Prebiotic fibres represent oligosaccharides that are resistant to human digestive enzymes but can be fermented by bacteria in the caeco-colon(Reference Delzenne1). Several studies have demonstrated that supplementing the diet, both standard chow(Reference Cani, Dewever and Delzenne2) and high fat(Reference Cani, Neyrinck and Maton3, Reference Maurer, Eller and Hallam4), of rodents with inulin, oligofructose or a combination of the two reduces energy intake and fat mass. Suggestions as to the mechanisms responsible for these effects have included alterations in satiety hormone secretion, delayed gastric emptying, energy dilution, increased energy expenditure and modulation of gut microflora.
The intestinal mucosa, primarily in the distal ileum, caecum and colon, contains endocrine L-cells that secrete peptides in response to nutrient stimulus(Reference Gevrey, Malapel and Philippe5–Reference Drucker7). Of particular interest, with respect to weight loss, are the two L-cell-derived anorexigenic peptides, glucagon-like peptide-1 (GLP-1) and peptide YY (PYY), and the X/A-like cell-derived orexigenic peptide ghrelin. Prebiotics have been shown to increase GLP-1 and PYY in human subjects(Reference Parnell and Reimer8, Reference Cani, Lecourt and Dewulf9) and rodent models(Reference Cani, Dewever and Delzenne2, Reference Cani, Neyrinck and Maton3, Reference Urias-Silvas, Cani and Delmee10–Reference Delzenne, Cani and Daubioul12). Expression of proglucagon, the precursor of GLP-1, can be up-regulated by SCFA, the end products of fibre fermentation in the gut(Reference Delzenne, Cani and Daubioul12, Reference Tappenden, Drozdowski and Thomson13). Ghrelin, which is acylated via the actions of ghrelin O-acyltransferase (GOAT)(Reference Yang, Brown and Liang14, Reference Gutierrez, Solenberg and Perkins15), has also been shown to decrease in response to prebiotic supplementation in rats(Reference Cani, Dewever and Delzenne2, Reference Cani, Neyrinck and Maton3) and human subjects(Reference Parnell and Reimer8).
Given that oligofructose-enriched diets stimulate the secretion of GLP-1, which in turn inhibits gastric motility via its actions as an ileal break(Reference Wettergren, Schjoldager and Mortensen16), it is plausible that prebiotic fibre may delay gastric emptying, although this suggestion lacks confirmation in an obese model. Furthermore, inulin-type fructans contain only 6·3 kJ/g as opposed to digestible carbohydrates, which have an energy value of 16·7 kJ/g(Reference Roberfroid17). As a result, supplementation with these fibres lowers the energy content of the diet. In general, rats will compensate for the lower energy content of a fibre-enriched diet with hyperphagia(Reference Daubioul, Rousseau and Demeure18); however, diets enriched in inulin-type fructans appear to be an exception(Reference Delzenne, Cani and Daubioul12). Whether the weight loss associated with prebiotic supplementation is due to increased satiety, energy dilution or a combination of the two remains controversial.
The human gut is colonised by large numbers of bacteria that utilise dietary substances that would otherwise be non-digestible(Reference Savage19). Recently, obesity has been linked to dysbiosis of the gut microbiota(Reference Cani and Delzenne20). Furthermore, the gut microbiota has also been shown to play a role in metabolic endotoxaemia, the low-grade inflammatory tone linked to obesity and many of its co-morbidities(Reference Cani, Possemiers and Van de Wiele21, Reference Cani, Bibiloni and Knauf22). Generally, obesity is associated with a reduction in Bacteroidetes and an increase in Firmicutes(Reference Ley, Turnbaugh and Klein23); nevertheless, this remains controversial(Reference Duncan, Lobley and Holtrop24, Reference Zhang, DiBaise and Zuccolo25). It has been proposed that changes at the smaller microbial community level rather than at the phylum level are involved in the development of obesity(Reference Cani and Delzenne20); however, this requires further investigation as does the potential for prebiotic fibres to modulate the gut microbiota to promote weight loss. A recent study has suggested that gut bifidobacteria are lower in obesity and with high-fat feeding and that supplementing the diet of mice with prebiotics restores bifidobacteria numbers and reduces metabolic endotoxaemia caused by a high-fat diet(Reference Cani, Neyrinck and Fava26). The effects of prebiotic fibres on other bacterial population in obesity remain understudied. Finally, metabolic rate is decreased in germ-free mice compared with conventionalised mice, suggesting that the microbial community may affect energy expenditure(Reference Backhed, Ding and Wang27).
The purpose of the present study was to examine the dose-dependent effects of prebiotic fibre on body-weight regulation in lean and obese littermates of the genetically obese JCR:LA-cp rat strain. Primary outcome measures following 10 weeks of inulin and oligofructose consumption included plasma satiety hormones, gastric emptying, energy expenditure and gut microbiota.
Methods
Animal model
Male lean (+/?; which includes cp/+ and +/+) and obese (cp/cp) JCR:LA-cp rats (8 weeks of age) were obtained from the colony of Dr Spencer Proctor (University of Alberta, Edmonton, AB, Canada). Due to the lack of a functional leptin receptor, they are severely obese and exhibit marked hyperphagia and hyperinsulinaemia in combination with altered intestinal physiology(Reference Koletsky28, Reference Parnell and Reimer29). All procedures were approved by the University of Calgary Animal Care Committee and conformed to the procedures set forth for the Care and Use of Laboratory Animals.
Dietary intervention
At 8 weeks of age, the animals were randomly assigned to ad libitum control (C) 0 %, fibre (F) 10 % or high-fibre (HF) 20 % inulin:oligofructose (w/w) diets (Table 1) for 10 weeks (eight animals per group). The composition of the diets and the energy contribution of the composite macronutrients are shown in Table 1. Food intake was recorded daily.
Table 1 Composition of the experimental diets*

* Based on the AIN-93 (Dyets, Inc., Bethlehem, PA, USA) diet for the maintenance of adult rats.
† 1:1 ratio of inulin:oligofructose supplied as Orafi Rafiline HP (Quadra Chemicals Limited, Burlington, ON, Canada) and Orafti Raftilose (Quadra Chemicals Limited).
‡ Digestible energy includes energy provided by carbohydrates, protein, fats and prebiotic fibre. For prebiotic fibre, a value of 6·3 kJ/g was used(Reference Roberfroid17).
Gastric emptying
Measurements of gastric emptying were performed in lean and obese rats from each diet group (n 6) in week 6 using the acetaminophen method(Reference Hatanaka, Kondoh and Kawarabayashi30). Following an overnight fast, acetaminophen (100 mg/kg) was added to 2 g of each group's respective diet and dissolved in distilled water at a 50 % concentration. A fasting blood sample was taken followed by administration of the mixture by oral administration. Additional blood samples were taken at times 15, 30, 60 and 90 min post-oral administration and assayed for acetaminophen concentrations by Calgary Laboratory Services (Calgary, AB, Canada).
