Insulin sensitivity decreases with advancing pregnancy, leading to abnormalities in glucose homoeostasis( Reference Catalano 1 ). Compared with lean or average-weight women, overweight and obese women have a greater reduction in insulin sensitivity, resulting in higher risk of developing glucose intolerance during pregnancy( Reference Catalano 1 ). A body of literature has demonstrated that the influence of maternal glucose concentrations on adverse perinatal outcomes extends throughout the range of glycaemia( 2 , Reference Aris, Soh and Tint 3 ). Thus, improvement in glycaemic levels during pregnancy, even among non-diabetic women, is expected to alleviate a number of detrimental health consequences in mothers and offspring( 2 ). At present, the use of dietary modification to control or improve maternal glycaemic levels has mostly focused on overall diet quantity and quality( Reference Han, Crowther and Middleton 4 ). Nevertheless, an elemental aspect of the diet that relates to the timing of feeding and the circadian pattern of food consumption has largely been ignored. In view of the fact that both food and light are powerful signals that entrain our body’s circadian clocks that control daily physiological events( Reference Oike, Oishi and Kobori 5 ), it is possible that timed feeding could serve as an important means to improve glucose tolerance.
Emerging evidence describing the metabolic risk of shift work, circadian misalignment and clock gene polymorphisms implies that inappropriate meal timing may induce impairment of glucose metabolism( Reference Oike, Oishi and Kobori 5 , Reference Bailey, Udoh and Young 6 ). Glucose tolerance and insulin secretion have been shown to oscillate in a diurnal pattern, with the lowest insulin sensitivity and pancreatic islet β-cell responsiveness to glucose in the evening( Reference Saad, Dalla Man and Nandy 7 ). It has been documented that the time of the day alters glucose profile following meal consumption, depending on the ability of timed feeding to synchronise local circadian rhythms( Reference Johnston 8 ). The overall effect of feeding on the circadian system appears to involve both the timing and quantity of food consumption( Reference Johnston 8 ). In the adult population, higher glycaemic levels and insulin resistance were found in those with greater energy consumption in the evening than in the morning despite similar total energy intake for the entire day( Reference Morgan, Shi and Hampton 9 , Reference Jakubowicz, Barnea and Wainstein 10 ).
In pregnant women, little information is available on the physiological adaptations of the circadian system to pregnancy( Reference Seron-Ferre, Mendez and Abarzua-Catalan 11 ). Much less is known about the response of this circadian system to environmental disturbance( Reference Seron-Ferre, Mendez and Abarzua-Catalan 11 ). Maternal feeding rhythm over a 24-h day–night cycle and the effects of timed feeding on metabolic outcomes during pregnancy are not widely explored. At present, there has been only one study that examined the association of meal timing with glucose metabolism during pregnancy( Reference Chandler-Laney, Schneider and Gower 12 ). In this study of low-income African-American pregnant women, energy consumption during night-time was inversely associated with dynamic β-cell response at late pregnancy( Reference Chandler-Laney, Schneider and Gower 12 ). When further analysis was carried out by stratifying women into normal and obese groups based on their weight status in early pregnancy, night-time energy consumption remained inversely associated with dynamic β-cell response in the obese group, but not in the normal-weight group( Reference Chandler-Laney, Schneider and Gower 12 ). However, generalisability of these findings may be limited by specific population demographics and distinctive diet quality.
Altogether, at this point, it is not known whether consuming energy content predominantly at night-time may further exacerbate the effect on insulin insensitivity during pregnancy and how this association may vary in response to different weight status. Growing Up in Singapore Towards healthy Outcomes (GUSTO) is a mother–offspring cohort study designed to test hypotheses related to the developmental pathways of metabolic diseases in Chinese, Malay and Indian populations( Reference Soh, Tint and Gluckman 13 ). In the present study, we asked the question as to whether the feeding patterns of women based on the timing of energy consumption throughout the day during pregnancy could influence maternal glycaemic levels in a multi-ethnic Asian context. We hypothesised that consuming higher energy content at night-time was associated with higher glucose concentrations in pregnant women who were overweight, as this group of women was more susceptible to insulin resistance compared with lean women.
Methods
Study participants
Details of the GUSTO cohort study have been reported previously( Reference Soh, Tint and Gluckman 13 ). In brief, pregnant women (18 years old and above) in their first trimester (<14 weeks of gestation) were recruited from KK Women’s and Children’s Hospital) and National University Hospital (NUH), between June 2009 and September 2010. Singapore citizens or permanent residents who were of Chinese, Malay or Indian ethnicity with homogeneous parental ethnic background were included. Women receiving chemotherapy, psychotropic drugs or with type 1 diabetes mellitus were excluded. All women provided their written informed consent. The Domain Specific Review Board of Singapore National Healthcare Group (reference D/09/021) and the Centralised Institutional Review Board of SingHealth (reference 2009/280/D) approved the GUSTO study protocol.
