 % of the subjects had S-25OHD values below 36 nmol/l.

Fig. 1 Association between serum 25-hydroxyvitamin D (S-25OHD) and serum intact parathyroid hormone (PTH) concentrations in a non-linear regression model. PTH = 153 − 25·7 × ln(S-25OHD), R 2 26, P < 0·0005.
Bone traits
Bone variables at most of the sites of the forearm were significantly higher in the Finnish women (Table 3). Most bone traits were significantly lower in the Somali group than in the other groups. In all ethnic groups of the present study, there was a significant correlation between S-25OHD concentrations and distal and proximal radius BMD (r 0·25, P = 0·008 and r 0·27, P = 0·001, respectively) and the stress–strain index (r 0·27, P = 0·001) after adjusting for age. We observed a progressive increase in all variables describing forearm bone mass with high S-25OHD concentrations (Table 4).
Table 3 Ethnic differences in forearm bone variables of the randomly assigned groups
(Mean values and standard deviations)

* Finnish v. Bangladeshi.
† Finnish v. Somali.
‡ P value adjusted for height.
§ Somali v. Finnish.
∥ Somali v. Finnish and Bangladeshi.
¶ Bangladeshi v. Somali and Finnish.
Table 4 Differences in forearm bone variables, and physical and biochemical variables in the subjects divided into two categories according to serum 25-hydroxyvitamin D (S-25OHD) concentration (≤36 or >36 nmol/l)
(Mean values and standard deviations)