Metabolic measurements
Measurements of energy expenditure were made 1 week before killing. Following an overnight fast, animals were placed in specially designed plastic cages and allowed free access to their respective diets throughout the test. Respiratory gas measurements were made over a 23 h period in which the air was drawn from the cages, dried and sent to an Oxymax Analyzing System (Columbus Instruments, Columbus, OH, USA) for O2 and CO2 analysis. To estimate the relative amount of carbohydrate and fat energy expenditure, the RER was calculated as the quotient of VCO2/VO2.
Body composition
Body weight was recorded weekly. On the day before killing, rats were lightly anaesthetised and a dual-energy X-ray absorptiometry scan was performed. Hologic QDR software for small animals was used to determine lean and fat mass (QDR 4500; Hologic, Inc., Bedford, MA, USA).
Meal tolerance test
Following an overnight fast, rats were anaesthetised (isoflurane) and a fasting blood sample drawn via cardiac puncture. Rats were allowed to awaken and then given an oral administration of 5 g of their respective diet dissolved in distilled water at 50 % concentration. Additional blood samples were taken at 15, 30, 60 and 90 min post-oral administration. Approximately, 0·5 ml of blood were collected from each rat into a chilled EDTA tube containing aprotinin (500 kallikrein inhibitor units/ml of blood; Sigma Chemical Company, Oakville, ON, Canada) and diprotin A (0·034 mg/ml of blood; MP Biomedical, Solon, OH, USA). After the final draw, animals were killed by over anaesthetisation of gaseous isoflurane and tissues were collected.
Gut characteristics
The stomach, small intestine, caecum and colon were analysed for weight and length (when appropriate). The small intestine was divided into three equal parts and a portion from the distal end of each was snap-frozen in liquid N2. Samples from the fundus of the stomach, the caecum and proximal colon were also frozen and all samples were stored at − 80°C.
Plasma analysis
Blood glucose concentrations were determined immediately at each time point using a ‘One Touch’ blood glucose meter (LifeScan Canada Limited, Burnaby, BC, Canada). Rat Endocrine LINCOplex kits were used to quantify GLP-1 (active), glucagon, amylin and insulin (Linco Research, Inc., Millipore, Billerica, MA, USA). The sensitivity of the multiplex kit is 55·6 pm for insulin and 6·2 pm for all other analytes. ELISA kits with a sensitivity of 12·5 fmol/ml were used to determine des-acyl ghrelin levels (Millipore, Billerica, MA, USA). Incremental area under the curve (iAUC) and total area under the curve (tAUC) were calculated(Reference Massimino, McBurney and Field31).
RNA isolation and real-time PCR
RNA isolation and real-time PCR were performed as described previously(Reference Parnell and Reimer29). Ghrelin, proglucagon and PYY mRNA were measured throughout the gastrointestinal tract. Additionally, ghrelin and GOAT were measured in the fundus of the stomach using previously published primer sequences(Reference Reimer, Maurer and Lau32). Within each genetic group (lean and obese), the control diet was used as the control condition. For comparisons between genetic groups, the lean group was used as the control. Fold change from control was calculated using the comparative cycle threshold method(Reference Livak and Schmittgen33).
Microbial profiling using quantitative PCR
Total bacterial DNA was extracted from approximately 200 mg of caecal samples using the QIAamp DNA Stool Mini Kit. According to the manufacturer, DNA yield is typically 15–60 μg and DNA concentration is typically 75–300 ng/μl (Qiagen, Mississauga, ON, Canada). Validation of the stool extraction kit was performed by spiking the stool samples with known amounts of gram+exogenous bacteria in two separate extraction experiments and measuring the colony-forming units by quantitative PCR. Recovery was ± 100 colony-forming units. This procedure was used to confirm that the extraction was consistent and able to lyse the more difficult gram+bacteria. DNA concentrations were determined using the PicoGreen DNA quantification kit (Invitrogen, Carlsbad, CA, USA). Bacterial groups were quantified by quantitative PCR using the BioRad iCycler (BioRad, Inc., Mississauga, ON, Canada) and the group-specific primers are shown in Table 2. Normal PCR was run on all primer sets to determine optimal conditions. Additionally, group-specific primers were run against all negative control bacteria. Amplification efficiency and the limit of detection were determined using serial dilutions of the standards. Primer sets were confirmed to be within 2 bp of known rat microbiota sequences by blasting primers against the 16S Ribosomal Database Project (http://rdp.cme.msu.edu/hierarchy/hb_intro.jsp;jsessionid = 568E9C48ED3A5164E1E1D69FEFC4C13C?timeout = true). The PCR was heated for 2 min at 95°C, followed by forty-two cycles of 95°C for 20 s, 55°C for 30 s and 72°C for 30 s and a final cycle of 72°C for 2 min. A melt curve analysis was run starting at 60°C and increased by 1°C every 8 s. Real-time PCR assays were run in duplicate and standard curves constructed for each experiment using 10-fold serial dilutions of standard bacterial genomic DNA of known concentration (American Type Culture Collection, Manassas, VA, USA) specific for each primer. Cell numbers of bacteria in faecal samples were calculated from threshold cycle values and are expressed as mean pg bacterial DNA/ng total genomic DNA.
Table 2 Groups of micro-organisms, primers and bacteria genomic DNA standards for quantitative PCR

F, forward; R, reverse.
* The primer set for C. leptum covers close relatives of C. leptum and does not cover the genus Rumminicocus.
Statistical analysis
All data are presented as means and standard errors. Differences in body composition, gut characteristics, gut microbiota, food and energy intake and area under the curve were determined using two-factor ANOVA (with diet and genetic group (lean v. obese) as variables). Changes in gene expression between the diets within the lean and obese groups were analysed using one-way ANOVA and Tukey's post hoc test. Parameters with serial measurements were analysed with a repeated-measures ANOVA (with time as a within-subject variable and diet and genetic group as between-subject variables) with Bonferroni adjustment when applicable. Pearson's correlation tests were used to evaluate the association of the gut microbiota with body weight and body fat as well as blood biochemistry and energy intake. Data were analysed using SPSS Statistics 17.0 (SPSS, Inc., Chicago, IL, USA). The result was considered significant when P ≤ 0·05.
Results
Prebiotics dose-dependently increase gut weight but do not alter body fat in JCR:LA-cp rats
Body weight did not differ with prebiotic supplementation in lean or obese rats over the 10-week study (Fig. 1(a)). Adjusting for the greater gut weight in the F groups (Table 3) did not alter these results. A 25 % reduction in percentage of body fat in lean rats fed the highest dose of fibre did not reach significance (P>0·1, Fig. 1(b)). No other parameters measured using dual-energy X-ray absorptiometry differed among groups including absolute lean and fat mass and bone mineral density (data not shown).
