Metabolic syndrome refers to a clustering of specific CVD risk factorsReference Kahn, Buse, Ferrannini and Stern1. There are several definitions of metabolic syndrome for adults2–7, such as the International Diabetes Federation definitionReference Alberti, Zimmet and Shaw4, WHO criteria7 and the Third Report of the National Cholesterol Education Program's Adult Treatment Panel (ATP III) criteria2. Paediatric metabolic syndrome has been reported in many populations, but the estimations are difficult to compare because a unanimous definition is lackingReference Chu, Rimm, Wang, Liou and Shieh8–Reference Yoshinaga, Tanaka, Shimago, Sameshima, Nishi, Nomura, Kawano, Hashiguchi, Ichiki and Shimizu13. Based on the ATP III criteria, Cook et al. Reference Cook, Weitzman, Auinger, Nguyen and Dietz14 proposed paediatric metabolic syndrome criteria for US children and adolescents defined as having three or more of the following abnormalities: high TAG level; low HDL-cholesterol level; high fasting glucose level; abdominal obesity; hypertension. In 2004, de Ferranti et al. Reference de Ferranti, Gauvreau, Ludwig, Neufeld, Newburger and Rifai15 developed less restrictive criteria analogous to ATP III. According to de Ferranti's definition, the prevalence of metabolic syndrome among US adolescents was 10 % and nearly 30 % among overweight/obese US adolescentsReference de Ferranti, Gauvreau, Ludwig, Neufeld, Newburger and Rifai15.
In China, paediatric overweight and obesity emerged only during the last few decades. Being very low in the 1980 s, the overall prevalence of overweight and obesity among children in China were approximately 5 % and 2 %, respectively, in 2002Reference Li, Chen, Kong, Yang, Zhai, Zhang and Ma16. Along with the rise of childhood obesity, related metabolic abnormalities began to be routinely observed and reportedReference Chu, Rimm, Wang, Liou and Shieh8–Reference Yoshinaga, Tanaka, Shimago, Sameshima, Nishi, Nomura, Kawano, Hashiguchi, Ichiki and Shimizu13, Reference de Ferranti, Gauvreau, Ludwig, Neufeld, Newburger and Rifai15. But no national representative data about metabolic syndrome of Chinese adolescents have been published. The purpose of the current study is to estimate the prevalence and distribution of metabolic syndrome among Chinese adolescents using the national representative survey data of the 2002 China National Nutrition and Health Survey.
Subjects and methods
Sampling
The 2002 China National Nutrition and Health Survey is a nationally representative cross-sectional survey that covered thirty-one provinces, autonomous regions and the municipalities directly affiliated to the central government (Hong Kong, Macao and Taiwan were not included). Detailed information of the sampling method has been described elsewhereReference Li, Zhai, Yang, Schouten, Hu, He, Luan and Ma17. Briefly, a multi-step cluster sampling method was used for subject selection. A total of 272 023 subjects aged 2–101 years old representative for the Chinese population were surveyed. The sample consisted of 8456 adolescents aged 15–19 years old and one third of the selected families (n 2913) were randomly selected to have blood samples drawn.
Among the 2913 adolescents, missing values for height/weight, waist, blood pressure and blood samples accounted for 1·8 %, 0·8 %, 2·1 % and 2·1 %, respectively. A total of 2761 adolescents had complete measurements of waist circumference, blood pressure, plasma glucose, TAG, HDL and were not currently pregnant. Children younger than 15 years were recruited, but did not have waist circumference and blood pressure measurements taken. There was no significant difference in age, weight, height, BMI and sex ratio between adolescents who had complete measurements and those who did not.
Among the 2761 subjects, 2469 adolescents' mothers (response 89·4 %) and 2186 adolescents' fathers (response 79·2 %) participated in the investigation, 2326 mothers (response 94·2 %) and 2064 fathers (response 94·4 %) had complete measurements for metabolic syndrome. Of them, 1861 adolescents had full information of both mother and father. There was no significant difference in weight, height and BMI between parents who had complete measurements and those who did not. Parents who completed the measurements were 0.4 years older than those who did not.