Data collection
Detailed interviews were conducted at the clinics at recruitment and at 26–28 weeks of gestation. Data on socio-economic status, educational attainment, physical activity and sleep duration were collected. Three types of physical activity were assessed, including light–moderate-, moderate- and vigorous-intensity activities. The total score of physical activity was computed from the summation of the duration (min) and frequency (d) of these three types of activity. The score was expressed in metabolic equivalents (MET-min/week)( Reference Padmapriya, Shen and Soh 14 ). Actual sleep duration at night was recorded using the Pittsburgh Sleep Quality Index questionnaire( Reference Buysse, Reynolds and Monk 15 ).
Anthropometric measurements
Maternal height was measured using a stadiometer (Seca 213; Seca). Self-reported pre-pregnancy weight and measured weight at the first antenatal visit (≤14 weeks of gestation) were collected. BMI was computed from weight (kg)/height (m2). Women were classified as lean (BMI<23 kg/m2) or overweight (BMI≥23 kg/m2) based on BMI cut-off points for Asian populations( 16 ). Strong agreements were observed between pre-pregnancy and first antenatal visit weight status (Cohen’s κ=0·82; P<0·001). Owing to some missing data for pre-pregnancy BMI (n 62, 6·3 %), maternal weight status classification based on BMI at the first antenatal visit was used for analysis.
Dietary assessments
A 24-h dietary recall was administered via face-to-face interviews by trained clinical staff at 26–28 weeks of gestation using the five-stage, multiple-pass interviewing technique( Reference Conway, Ingwersen and Vinyard 17 ), which includes reporting an uninterrupted listing of all foods and beverages consumed, answering a forgotten food list tailored for the local population, providing details of time, occasions and descriptions of foods and amounts eaten, and a final probe review. Standardised household measuring utensils and food pictures of various portion sizes were used to assist women in quantifying their food and beverage intakes. Daily energy and macronutrient intakes were assessed using a nutrient analysis software (Dietplan; Forestfield Software) with a food composition database of locally available foods( 18 ) and modifications made on inaccuracies found. For mixed dishes not found in the local database, nutrient analyses of recipes were conducted using the nutrient software. For other food items not found in the database, nutrient information was obtained from either food labels or the United States Department of Agriculture (USDA) national nutrient database( 19 ).
Feeding patterns
Sunlight has been reported as a strong environmental signal for the human circadian clock( Reference Wright, McHill and Birks 20 ). In Singapore (1.3° North, 103.8° East)( 21 ), sunrise and sunset occur at approximately 07.00 and approximately 19.00 hours, respectively, throughout the year, with a fairly constant day length of 12 h all year round( 22 ). Therefore, we categorised women as (i) predominantly daytime (pDT) feeders who consumed >50 % of their total energy intake from 07.00 to 18.59 hours (from sunrise to sunset), and (ii) predominantly night-time (pNT) feeders who consumed >50 % of their total energy intake from 19.00 to 06.59 hours (from sunset to sunrise).
Glucose concentrations
Overnight fasting blood samples were collected at 26–28 weeks of gestation. At the same visit, women underwent 75 g oral glucose tolerance test (OGTT). Fasting glucose (FG) and 2-h postprandial glucose (2HPPG) concentrations were measured by colorimetry (Advia 2400 Chemistry system (Siemens Medical Solutions Diagnostics) and Beckman LX20 Pro-analyzer (Beckman Coulter)).
Statistical analyses
Differences in maternal characteristics between included and excluded women in this study, as well as lean and overweight women, were compared using Pearson’s χ 2 test for categorical variables or independent t test for continuous variables. The interaction effect between BMI status and feeding pattern on glucose concentration was tested. Multivariate linear regression analysis was performed to assess the associations between feeding patterns and glucose concentrations, adjusting for confounders. The confounding variables included maternal age, education, ethnicity, physical activity, sleep duration and total energy intake. These confounders were selected a priori based on literature review( Reference Chandler-Laney, Schneider and Gower 12 , Reference Barrett-Connor, Schrott and Greendale 23 , Reference Loy, Lek and Yap 24 ). Total energy intake was adjusted for using standard multivariate approach( Reference Willett, Howe and Kushi 25 ) in order to examine the association of feeding pattern with glucose concentration in an isoenergetic condition. In view of the difference in carbohydrate intakes between groups, additional adjustment for proportion of carbohydrates was performed. All the statistical analyses were performed using IBM SPSS statistics, version 20 (IBM). Two-sided tests were used, and a value of P<0·05 was considered to be statistically significant.