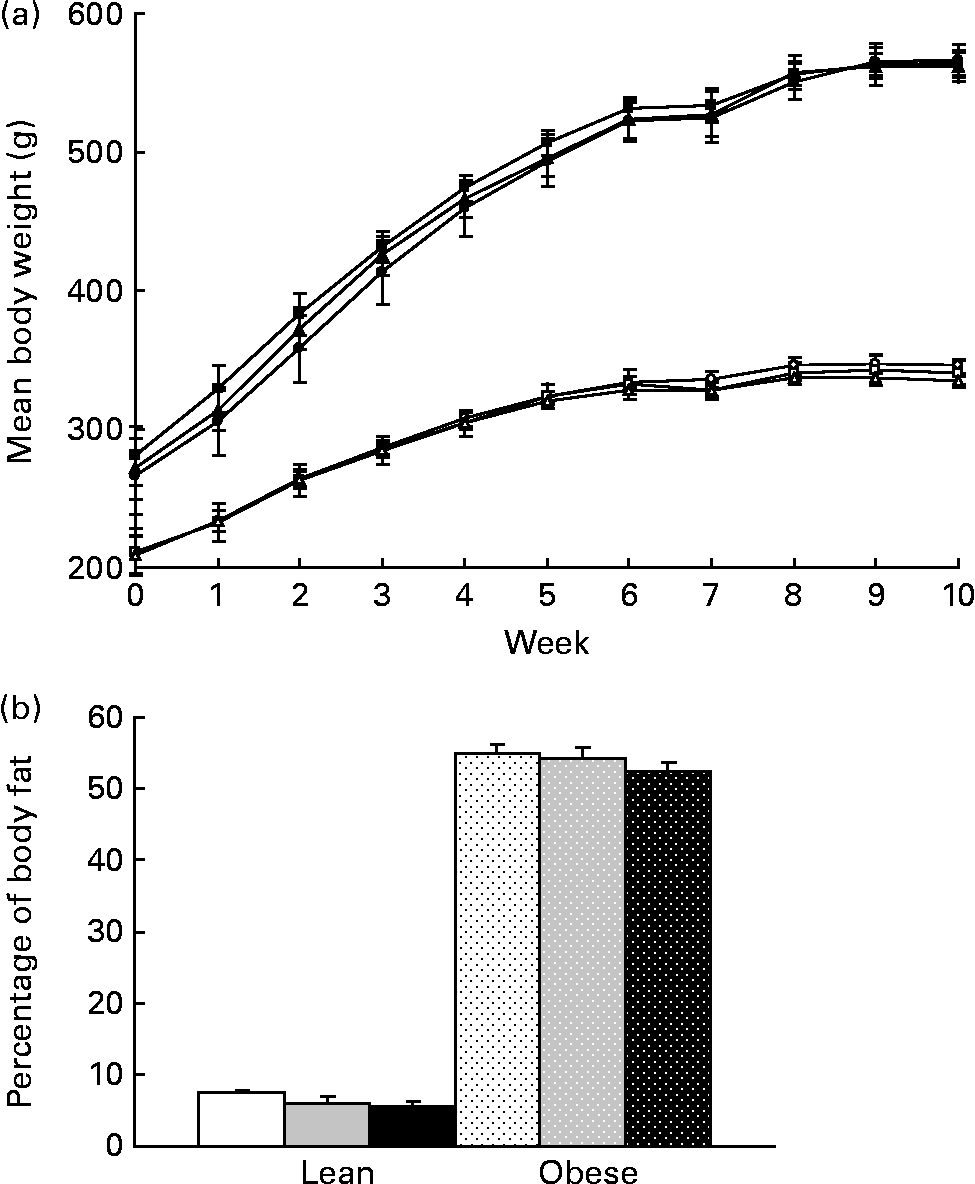
Fig. 1 (a) Weekly body weights and (b) percentage of body fat as determined by dual-energy X-ray absorptiometry for lean and obese rats on control (0 %), fibre (10 %) and high-fibre (20 %) prebiotic diets. Values are means, with their standard errors represented by vertical bars (n 8). Mean values were not significantly different within genetic groups (P>0·05; two-way ANOVA and repeated-measures ANOVA). LC ((a) ![]() ; (b) □), lean control; LF ((a)
; (b) □), lean control; LF ((a) ![]() ; (b)
; (b) ![]() ), lean fibre; LHF ((a)
), lean fibre; LHF ((a) ![]() ; (b) ■), lean high fibre; OC ((a)
; (b) ■), lean high fibre; OC ((a) ![]() ; (b)
; (b) ![]() ), obese control; OF ((a)
), obese control; OF ((a) ![]() ; (b)
; (b) ![]() ), obese fibre; OHF ((a)
), obese fibre; OHF ((a) ![]() ; (b)
; (b) ![]() ), obese high fibre.
), obese high fibre.
Table 3 Physical characteristics and daily energy intake
(Mean values with their standard errors, n 8)

LC, lean control; LF, lean fibre; LHF, lean high fibre; OC, obese control; OF, obese fibre; OHF, obese high fibre; SI, small intestine; TG, total gut; GB, gutless body weight.
* Mean values were significantly different from the control diet within the respective lean and obese groups (P < 0·05; two-way ANOVA).
† Mean values were significantly different between 10 % and 20 % fibre within the lean and obese groups (P < 0·05; two-way ANOVA).
‡ Mean values were significantly different between lean and obese within the respective diet groups (P < 0·05; two-way ANOVA).
§ Caecal weight refers to the caecal tissue and its contents.
∥ TG weight was calculated by adding the weight of the stomach, SI, caecum and colon.
¶ Body weight without the gut was determined by subtracting total gut weight from body weight.
Small-intestinal length was 17 % longer in obese v. lean rats (P < 0·01; Table 3). The HF diet also increased small-intestinal length compared with the C and F diets. In lean and obese rats, the weight of the caecum and colon increased dose-dependently with F supplementation (P < 0·001). Total gut weight was increased in obese v. lean rats as well as dose-dependently with F supplementation (P < 0·01).
Prebiotic enhances glucagon-like peptide-1 and blunts ghrelin response to a meal
There was a significant effect of the prebiotic on GLP-1 during the meal tolerance test with a higher tAUC in the HF group compared with the C group (P < 0·01). tAUC for GLP-1 was increased in lean 20 % fibre (LHF) compared with lean 0 % fibre (LC) and in obese 20 % fibre (OHF) compared with obese 0 % fibre (OC; P = 0·01, Fig. 2(b)). There was a significant diet effect for ghrelin with tAUC in the LHF (P = 0·007) and lean 10 % fibre (LF; P = 0·04) groups lower than the LC group. Ghrelin concentrations at fasting, 15 and 30 min were lower in obese v. lean rats (P < 0·001; Fig. 2(a)) and showed minimal response to the meal.