Measurements
The fasting body weight, height, waist circumference and blood pressure of the adolescents and their (biological) parents were measured following standardized procedures by trained interviewers. The waist circumference was measured to the nearest 0·1 cm at the midpoint between the bottom of the rib cage and the top of the iliac crest at the end of exhalationReference Wang18. Subjects' seated resting blood pressure was measured twice to the nearest 2 mmHg. The first and fifth Korotkoff sounds were used to represent the systolic and diastolic blood pressureReference Wang18. The mean of these two measurements was recorded.
Fasting blood samples (5 ml) of the adolescents and their (biological) parents were collected after approximately 10–14 h overnight fast, drawn into tubes containing heparin as an anticoagulant for preparation of plasma. After the blood was drawn, the tubes were gently shaken and then separated by centrifugation at 3200 rpm for 10–15 min. Plasma glucose level was measured with a spectrophotometer within 4 h after a fasting blood sample was obtained. Other plasma samples were moved into airtight storage tubes and stored at − 80°C prior to shipment on dry ice to the Chinese Center for Disease Control and Prevention for lipid measurements. Plasma total cholesterol, TAG and HDL-cholesterol were measured enzymatically with a Hitachi 7060, 7180 auto-analyzer (Hitachi, Tokyo, Japan)Reference Wang18.
The protocol of the survey was approved by the Ethical Committee of the National Institute for Nutrition and Food Safety, Chinese Center for Disease Control and Prevention. Signed consent forms were obtained from both their parents or guardians and the adolescents themselves.
Quality control of physical measurements
Fasting body weight, height and blood pressure were all objectively measured by trained investigators and the duplicate measurements in subgroups showed very high reproducibility (correlation coefficients of duplicate measurements were 0·99 for height and 0·98 for weight). Plasma was separated immediately and all fasting glucose samples were measured within 4 h. Every tenth sample was measured twice (correlation coefficient of duplicate measurements was 0·98), at the same time, one reference sample, one quality control sample and one blind sample were measured before every thirtieth sampleReference Wang18. Blind serum samples provided by the US Center for Disease Control were analysed in our laboratory (China Center for Disease Control) seven times at regular intervals over the course of the analysis of the samples from the 2002 China National Nutrition and Health Survey; the relative difference ranged from − 7.02 % to 1·60 % for TAG and from − 1·05 % to 4·21 % for HDL-cholesterolReference Wang18.
Definition
Metabolic syndrome was defined, using the criteria proposed by de Ferranti et al. Reference de Ferranti, Gauvreau, Ludwig, Neufeld, Newburger and Rifai15, as three or more of the following variables and cut-off points: (1) fasting TAG ≥ 1·1 mmol/l (100 mg/dl); (2) HDL-cholesterol boys < 1·2 mmol/l (45 mg/dl); girls < 1·3 mmol/l (50 mg/ dl); (3) fasting glucose ≥ 6·1 mmol/l (110 mg/dl); (4) waist circumference >75th percentile for age and sex for US adolescents; (5) systolic blood pressure and/or diastolic blood pressure >90th percentile for sex, age and height recommended by the National Heart, Lung, and Blood Institute (USA)19. The ATP III criteria2 were used for the definition of the adults (parents) metabolic syndrome. BMI was calculated as body weight divided by the square of height (kg/m2), in order to compare with US adolescents, the BMI status was classified by age-, sex- specific BMI standards for US adolescents ( < 85th, 85th–95th and ≥ 95th percentile by age and sex)20.
Statistical analysis
The prevalence of metabolic syndrome was estimated overall, by sex, by region (urban or rural), by BMI and stratified by the presence of parental metabolic syndrome. BMI was estimated using the US historical percentiles that were derived from the NHANES III survey: normal weight < 85th; at risk of overweight: 85th–95th; overweight: ≥ 95th percentile of US adolescents by age and sex20. Prevalence values were compared using χ2 tests. Sampling weights were applied to national estimates according to the data of the Fifth National Population Census21. All statistical analyses were done with SAS (8.2e for Windows; SAS Institute Inc. Cary, NC, USA), and the significance level was set at 0·05.
Results
Characteristics of adolescents and their parents are shown in Table 1. A total of 2761 adolescents (boys 1478; girls 1283) aged 17·1 (sd 1·5) (15–19) years participated in the investigations. A total of 2326 adolescents' mothers aged 42·7 (sd 4.9) years and 2064 adolescents' fathers aged 44·9 (sd 5·6) years finished all measurements for metabolic syndrome; among them, there were 1861 couples.