Results
Participant characteristics
Of the 1237 recruited singleton pregnant women, 79 (6·4 %) had incomplete 24-h dietary recalls, 146 (11·8 %) missed their blood glucose tests and 154 (12·4 %) did not have their first antenatal recorded weights; these women were excluded from the analyses. We further excluded women with implausible energy intakes( Reference Qiu, Frederick and Zhang 26 , Reference Lim, Yoo and Kim 27 ), which was <2092 kJ/d (<500 kcal/d) (n 4) and >14 644 kJ/d (>3500 kcal/d) (n 10), leaving a final sample of 985 women in this study.
No statistically significant differences in characteristics were observed between included and excluded pregnant women (online Supplementary Table S1).
The study sample included a higher proportion of lean (54·2 %) than overweight (45·8 %) women. Overall, there were 838 (85·1 %) pDT feeders and 147 (14·9 %) pNT feeders. The hourly energy consumption patterns throughout the day for these two groups of feeders are presented in Fig. 1. A substantial rise in energy consumption was observed during 19.00–19.59 hours for pNT feeders. The proportions of pDT and pNT feeders were not significantly different between lean and overweight women (P=0·553). The majority of lean women were Chinese (P<0·001), attained higher education (P<0·001) and slept for longer duration at night (P=0·043) as compared with overweight women. The majority of overweight women were multiparous (P=0·004), diagnosed with gestational diabetes mellitus (P<0·001), had higher FG (P<0·001) and 2HPPG concentrations (P<0·001) and had lower total daily energy intakes (P<0·001), but with similar proportions of protein (P=0·592), fat (P=0·174) and carbohydrate intakes (P=0·401), as compared with lean women. There were no differences in the level of physical activity between these two groups of women (P=0·717). Maternal characteristics were similar between pDT and pNT feeders, except for total energy intake and proportion of carbohydrate intake, which were significantly higher in pDT feeders, whereas the proportion of fat intake was significantly higher in pNT feeders (Table 1).

Fig. 1 The hourly energy consumption patterns throughout the day of predominantly daytime (pDT, ![]() ) and predominantly night-time (pNT,
) and predominantly night-time (pNT, ![]() ) feeders.
) feeders.
Table 1 Maternal characteristics during pregnancy (Numbers and percentages; mean values and standard deviations)

pDT, predominantly daytime; pNT, predominantly night-time; MET, metabolic equivalents.
* P values are based on the χ 2 test or the independent t test as appropriate.
A statistical trend towards significance was observed for interaction between BMI status and feeding pattern on FG (P=0·056), but not on 2HPPG (P=0·315). Lean pNT feeders were found to have higher FG than lean pDT feeders (4·36 (sd 0·38) v. 4·22 (sd 0·35) mmol/l; P=0·002); however, such differences were not observed between overweight pDT and pNT feeders (4·49 (sd 0·60) v. 4·46 (sd 0·45) mmol/l; P=0·717). For 2HPPG, there were no significant differences between pDT and pNT feeders in both lean (6·32 (sd 1·36) v. 6·22 (sd 1·58) mmol/l; P=0·564) and overweight groups (6·86 (sd 1·58) v. 6·49 (sd 1·52) mmol/l; P=0·078). With respect to the proportions of daily macronutrient intake, no significant differences were found between pDT and pNT feeders, apart from a lower proportion of carbohydrate intake among pNT feeders in the overweight group (Table 2).
Table 2 Comparison of glucose concentrations, energy and macronutrients distribution between predominantly daytime (pDT) and night-time (pNT) feeders by weight status (Mean values and standard deviations)

* P values are based on independent t test.
Associations between maternal feeding patterns and glucose concentrations
Table 3 shows the association between maternal feeding pattern and glucose concentration. An association of pNT feeding with higher FG was observed in the lean group (β=0·16 mmol/l; 95 % CI 0·05, 0·26; P=0·003), but not in the overweight group (β=0·02 mmol/l; 95 % CI −0·17, 0·20; P=0·879), after adjusting for maternal age, education, ethnicity, physical activity, sleep duration and total energy intake (Table 3). Similar findings were obtained after further adjustment for proportion of carbohydrate intake (lean group: β=0·15 mmol/l; 95 % CI 0·05, 0·26; P=0·003; overweight group: β=0·02 mmol/l; 95 % CI −0·17, 0·21; P=0·842). On the other hand, no significant association was found between maternal feeding pattern and 2HPPG in both lean and overweight groups (lean group: β=−0·24 mmol/l; 95 % CI −0·64, 0·16; P=0·232; overweight group: β=−0·31 mmol/l; 95 % CI −0·83, 0·21; P=0·246).