Fig. 2 Glucagon-like peptide-1 (GLP-1; active) and des-acyl ghrelin in lean and obese rats fed increasing doses of prebiotic. Values are means, with their standard errors represented by vertical bars (n 8). (a) Serial values during the 90 min meal tolerance test. * Mean values were significantly different between the lean and obese groups from those of control diets (P ≤ 0·05; two-way ANOVA and repeated-measures ANOVA). † Mean values were significantly different between the lean and obese groups from those of 10 % fibre diets (P ≤ 0·05; two-way ANOVA and repeated-measures ANOVA). ‡ Mean values were significantly different between the lean and obese groups from those of 20 % fibre diets (P ≤ 0·05; two-way ANOVA and repeated-measures ANOVA). (b) Total area under the curve (tAUC). * Mean values were significantly different from those of the control within the lean or obese groups (P ≤ 0·05; two-way ANOVA and repeated-measures ANOVA). LC ((a) ![]() ; (b) □), lean control; LF ((a)
; (b) □), lean control; LF ((a) ![]() ; (b)
; (b) ![]() ), lean fibre; LHF ((a)
), lean fibre; LHF ((a) ![]() ; (b) ■), lean high fibre; OC ((a)
; (b) ■), lean high fibre; OC ((a) ![]() ; (b)
; (b) ![]() ), obese control; OF ((a)
), obese control; OF ((a) ![]() ; (b)
; (b) ![]() ), obese fibre; OHF ((a)
), obese fibre; OHF ((a) ![]() ; (b)
; (b) ![]() ), obese high fibre.
), obese high fibre.
Proglucagon and peptide YY mRNA levels are increased with prebiotic intake while ghrelin O-acyltransferase mRNA is decreased in obese rats at high intake
In lean rats, prebiotics increased proglucagon and PYY mRNA levels in the caecum with a 2-fold increase in the LF group and a 3-fold increase in the LHF group. PYY was up-regulated in the middle segment of the small intestine in the LHF group (Fig. 3). Ghrelin was down-regulated 40 % in the ileum of both LF and LHF groups compared with the LC group.

Fig. 3 (a) Proglucagon, (b) ghrelin and (c) peptide YY (PYY) mRNA levels in lean and obese rats exposed to a control, fibre or high-fibre diet. Values are means, with their standard errors represented by vertical bars (n 8). * Mean values were significantly different from the control diet (P ≤ 0·05; one-way ANOVA). † Mean values were significantly different between the fibre and high-fibre diets within genetic groups (P ≤ 0·05; one-way ANOVA). LC (□), lean control; LF (![]() ), lean fibre; LHF (■), lean high fibre; OC (
), lean fibre; LHF (■), lean high fibre; OC (![]() ), obese control; OF (
), obese control; OF (![]() ), obese fibre; OHF (
), obese fibre; OHF (![]() ), obese high fibre; SI, small intestine.
), obese high fibre; SI, small intestine.
In obese rats, proglucagon and PYY mRNA levels were increased in the caecum with the obese 10 % fibre (OF) and OHF diets. There was a dose–response in colonic proglucagon mRNA with a 1·7-fold increase in the OF group and a 2·5-fold increase in the OHF group. A similar trend was observed with PYY expression. In the proximal small intestine, OHF increased proglucagon and PYY expression by 1·5-fold and 9-fold, respectively, compared with the other diets. There was a approximately 4-fold increase in PYY mRNA levels in the middle small intestine and an approximately 2-fold increase in the distal small intestine with OHF supplementation (Fig. 3). There was a 50 % decrease in ghrelin mRNA in the proximal small intestine in the OHF group (P < 0·05). Finally, there were 45 and 32 % decreases in ghrelin mRNA levels with OF and OHF supplementation, respectively, in the middle segment of the small intestine (Fig. 3).
In obese rats, GOAT mRNA expression was significantly decreased (P = 0·05) with OHF supplementation compared with OC and OF supplementation (Fig. 4). When all obese rats were compared with all lean rats, there was a significant 2·0-fold higher expression of GOAT mRNA in the stomach in obese v. lean rats (P = 0·03).
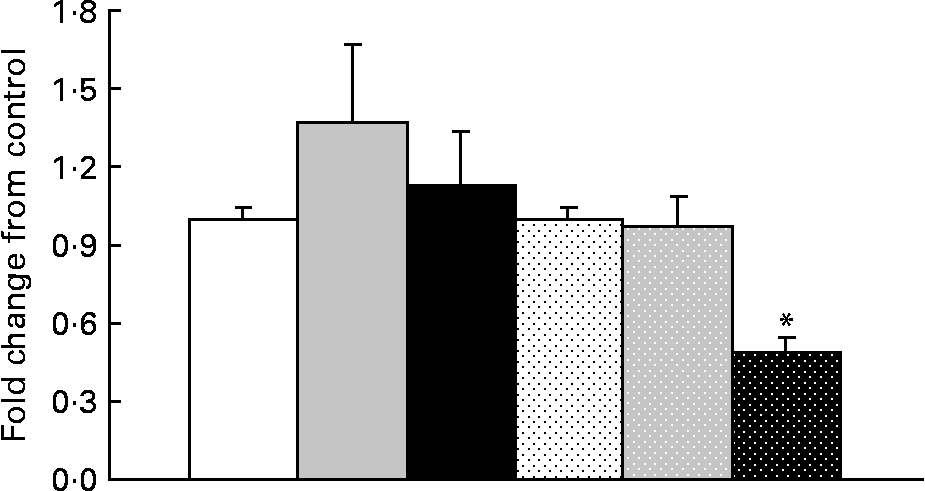
Fig. 4 Ghrelin O-acyltransferase mRNA levels in lean and obese rats exposed to a control, fibre or high-fibre diet. Values are means, with their standard errors represented by vertical bars (n 8). * Mean values were significantly different from the control diet within the obese groups (P < 0·05). LC (□), lean control; LF (![]() ), lean fibre; LHF (■), lean high fibre; OC (
), lean fibre; LHF (■), lean high fibre; OC (![]() ), obese control; OF (
), obese control; OF (![]() ), obese fibre; OHF (
), obese fibre; OHF (![]() ), obese high fibre.
), obese high fibre.
Energy intake but not energy expenditure or gastric emptying is altered with prebiotic
Food intake was greater in obese v. lean rats and increased with prebiotic in the HF group compared with the C group (P = 0·02). Energy intake (grams of food consumption multiplied by energy density of the diet) was reduced in the F and HF groups compared with the C group. Prebiotic fibre resulted in a 6 % decrease in energy intake in the LF and OF groups, an 11 % decrease in the LHF group and an 8 % decrease in the OHF group compared with the OC group (P < 0·05; Fig. 5). The mean daily food intake for lean rats in the C, F and HF groups was 15·1 (sd 0·6), 15·2 (sd 0·7) and 15·7 (sd 1·1) g/d compared with obese rats in the C, F and HF groups at 24·8 (sd 1·8), 25·0 (sd 1·9) and 26·7 (sd 2·0) g/d, respectively. Conversely, the mean energy intake for lean rats in the C, F and HF groups was 239·3 (sd 9·2), 225·1 (sd 10·3) and 217·1 (sd 14·9) kJ/d compared with obese rats in the C, F and HF groups at 394·9 (sd 28·5), 370·7 (sd 28·49) and 369·0 (sd 27·9) kJ/d, respectively. For both food intake and energy intake, there was a significant effect of genetic group and diet.