Table 1 Characteristics of adolescents and their parents*
(Mean values and standard deviations)
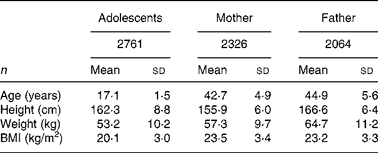
* For details of subjects and procedures, see Subjects and methods.
Prevalence of metabolic syndrome is shown in Table 2. The overall prevalence of metabolic syndrome in Chinese adolescents aged 15–19 years was 3·7 %. Based on population weighting, we have estimated that more than three million Chinese adolescents have metabolic syndrome. Metabolic syndrome was present in 35·2 % of overweight adolescents (BMI ≥ 95th percentile) compared with 23·4 % of at-risk adolescents (BMI 85th to < 95th percentile) and 2·3 % of those with a BMI below the 85 percentile (P < 0·01). The prevalence of metabolic syndrome by sex and region is shown in Fig. 1. Urban boys had the highest rate at 5·8 %, whereas their rural counterparts (2·9 %) had the lowest rate (P < 0·01). Urban girls had comparable prevalence as their rural counterparts (3·5 % v. 3·7 %).
Table 2 Number of metabolic syndrome risk factors among Chinese adolescents aged 15–19 years, 2002CNNHS (%)†

CNNHS, China National Nutrition and Health Survey, MetS, metabolic syndrome, with three or more risk factors.
χ2-test of prevalence of metabolic syndrome risk factors between subgroups:
* P < 0·05;
** P < 0·01.
† For details of subjects and procedures, see Subjects and methods.
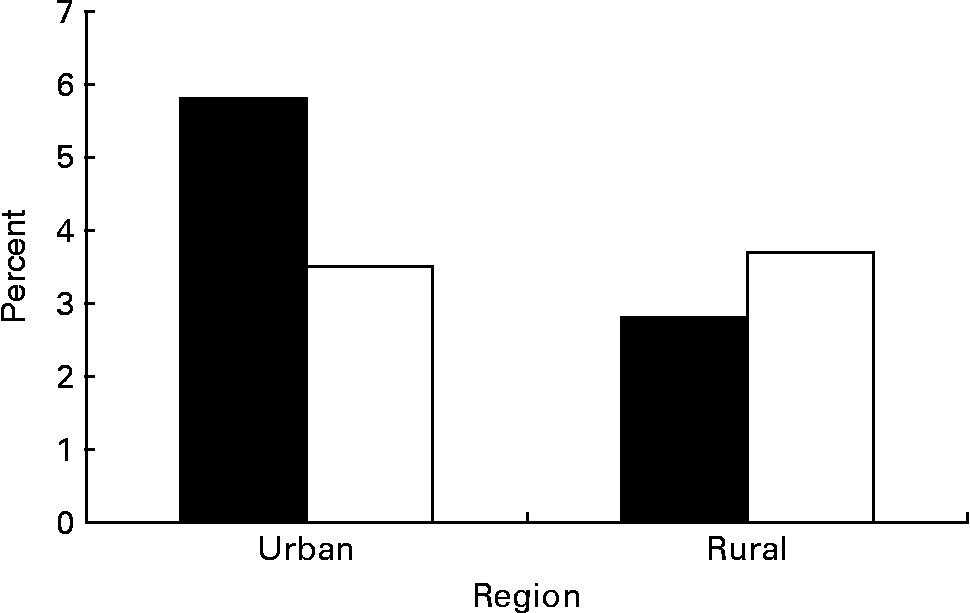
Fig. 1 Prevalence of the metabolic syndrome by sex (boys ■; girls □) and region. For details of subjects and procedures, see Subjects and methods.
Adolescents having parent(s) with metabolic syndrome had a higher rate of metabolic syndrome than their counterparts (10·7 % v. 3·0 %, P < 0·01; Table 2). The prevalence of metabolic syndrome by adolescents' BMI status and parental metabolic syndrome status is shown in Fig. 2. Among normal weight adolescents, metabolic syndrome prevalence was low whether they had parents with (5·1 %) or without (2·1 %) metabolic syndrome. Once they became overweight or were at risk of overweight, the prevalence increased dramatically, especially if their parent(s) had metabolic syndrome. More than 40 % (46·4 %) of adolescents who had BMI ≥ 85th percentile and parent(s) with metabolic syndrome met the criteria of metabolic syndrome.