S-iPTH, serum intact parathyroid hormone.
Discussion
To the best of our knowledge, the present study is the first to detail the prevalence of hypovitaminosis D, SHPT and forearm bone status among pre-menopausal immigrant women and compare the data with those of ethnic Finnish women. We observed the relationship between S-25OHD, S-iPTH and forearm bone mass in these subject groups.
The problem of high prevalence of hypovitaminosis D and vitamin D deficiency among Europeans is not a new finding. Substantial studies have been carried out since 1970 among ethnic European and immigrant populations. Immigrant status has been reported to be an additional risk factor for vitamin D deficiency. However, the most important finding in the present study was the unexpectedly high prevalence of hypovitaminosis D (S-25OHD < 50 nmol/l) in both immigrants and ethnic Finnish subjects. Subjects with the Somali background were the most vulnerable group in the present study, since low S-25OHD concentrations were most frequently observed in these subjects.
In fact, low S-25OHD is a matter of concern, because it has clinical importance and may result in SHPT and increased bone loss. In the present study, we observed severe vitamin D depletion in the immigrants, Somali subjects in particular. The lower S-25OHD concentrations in this group may trigger higher S-iPTH concentrations that may stimulate bone resorption in these subjects. The present data confirm the high prevalence of vitamin D insufficiency and SHPT among female immigrant populations living in Helsinki (60°N), which is also in line with previous studies among immigrants in Denmark, Norway and The Netherlands(Reference Henriksen, Brunvand and Stoltenberg6, Reference Meyer, Falch and Sogaard9, Reference Holvik, Meyer and Haug14–Reference Madar, Stene and Meyer17). The data also indicate the most adverse impact of vitamin D insufficiency on forearm bone status among Somali subjects.
In the present study, we observed that higher dietary vitamin D intake was not associated with higher S-25OHD concentrations (Table 1). The results of previous studies also indicated that dietary vitamin D intake became less of a contributing factor to vitamin D status when supplement consumption and sunlight exposure were increased(Reference Jacques, Felson and Tucker18, Reference Outila, Karkkainen and Lamberg-Allardt19). Although 50 % of the subjects in the Finnish group reported their intake of vitamin D supplements within 3 months, the rate of supplement consumption was very low in immigrant subjects. Only three in the Bangladeshi group and five in the Somali group reported their intake of vitamin D supplements. In Helsinki, Finland (60°N), no UV-B radiation is found between 15 October and 15 March. Due to this high latitude and limited exposure to sunlight, cutaneous vitamin D production is absent during part of the year, so that the population living in this area is likely to be dependent on dietary sources of vitamin D. Since the natural sources of vitamin D are limited, consumption of vitamin D supplement was also recommended for this population during winter. However, the low dietary intake of vitamin D, no use of vitamin D supplements and no cutaneous vitamin D production in winter are probably the underlying reasons for the unexpectedly high prevalence of vitamin D insufficiency among immigrant subjects.
Several studies(Reference Serhan and Holland20, Reference Sakuma, Endo and Oinuma21) have reported that vitamin D deficiency may cause SHPT and low bone mass among women, which agree with the present findings in immigrant women. However, our findings among the subjects with a Finnish background were in contrast to these results and agree with reports suggesting that low S-25OHD does not always lead to an increase in the level of S-iPTH(Reference Chapuy Preziosi, Maamer and Arnaud22, Reference Islam, Shamim and Kemi23). In fact, a comparison of the present findings on S-25OHD and S-iPTH with previous studies may not be fully appropriate, because the studies were conducted in different seasons, with subject groups of varying ethnic backgrounds and using various commercial assays in different laboratories.
The negative association between S-25OHD and S-iPTH is commonly used to establish an adequate serum vitamin D concentration. There is some disagreement concerning the S-25OHD concentrations that trigger the increase in S-iPTH, which varies from 30 to 125 nmol/l for S-25OHD(Reference Chapuy Preziosi, Maamer and Arnaud22, Reference Grant and Holick24–Reference Lamberg-Allardt, Outila and Kärkkäinen26). Some researchers(Reference Chapuy Preziosi, Maamer and Arnaud22, Reference Heaney, Dowell and Hale27) have concluded that S-25OHD of >80 nmol/l was required to obtain maximal intestinal Ca absorption. Holick et al. (Reference Holick, Siris and Binkley28) also reported that their consistent findings on S-25OHD for maximal intestinal Ca absorption. Malabanan et al. (Reference Malabanan, Veronikis and Holick29) concluded that S-25OHD concentration of 50 nmol/l was the minimum value required to optimise S-iPTH levels and prevent SHPT. There is no disagreement concerning the association between the S-25OHD and S-iPTH concentrations. The correlation coefficient between the two indices is of relatively low magnitude, indicating that only a small part of variation in S-iPTH concentration is explained by the differences in S-25OHD concentrations. The present study indicated that S-25OHD and ethnicity together explained 30 % of the variation in S-iPTH and 24 % of the total variability in S-iPTH was explained by the variation in S-25OHD alone. Thus, the present results suggest that to maintain S-iPTH in balance throughout the year to prevent SHPT and to maintain healthy bone mass, S-25OHD concentrations could be considered as one of the important factors and should be maintained at a sufficient level throughout the year, both for immigrants and ethnic Finnish women.
Debate over the most appropriate cut-off value for identifying vitamin D deficiency or insufficiency is still open. However, most experts recommended at a recent consensus conference that serum concentrations of vitamin D higher than 75 nmol/l may be associated with better health outcomes in terms of higher BMD and less risk of many common and serious diseases, including osteoporosis, some common cancers, type 1 diabetes, multiple sclerosis, rheumatoid arthritis, schizophrenia and CVD. If the 75 nmol/l cut-off for the present study subjects is used, only 9 % of the subjects would enjoy optimal S-25OHD concentrations in winter. The US Institute of Medicine(30) suggested S-25OHD levels of 50 nmol/l to cover the requirements of 97 % of the population. We observed that only 32 % of the subjects had this level of S-25OHD.
The present data are consistent with previous findings(Reference Roy, Berry and Pye10, Reference Holvik, Meyer and Haug14, Reference Madar, Stene and Meyer17, Reference Proto31, Reference Huntington, Shafer and Pudwill32) in European countries and elsewhere that female immigrants with Asian and African backgrounds have a high prevalence of S-25OHD insufficiency. In terms of the relationship between S-25OHD and S-iPTH or BMD, there are conflicting results observed in previous studies. However, a remarkable number of studies(Reference Arya, Bhambri and Godbole33–Reference Viljakainen, Natri and Kärkkäinen37) have shown the positive correlation between S-25OHD and BMD. Vitamin D insufficiency influences bone mass in ethnic Finnish subjects and the present data are consistent with this finding(Reference Outila, Karkkainen and Lamberg-Allardt19, Reference Lehtonen-Veromaa, Mottonen and Nuotio38).
In all ethnic groups of the present study, there was a significant correlation among S-25OHD concentrations and several bone traits. This suggests that subclinical vitamin D deficiency could be a factor responsible for weak bones with low bone mass and a risk factor for osteoporosis.
Limitations to the study
The subjects included in the present study may have vitamin D status different from that of the population in general. A population-based study and random selection criteria of subjects could provide better evidence on vitamin D status. We used the reference range for S-iPTH (10–65 ng/l) and the thresholds for SHPT (>65 ng/l) given by the manufacturer of the assay, which is controversial. The frequency of SHPT in the different subgroups studied and the concentration of 25OHD, at which the curved regression line between S-iPTH and S-25OHD cuts the line separating ‘normal parathyroid hormone’ concentration from SHPT (Fig. 1), could have been increased significantly if a lower and more authentic cut-off level for SHPT is used(Reference Rejnmark, Vestergaard and Heickendorfft39, Reference Souberbielle, Cormier and Kindermans40).
Conclusions
Regardless of the ethnic background, the present data confirm the unexpectedly high prevalence of vitamin D deficiency or insufficiency during winter among immigrant women and ethnic Finnish women. In the present study, Somali subjects were the most vulnerable group at risk of low bone mass, osteomalacia and osteoporosis. Public health measures to increase the level of S-25OHD and to prevent SHPT are urgently required. Therefore, both health policy makers and health professionals should pay attention without delay to this silent epidemic. To prevent vitamin D deficiency during winter, the increased consumption of vitamin D-rich food, such as fish, and consumption of vitamin D supplements are recommended. An immediate plan to maintain optimal vitamin D status and gain increased bone mass could prove beneficial in alleviating future strain on the health care system of Finland by reducing morbidity caused by osteoporosis and other problems related to vitamin D deficiency. The present results suggest that an intervention trial to improve vitamin D and BMD status and to minimise osteoporotic risk is essential in these segments of the population.
Acknowledgements
The present study was supported by a research grant of the Ella and Georg Ehrnrooth Foundation, Finland. The authors are grateful to members of the directory board of the foundation. We thank the 143 volunteer subjects who participated in the study, making the research possible. Md Z. I., C. L.-A. and K. L. designed the study, secured funding and participated in carrying out the study. Md Z. I. was the principal investigator of the study, providing guidance in data collection and participating in data entry, statistical analysis and writing the manuscript. H. T. V. and E. S. contributed to field data collection. C. L.-A. checked the manuscript and approved the final version. M. U. M. K. participated in the statistical analysis and interpretation. None of the contributing authors had any financial or personal conflicts of interest.