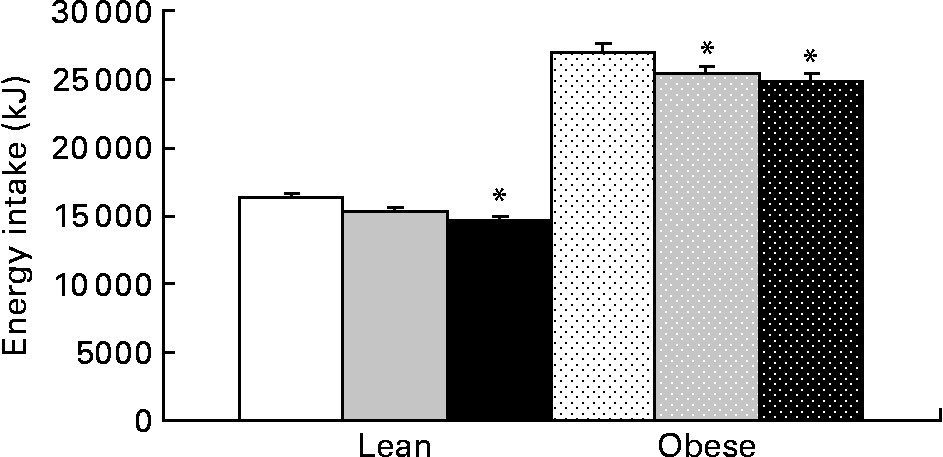
Fig. 5 Cumulative energy intake for lean and obese rats on the control, fibre and high-fibre prebiotic diets over 10 weeks. Values are means, with their standard errors represented by vertical bars (n 8). * Mean values were significantly different from the control within the respective lean and obese groups (P ≤ 0·05; two-way ANOVA). LC (□), lean control; LF (![]() ), lean fibre; LHF (■), lean high fibre; OC (
), lean fibre; LHF (■), lean high fibre; OC (![]() ), obese control; OF (
), obese control; OF (![]() ), obese fibre; OHF (
), obese fibre; OHF (![]() ), obese high fibre.
), obese high fibre.
No difference in the relative amount of carbohydrate and fat energy expenditure was detected among the groups during the 23 h test in lean or obese rats. RER values for the LC, LF, and LHF groups were 0·94 (sd 0·01), 0·93 (sd 0·01) and 0·94 (sd 0·01), respectively, while those for the OC, OF, and OHF groups were 0·95 (sd 0·01), 0·94 (sd 0·01) and 0·95 (sd 0·01), respectively. Gastric emptying was accelerated in obese v. lean rats at individual time points during the test as well as tAUC (P < 0·001; Fig. 6). Prebiotic did not alter acetaminophen appearance in plasma at any point over the 90 min test nor was tAUC changed.
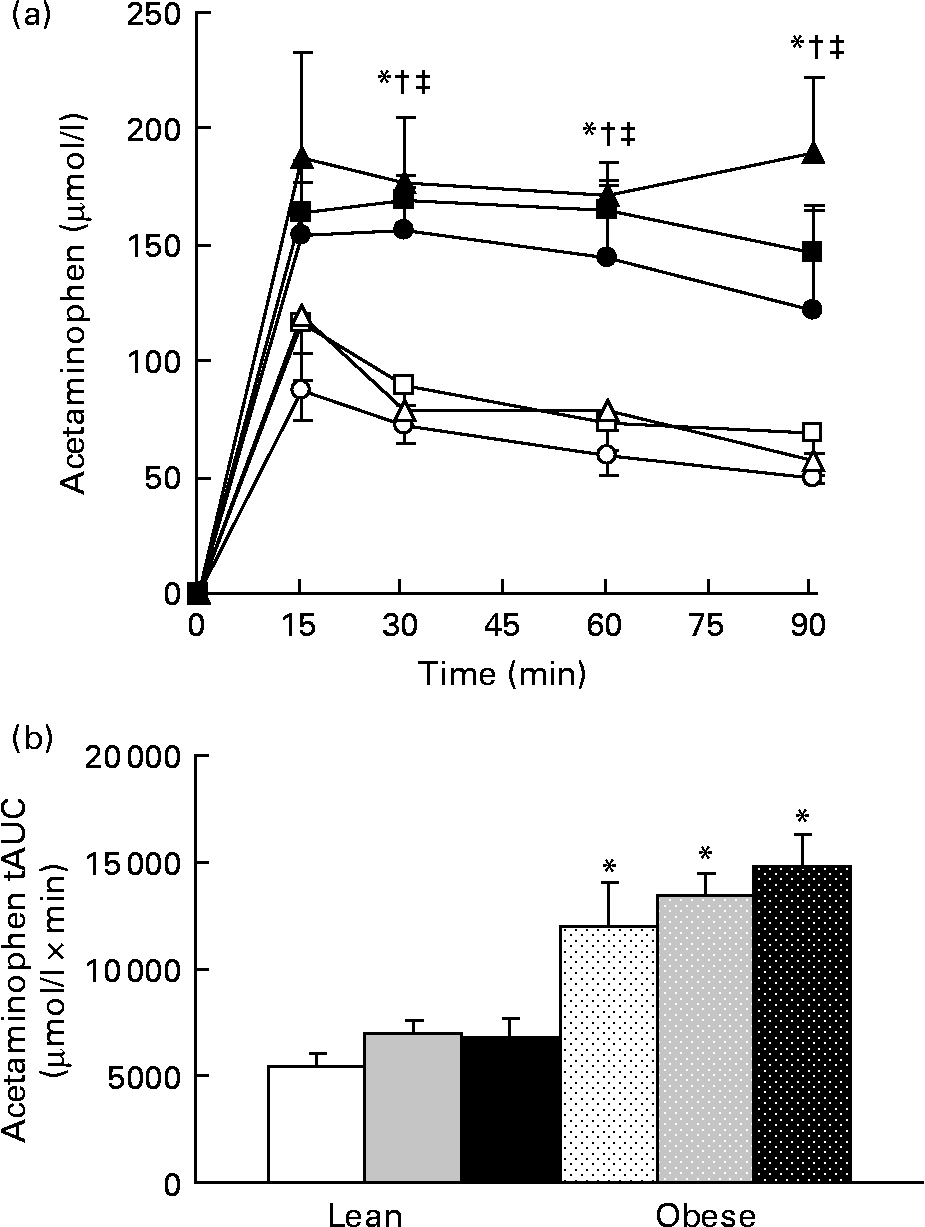
Fig. 6 Acetaminophen levels during a gastric emptying test. Values are means, with their standard errors represented by vertical bars (n 6). (a) Serial values during the 90 min test. * Mean values were significantly different between the lean and obese groups from those of the control diets (P ≤ 0·05; two-way ANOVA and repeated-measures ANOVA). † Mean values were significantly different between the lean and obese groups from those of the 10 % fibre diets (P ≤ 0·05; two-way ANOVA and repeated-measures ANOVA). ‡ Mean values were significantly different between the lean and obese groups from those of the 20 % fibre diets (P ≤ 0·05; two-way ANOVA and repeated-measures ANOVA). (b) Total area under the curve (tAUC). * Mean values were significantly different between obese and lean within a diet group (P ≤ 0·05; two-way ANOVA and repeated-measures ANOVA). LC ((a) ![]() ; (b) □), lean control; LF ((a)
; (b) □), lean control; LF ((a) ![]() ; (b)
; (b) ![]() ), lean fibre; LHF ((a)
), lean fibre; LHF ((a) ![]() ; (b) ■), lean high fibre; OC ((a)
; (b) ■), lean high fibre; OC ((a) ![]() ; (b)
; (b) ![]() ), obese control; OF ((a)
), obese control; OF ((a) ![]() ; (b)
; (b) ![]() ), obese fibre; OHF ((a)
), obese fibre; OHF ((a) ![]() ; (b)
; (b) ![]() ), obese high fibre.