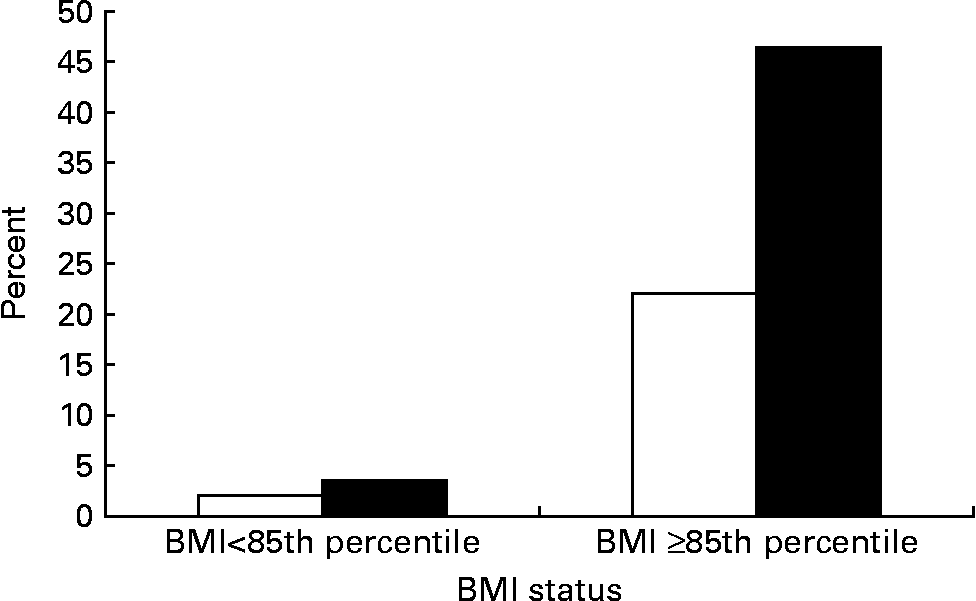
Fig. 2 Prevalence of adolescent's metabolic syndrome by their BMI status and parental metabolic syndrome (MetS) status (□, no parents with MetS; ■, one or more parents with MetS). For details of subjects and procedures, see Subjects and methods.
The proportion of subjects with one or more metabolic abnormalities is also shown in Table 1, 96·3 % overweight adolescents had at least one and 74·1 % had at least two abnormalities of metabolic syndrome. The distribution of each metabolic abnormality is shown in Table 3. Overall, low HDL-cholesterol levels were the most common, whereas high fasting glucose levels and abdominal obesity were the least common abnormality. Central obesity, high fasting glucose, high TAG level and hypertension all significantly worsened with increasing body weight status.
Table 3 Prevalence of individual metabolic syndrome risk factors among Chinese adolescents aged 15 to 19 years, 2002 CNNHS (%)†

CNNHS: China National Nutrition and Health Survey; MetS, Metabolic syndrome, with three or more risk factors.
χ2-test of prevalence of individual metabolic syndrome between subgroups:
* P < 0·05,
** P < 0·01.
† For details of subjects and procedures, see Subjects and methods.
The prevalence of metabolic syndrome of children with a high family income was significantly higher than their counterparts whose family incomes were low or intermediate. No significant association was found between mother's educational level and the child's metabolic syndrome (Table 2). Family income status was also positively associated with the risk of having four metabolic abnormalities, but the children from poor families were at a significantly higher risk of having at least one metabolic abnormality. Family income status was also positively related to the prevalence of abdominal obesity and negatively related to HDL-cholesterol level. The prevalence of low HDL-cholesterol level among children whose mother's educational level was low (49·6 %) or high (45·8 %) was both lower than those whose mother's educational level was medium (54·2 %) (Table 3).
Discussion
This is the first study examining the prevalence and distribution of metabolic syndrome among 15- to 19-year-old Chinese adolescents using a national representative sample. Compared with their US counterparts, the prevalence of metabolic syndrome is still low in China (about 10 % in the USA v. 3·7 % in China)Reference de Ferranti, Gauvreau, Ludwig, Neufeld, Newburger and Rifai15. The prevalence of metabolic syndrome among overweight adolescents is quite similar in China and in the USA (27·7 % v. 31·2 %). Overweight adolescents who had one, two, three and four items of the metabolic abnormalities are also comparable between Chinese (75·9 %, 51·9 %, 20·4 % and 5·6 %) and US adolescents (88·5 %, 56·0 %, 28·7 % and 5·8 %) (both applying the criteria developed by Cook et al.)Reference Cook, Weitzman, Auinger, Nguyen and Dietz14.