Table 3 Association between maternal feeding pattern and glucose concentration (β Coefficients and 95 % confidence intervals)
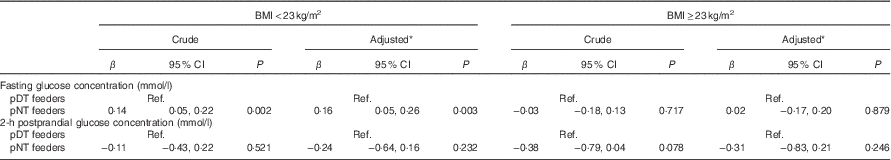
Ref. referent values; pDT, predominantly daytime; pNT, predominantly night-time.
* Adjusted for maternal age, education, ethnicity, physical activity, sleep duration and total energy intake.
Discussion
In this large multi-ethnic cohort, we showed that one in seven pregnant women were pNT feeders in the late second trimester. We found that pNT feeders were positively associated with higher FG concentration among women who were lean at the start of pregnancy. In contrast, such an association was not observed in overweight women. These findings are at odds with our hypothesis and suggest that risk of glucose intolerance was more susceptible to feeding pattern only in lean women. In overweight women, feeding pattern had no significant effect on glucose metabolism.
A few studies have examined the timing of daily energy intake with glucose regulation. A clinical trial of Spanish women showed that delaying meal timing resulted in decreased carbohydrate oxidation and glucose tolerance( Reference Bandin, Scheer and Luque 28 ). In an experimental study among Israeli women, FG and insulin resistance were higher in participants with high-energy dinner (18.00–21.00 hours) intake than in those with high-energy breakfast (06.00–09.00 hours) intake after a 12-week intervention, despite consuming an isoenergetic diet on a daily basis( Reference Jakubowicz, Barnea and Wainstein 10 ). These findings are similar to our results, which demonstrated that lean women who consumed greater proportions of energy content at night were more likely to exhibit higher FG concentrations. In support of this, previous reports have indicated that there is a progressive reduction in insulin sensitivity, β-cell response and glucose tolerance throughout the day, with insulin sensitivity reaching a nadir at night-time( Reference Saad, Dalla Man and Nandy 7 , Reference Morgan, Shi and Hampton 9 , Reference Lee, Ader and Bray 29 ). A recent study with the sample size of forty African-American women found that night-time (20.00–05.59 hours), but not daytime (06.00–19.59 hours), energy consumption was inversely associated with dynamic β-cell response but not with glucose tolerance or insulin action during late pregnancy( Reference Chandler-Laney, Schneider and Gower 12 ).
A previous report found that glucose tolerance declined in the evening in normal-weight adults, but such a rhythm was absent in the obese( Reference Lee, Ader and Bray 29 ). It was suggested that the marked suppression of insulin sensitivity in the morning in obese subjects may lead to failure for detection with further reduction in insulin sensitivity( Reference Lee, Ader and Bray 29 ). This may probably explain the reason why overweight pNT feeders in our study did not show significant difference in glycaemic response related to feeding patterns. It is therefore speculated that diurnal rhythm in insulin sensitivity and secretion may be adiposity dependent. Specifically, we showed that FG but not 2HPPG concentration was associated with feeding patterns. This suggests that the 2-h glucose measurement is less likely to be influenced by the timed feeding, although the variability of the 2-h glucose measurement was larger than FG. Nonetheless, as we did not ascertain any glucose measurements between FG and 2HPPG, we were unable to determine the post-OGTT response using the trapezoid method( Reference Matthews, Altman and Campbell 30 ), which serves as a better indicator for glucose tolerance.
Limited research has been conducted to examine the diet quality in those with delayed temporal distribution of food intake( Reference Gallant, Lundgren and Drapeau 31 ). With respect to the daily macronutrient distribution, overweight pNT feeders had lower proportion of carbohydrate consumption than their counterparts. This is consistent with a report that indicated an association between evening chronotypes and less carbohydrate consumption( Reference Kanerva, Kronholm and Partonen 32 ). Such difference in carbohydrate intake did not seem to attenuate the association between feeding pattern and FG in the overweight group. Similar results remained with adjustment for proportion of carbohydrate intake in the model. In contrast to these observations, two studies reported no differences in the daily macronutrient distribution between early and late eaters( Reference Garaulet, Gómez-Abellán and Alburquerque-Béjar 33 , Reference Lucassen, Zhao and Rother 34 ), which is similar to our findings in lean women. Altogether, this suggests that the association between feeding patterns and glucose concentration may not be confounded by diet quality in terms of macronutrient distribution.