), obese high fibre.
Glucagon but not glucose or insulin is decreased by prebiotic
Plasma glucose was increased in obese v. lean rats, suggesting impaired glucoregulation (Fig. 7(a)). Although glucose iAUC was 14 % lower in the LF group than in the LC group and 25 % lower in the LHF group than in the LC group, this difference was not significant (Fig. 7(b)). Insulin was significantly higher in obese v. lean rats (P < 0·05). Within the obese groups, insulin was increased in the OF group compared with the OC group at 0 and 30 min but no differences were seen in iAUC. Glucagon tAUC was decreased in the F v. HF group (P = 0·03); however, no differences were observed when the results were stratified by genetic group. No differences were seen in amylin.
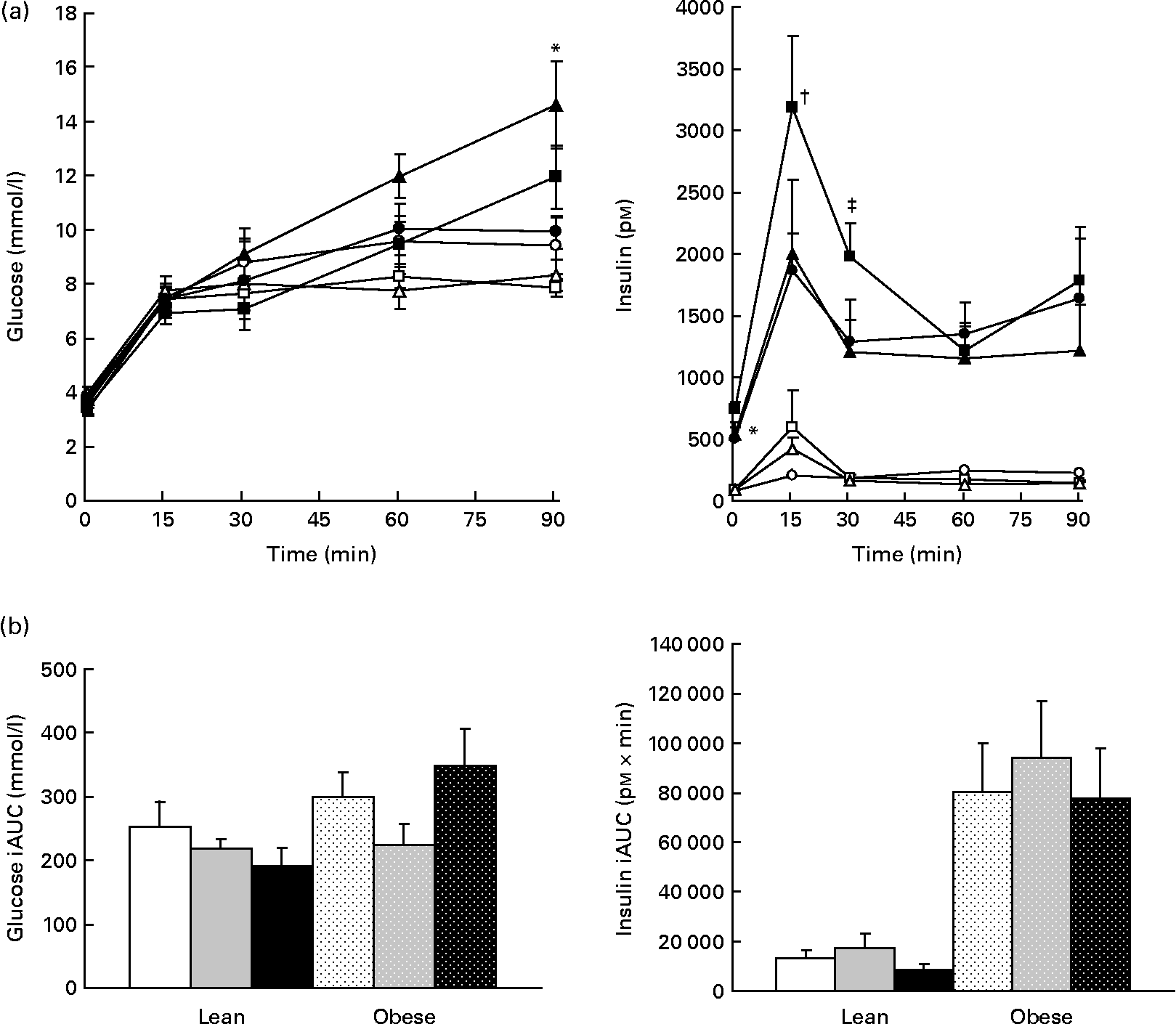
Fig. 7 Whole blood glucose values and plasma insulin. Values are means, with their standard errors represented by vertical bars (n 8). (a) Serial values during the 90 min meal tolerance test. * Mean values were significantly different between the OC and OF diets (P ≤ 0·05; two-way ANOVA and repeated-measures ANOVA). † Mean values were significantly different between the OC and OHF diets (P ≤ 0·05; two-way ANOVA and repeated-measures ANOVA). ‡ Mean values were significantly different between the OF and OHF diets (P ≤ 0·05; two-way ANOVA and repeated-measures ANOVA). (b) Incremental area under the curve (iAUC). LC ((a) ![]() ; (b) □), lean control; LF ((a)
; (b) □), lean control; LF ((a) ![]() ; (b)
; (b) ![]() ), lean fibre; LHF ((a)
), lean fibre; LHF ((a) ![]() ; (b) ■), lean high fibre; OC ((a)
; (b) ■), lean high fibre; OC ((a) ![]() ; (b)
; (b) ![]() ), obese control; OF ((a)
), obese control; OF ((a) ![]() ; (b)
; (b) ![]() ), obese fibre; OHF ((a)
), obese fibre; OHF ((a) ![]() ; (b)
; (b) ![]() ), obese high fibre.
), obese high fibre.