Comparing the different individual metabolic abnormalities between Chinese and US adolescentsReference Cook, Weitzman, Auinger, Nguyen and Dietz14, Reference de Ferranti, Gauvreau, Ludwig, Neufeld, Newburger and Rifai15, the prevalence of abdominal obesity and high TAG levels was lower, while the prevalence of low HDL-cholesterol level and elevated blood pressure was higher among Chinese than US adolescents. The differences may be caused by genetic factors, dietary habits, physical activity patterns or other lifestyle differences that need further research. High levels of HDL-cholesterol have previously been reported in some Chinese populationsReference Kesteloot, Huang, Yang, Yang, Claes, Rosseneu, Geboers and Joossens22, more recent data, however, suggested that the lipid profile of Asian populations is changing with a trend toward decreased HDL-cholesterol and increased total cholesterol and LDL-cholesterol, possibly as a result of changing lifestylesReference Chen, Peto, Collins, MacMahon, Lu and Li23, Reference Davis, Williams, Oganov, Tao, Rywik, Stein and Little24. Serum lipid concentrations of various Chinese population samples are now closer to that of Western populationsReference Campbell, Parpia and Chen25, Reference McGladdery, Pimstone, Clee, Bowden, Hayden and Frohlich26. In order to compare with the US adolescents, we used the old cut-point for high glucose level of 110 mg/dl. When the new cut-point of 100 mg/dl was applied, 2·8 % of Chinese adolescents had a high glucose level.
One limitation of the present study is that the estimation of metabolic syndrome is based on a proposed definition for US adolescents using the historical cut-points from the NHANES III due to the lack of a metabolic syndrome definition for Chinese adolescents. Whether the US criteria are applicable for Chinese adolescents is still questionable. As previous studies found a higher body fat percentage at the same BMI among Asian adults, specific overweight (BMI ranging from 24·0 kg/m2 to 27·9 kg/m2) and obesity (BMI equal or above 28 kg/m2) definitions were then developed27–29. Using the US upper limits for waist circumference may have underestimated the prevalence, while using the US lower limit for HDL-cholesterol level may have overestimated the prevalence, considering the generally lower adult weight and waist circumference of Chinese compared with US adults. Another limitation of the present study is that we compared the Chinese adolescents aged 15–19 years with US adolescents aged 12–19 years. As similar results were found for older and younger adolescents in the USAReference Cook, Weitzman, Auinger, Nguyen and Dietz14, Reference de Ferranti, Gauvreau, Ludwig, Neufeld, Newburger and Rifai15, our comparison may still be valid.
Strengths of the study were the national representativeness and the high quality control of physical examination and laboratory analysisReference Wang18. High reproducibility was observed in our duplicate measurements; moreover, there is good agreement between Chinese and American laboratory measurements in TAG and HDL-cholesterol. Evaluating parental history of metabolic syndrome was also a strength of the present study.
In conclusion, the prevalence of metabolic syndrome is lower among Chinese than US adolescents. But among overweight subjects, the prevalence of metabolic syndrome is comparable. It is estimated that more than three million Chinese adolescents aged 15 to 19 years have metabolic syndrome and 40 % of them have elevated body weight. Of the adolescents having parents with metabolic syndrome, once they are overweight or at risk of overweight, their risk of metabolic syndrome would be much higher. The present findings highlight the need for effective childhood obesity prevention strategies and actions in China, especially among the adolescents having parents with metabolic syndrome.
Acknowledgements
We thank all the team members and all participants from thirty-one provinces of China. Special thanks are for Professor Evert G. Schouten for his contribution to this paper. None of the authors had any personal or financial conflicts of interest. The ‘2002 China National Nutrition and Health Survey’ was supported by Ministry of Health and Ministry of Science and Technology, China (2001DEA30035, 2003DIA6N008). UNICEF, WHO, Unilever China and Danone Nutrition Institute China.