This study provides an insight into the influence of feeding patterns on glycaemic levels in a large sample of pregnant Asian women. However, our findings were limited by the lack of data on serum insulin concentrations, dietary glycaemic index and maternal genotype, which would have allowed the assessment on insulin sensitivity, quality of carbohydrates and clock gene polymorphisms. Furthermore, only one free-living 24-h dietary recall was collected and might not reflect habitual consumption patterns. Fluctuation of food intakes from day to day could have led to misclassification of women according to feeding patterns.
In conclusion, pNT feeding was associated with higher FG concentrations in lean but not in overweight pregnant women. This suggests that the effect of feeding patterns on glucose tolerance during pregnancy may be adiposity dependent. Further investigation is required to identify the underlying mechanism of such differences. Nonetheless, our findings are important to serve as a basis for developing novel nutritional strategies to improve glucose tolerance during pregnancy. Therefore, intervention studies targeting pregnant women at risk of glucose intolerance and examination of changes in metabolic profile should be performed to better elucidate the effectiveness of using this time-related dietary approach. It will also be interesting to examine the changes in feeding patterns across different trimesters of pregnancy. In addition, the potential long-term health benefits of consuming higher energy content during the day for both mother and offspring need to be assessed. Undoubtedly, future research will shed more light on the interaction between the circadian timing system, nutrition and metabolism to improve human health, calling for more attention to the role of chrononutrition.
Acknowledgements
The authors thank the study subjects and their families for their participation. The authors also thank the GUSTO study group, which includes Pratibha Agarwal, Arijit Biswas, Choon Looi Bong, Birit F. P. Broekman, Shirong Cai, Yiong Huak Chan, Cornelia Yin Ing Chee, Helen Chen, Amutha Chinnadurai, Chai Kiat Chng, Shang Chee Chong, Mei Chien Chua, Doris Fok, Marielle V. Fortier, Anne Eng Neo Goh, Yam Thiam Daniel Goh, Joshua J. Gooley, Wee Meng Han, Mark Hanson, Christiani Jeyakumar Henry, Joanna D. Holbrook, Chin-Ying Hsu, Neerja Karnani, Jeevesh Kapur, Ivy Yee-Man Lau, Bee Wah Lee, Yung Seng Lee, Sok Bee Lim, Iliana Magiati, Lourdes Mary Daniel, Michael Meaney, Cheryl Ngo, Krishnamoorthy Niduvaje, Wei Wei Pang, Anqi Qiu, Boon Long Quah, Victor Samuel Rajadurai, Mary Rauff, Salome A. Rebello, Jenny L. Richmond, Anne Rifkin-Graboi, Lynette Pei-Chi Shek, Allan Sheppard, Borys Shuter, Leher Singh, Shu-E. Soh, Walter Stunkel, Lin Lin Su, Kok Hian Tan, Oon Hoe Teoh, Mya Thway Tint, Hugo P. S. van Bever, Rob M. van Dam, Inez Bik Yun Wong, P. C. Wong and George Seow Heong Yeo.
This study was supported by the Singapore National Research Foundation under its Translational and Clinical Research Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council, Singapore-NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding was provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), Singapore. K. M. G. is supported by the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre and by the European Union’s Seventh Framework Programme (FP7/2007-2013), project EarlyNutrition under grant agreement no. 289346. J. K. Y. C. received salary support from the Ministry of Health’s National Medical Research Council, Singapore (NMRC/CSA/043/2012).
K. M. G., P. D. G., K. K., S. M. S. and Y.-S. C. designed the GUSTO cohort study. S. L. L. and F. Y. designed the present study. S. L. L., T. S. C., M. T. C., N. P. and F. M.-R. performed data management and analysis. Y. B. C. advised on the statistical analysis. S. L. L., T. S. C., Y. B. C., N. L., F. Y., M. F.-F. C. and J. K. Y. C. interpreted the findings. S. L. L. and T. S. C. drafted the paper. All the authors participated in the critical review, revision and approval of the final manuscript.
P. D. G., K. M. G. and Y.-S. C. report receiving reimbursement for speaking at conferences sponsored by companies selling nutritional products. P. D. G., K. M. G. and Y.-S. C. report being part of an academic consortium that has received research funding from Abbott Nutrition, Nestle and Danone. No other disclosures were reported.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516000441