Composition of the gut microbiota changes with prebiotic in lean and obese JCR:LA-cp rats
There was a significant effect of genetic group (P < 0·01) for all bacterial populations examined except for Clostridium coccoides and Enterobacteriaceae (Table 4). Total bacteria and Bacteroides were significantly lower in obese v. lean rats. There was a significant effect of diet for all populations examined except total bacteria and C. coccoides. There was a significant interaction between diet and genetic group for Bifidobacterium spp., Enterobacteriaceae, total bacteria, C. coccoides, Clostrium leptum and Lactobacillus spp. (P < 0·05). Bifidobacterium spp. were dose-dependently increased with prebiotic in both lean and obese rats (P < 0·05). Lactobacillus spp. were significantly increased in the OHF group v. OC and OF groups. The C. coccoides group was reduced in the OF group compared with the OHF group but not compared with the C group (P < 0·05). The Bacteroides group was increased in the HF groups in both lean and obese rats compared with the C groups, whereas Clostridium leptum increased in the OHF group compared with the OC and OF groups but decreased in the LHF group compared with the LC group. There were no differences in Enterobacteriaceae in lean rats but a significant increase in the OHF group compared with the OC and OF groups (P < 0·05). Independent of genetic group, prebiotic fibre dose-dependently increased the Bacteroidetes groups measured in the 0, 10 and 20 % prebiotic groups. Likewise, diet and genetic group affected the Firmicutes groups quantified here with an increase in obese v. lean rats. Independent of genetic group, prebiotic fibre decreased the Firmicutes groups measured with the 10 and 20 % dose showing equal effects.
Table 4 Faecal microbiota composition of lean and obese JCR:LA-cp rats fed a 0, 10 or 20 % prebiotic fibre diet for 10 weeks§
(Mean values with their standard errors, n 6–8)

* Mean values were significantly different from the control diet within the respective lean and obese groups (P < 0·05; two-way ANOVA).
† Mean values were significantly different between the 10 % and 20 % fibre diets within the lean and obese groups (P < 0·05; two-way ANOVA).
‡ Mean values were significantly different between lean and obese within the respective diet groups (P < 0·05; two-way ANOVA).
§ Data are expressed as pg bacterial DNA/ng total genomic DNA.
Gut microbiota correlate with biomarkers of obesity
Bacteriodes and total bacteria were negatively correlated with percentage of body fat and body weight, whereas levels of Lactobacillus spp. were positively correlated with body weight and fat as well as with total energy intake and glucose iAUC (P < 0·05). Enterobacteriaceae increased in conjunction with glucose iAUC and GLP-1 tAUC. Bacteroides and total bacteria were positively correlated with ghrelin tAUC yet negatively correlated with fasting insulin, insulin iAUC and energy intake.
Discussion
Diets enriched with prebiotic fibre at a dose of 10 % have previously been shown to reduce weight gain and fat mass development in rats(Reference Cani, Neyrinck and Maton3). The present study was undertaken to determine whether the effects of prebiotics are regulated dose-dependently and to specifically determine whether gut microbiota beyond the traditionally measured Bifidobacterium and Lactobacillus spp. are modulated by prebiotics in a monogenic model of obesity. Our findings suggest that prebiotics chiefly affect energy balance by reducing energy intake. Both satiety hormone levels and numerous gut bacterial groups not previously described in supplemented obese models are modulated by prebiotics in a dose-dependent manner.
Ingestion of prebiotics alters the physical characteristics of the gut, most notable of which is an increase in gut weight and length, particularly the caecum and colon. This proliferative effect of fibre has important implications with respect to satiety hormone secretion, as these are the primary tissues involved in GLP-1 and PYY production. Augmented gut proliferation is supported in the present study by elevated expression of proglucagon, the precursor of GLP-2, a gut trophic hormone(Reference Drucker7). While the increase in gut weight that we observed with prebiotics has previously been reported(Reference Delzenne, Cani and Daubioul12), the lack of reduction in body weight and fat mass is contrary to many but not all reports with oligofructose supplementation(Reference Urias-Silvas, Cani and Delmee10, Reference Boyle, Wrenn and Marsh34).
It is well established that one of the metabolic by-products of prebiotic fibre metabolism by gut microbiota is SCFA. Although we did not measure SCFA in the present study, others have reported that prebiotic fibres increase the levels of SCFA and link this to increased expression of proglucagon(Reference Delzenne, Cani and Daubioul12, Reference Tappenden, Drozdowski and Thomson13, Reference Tappenden, Thomson and Wild35, Reference Drozdowski, Dixon and McBurney36). Indeed, we report increases in both proglucagon and plasma GLP-1. There are several probable explanations for the contradictory results between the significant increase in GLP-1 and lack of weight loss. Although the increase in proglucagon expression and GLP-1 secretion is statistically significant, it is possible that it is insufficient to induce a physiologically meaningful change in body weight in this specific model of monogenic obesity. We have previously observed a similar increase in GLP-1 and decrease in energy intake without concomitant weight loss in the JCR:LA-cp rat fed a combination of a high-protein/HF diet(Reference Reimer and Russell37). Additionally, although it has been well established that exogenous GLP-1 regulates satiety and promotes weight loss, GLP-1R− / − mice have normal food intake and body weight consuming either a normal or high-fat diet(Reference Scrocchi, Brown and MaClusky38, Reference Scrocchi and Drucker39), suggesting that other factors may override the satiogenic effects of GLP-1 secretion.
Plasma des-acyl ghrelin and ghrelin mRNA in the distal small intestine were decreased with prebiotic fibre in lean rats. In addition to the diet-induced effects, there were also notable differences in ghrelin secretion between lean and obese rats, with obese rats showing attenuated ghrelin levels both at fasting and in response to a meal. Other studies have supported an attenuated ghrelin response to food stimulus in human subjects with obesity(Reference English, Ghatei and Malik40, Reference Zwirska-Korczala, Konturek and Sodowski41). While gastric ghrelin mRNA did not change with prebiotics in lean or obese rats, GOAT mRNA in the stomach was significantly reduced by the 20 % fibre dose in obese rats. Obese rats also showed a 2·0-fold higher expression of GOAT mRNA than lean rats. GOAT, which acylates ghrelin and gives it potent orexigenic and growth hormone-stimulating actions(Reference Yang, Brown and Liang14, Reference Gutierrez, Solenberg and Perkins15), has not been described by others in the JCR:LA-cp rat but Kirchner et al. (Reference Kirchner, Gutierrez and Solenberg42) and Gahete et al. (Reference Gahete, Cordoba-Chacon and Salvatori43) did demonstrate an approximate 1·7-fold increase in GOAT mRNA in ob/ob mice compared with age-matched wild-type mice which did not reach statistical significance. While a comprehensive understanding of the roles and regulation of GOAT is not yet available, Kirchner et al. (Reference Kirchner, Gutierrez and Solenberg42) recently showed that production of active ghrelin in vivo is dependent on GOAT and diet composition, specifically medium-chain TAG availability. Some have suggested that blocking GOAT could be a strategy to prevent diet-induced obesity(Reference Gonzalez, Vazquez and Lopez44).
Food intake was either unaffected or increased with prebiotic fibres; however, due to the lower energy value of the fibre diets, overall energy intake was reduced(Reference Roberfroid17). This supports the theory of energy dilution as a mechanism of action of prebiotic fibres. We cannot rule out that the lower energy value of the diets and altered food intake did not also indirectly influence overall energy balance via changes in gut microbiota. Conversely, energy expenditure was not altered with prebiotic supplementation, indicating that changes in metabolic rate do not occur with increased prebiotic intake in the JCR:LA-cp rat. Gastric emptying was not affected with prebiotic consumption but was increased in obese v. lean rats. Although controversial(Reference Gallagher, Geoghegan and Baird45), increased rates of gastric emptying in obesity have been previously reported(Reference Cardoso-Junior, Coelho and Savassi-Rocha46) and may be linked to reduced satiety in obesity. Of special note with regard to satiety is that all of our diets were formulated to provide an equivalent amount of energy from fat and protein, thereby increasing the likelihood that changes in satiety were due to the addition of prebiotic fibre rather than alterations in protein or fat contribution to total energy of the diet.
In addition to disrupted gut satiety endocrine function, recent evidence suggests that analogous differences in the distal gut bacteria of obese v. lean mice and human subjects also play a role in obesity and contribute to the low-grade inflammatory state of obesity(Reference Ley, Turnbaugh and Klein23, Reference Turnbaugh, Ley and Mahowald47, Reference Backhed, Manchester and Semenkovich48). We confirm the decrease in Bacteriodetes and the corresponding increase from the division Firmicutes that has been described for obese mice and human subjects(Reference Ley, Turnbaugh and Klein23, Reference Turnbaugh, Ley and Mahowald47, Reference Backhed, Manchester and Semenkovich48). Furthermore, we demonstrate a significant shift in the Bacteroidetes and Firmicutes groups that is brought about with increasing doses of prebiotic fibre intake. Where our work specifically adds to the current body of knowledge is its assessment of the effects of obesity and prebiotics on other bacteria groups not traditionally measured. The impact of these strains on obesity requires further investigation; however, one study has reported that weight loss in overweight adolescents reduced Bifidobacterium spp. levels(Reference Santacruz, Marcos and Warnberg49), which supports our results.
Given that prebiotics alter the composition of the gut microbiota, there is significant interest in their ability to do so in a manner that promotes weight loss. Here, we report increases in Lactobacillus spp. with prebiotic supplementation. These results are promising as perinatal probiotic treatment with Lactobacillus rhamnosus has been shown to attenuate excessive weight gain in the first years of life(Reference Luoto, Kalliomaki and Laitinen50) and Lactobacillus gasseri reduced abdominal adiposity and body weight in adults with obese tendencies(Reference Kadooka, Sato and Imaizumi51). Our 1:1 mixture of inulin and oligofructose also increased Bacteroides, suggesting that prebiotics may be able to normalise the reduced Bacteroidetes numbers reported in obesity. While others have shown that prebiotic fibres increase gut Bifidobacterium spp. numbers(Reference Cani, Neyrinck and Fava26), the present study is the first to show that this response is dose-dependent in lean and obese JCR:LA-cp rats. The gut microbiota has been linked to body fat through a variety of mechanisms including increased energy harvesting, hepatic de novo lipogenesis, adipocyte fatty acid storage, presumably through lipoprotein lipases, and suppression of AMP-activated protein kinase-dependent fatty acid oxidation(Reference Cani and Delzenne20). Cani et al. (Reference Cani, Amar and Iglesias52) have shown that a high-fat diet increases intestinal permeability, which is considered a chief contributor to higher plasma lipopolysaccharide levels and consequent metabolic endotoxaemia. It has been proposed that prebiotics reduce the impact of high-fat diet-induced metabolic endotoxaemia in part by increasing the gut trophic factor GLP-2 and lowering intestinal permeability(Reference Cani, Neyrinck and Fava26). The increased proglucagon mRNA levels that we report with prebiotic would support such an increase in GLP-2 production. Furthermore, the correlation between the gut microflora and satiety hormones, energy intake and insulin levels is novel and emphasises the complexity of the system. In summary, the shift in microbial profile that we induced with an increasing dose of prebiotic probably contributes to overall improvements in health, particularly those aspects related to the development and maintenance of obesity.
We are aware of certain limitations of the present study. Obesity and the metabolic syndrome in humans are multifactorial and, as a result, make the identification or development of rodent models that fully represent the human condition difficult. While some monogenic models of obesity such as the Zucker fa/fa rat or our JCR:LA-cp rat do in fact develop anomalies similar to those characterising patients with obesity and/or the metabolic syndrome(Reference Aleixandre de Artiñano and Miguel Castro53), it is likely that a diet-induced obesity model(Reference Sampey, Vanhoose and Winfield54) more closely mimics these conditions in human subjects. Interpreting our findings in light of the genetic background of the JCR:LA-cp rat is prudent. It is also difficult to isolate the independent effects of the prebiotic on the gut microbiota from the lower energy intake associated with consumption of these diets. Studies have demonstrated a significant shift in gut bacterial groups following energy restriction and weight loss(Reference Santacruz, Marcos and Warnberg55, Reference Ley, Turnbaugh and Klein56). When energy restriction was achieved with an energy-restricted diet and increased physical activity(Reference Santacruz, Marcos and Warnberg55) or bariatric surgery(Reference Furet, Kong and Tap57), decreases in bifidobacteria were observed, which contrasts to the increases that we demonstrated in the present study. This as well as in vitro studies, showing significantly increased bifidobacteria with batch culture fermentations of prebiotics(Reference Beards, Tuohy and Gibson58), suggests that prebiotics have effects independent of energy dilution. Furthermore, it should be noted that our methods have not quantified all of the bacteria groups comprising the rodent gut microbiome(Reference Fleissner, Huebel and Abd El-Bary59). Additionally, the differences between rats and humans with respect to gut bacteria must be acknowledged. These data are significant; however, as they provide justification for additional studies in diet-induced obese models and human clinical trials.
In conclusion, the present study represents a systematic dose–response analysis of the effects of prebiotic fibres in both lean and obese states. There was a significant increase in circulating GLP-1 levels and a concomitant up-regulation of proglucagon and PYY mRNA with prebiotic intake in the present study. The results are limited, however, in the use of the monogenic rat model and its specific hormonal milieu, particularly satiety hormones. Furthermore, it should be noted that our methods have not quantified all of the bacteria groups comprising the rodent gut microbiome(Reference Fleissner, Huebel and Abd El-Bary59). Additionally, the differences between rats and humans with respect to gut bacteria must be acknowledged. These data are significant, however, as they provide justification for additional studies in diet-induced obese models and human clinical trials. Alterations in the gut microflora of these animals suggest that prebiotics may be able to ‘shape’ the bacterial communities in lean and obese animals to optimise metabolic health. The significance of the present study lies in the tremendous need for effective dietary strategies to combat the increasingly burdensome obesity epidemic. From a practical standpoint, numerous foods containing prebiotics have appeared on the market recently and additional research is required to fully understand their mechanism of action, safety and effectiveness.
Acknowledgements
The present study was supported in part by the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, and Mount Royal University Internal Grants. J. A. P. declares no conflict of interest. R. A. R. holds funding from the company that manufactures the prebiotics, Raftilose and Raftiline, for a project distinct from the present study. J. A. P. was involved in the study design and concept, acquisition and analysis of the data, and drafting of the manuscript. R. A. R. was involved in the study design and concept, analysis of the data, and revision of the manuscript. The authors would like to thank Kristine Lee and Kristine Cannon for their assistance with the laboratory work.













