Breast milk is the most important source of nutrients for infants, significantly influencing growth and development. Previously, breast-feeding had been reported to prevent allergy in children(Reference Gdalevich, Mimouni and David1), but recent observational studies of breast milk components have revealed various immunoactive agents with positive and negative roles in the onset of allergic disorders(Reference Snijders, Damoiseaux and Penders2–Reference Higashi, Shimojo and Suzuki5). Intervention studies of food products have revealed that the consumption of fish oil and probiotics by pregnant and lactating women increases the concentrations of anti-allergic immunoactive agents such as soluble cluster of differentiation 14 (CD14), IgA and transforming growth factor (TGF)-β in breast milk(Reference Dunstan, Roper and Mitoulas6, Reference Prescott, Wickens and Westcott7). The consumption of probiotics during pregnancy and lactation may help prevent atopic dermatitis, suggesting that probiotic administration in mothers induces the production of immunoactive agents in breast milk.
Prebiotics are defined as ‘selectively fermented ingredients that allow specific changes, in both the composition and/or activity in the gastrointestinal microbiota that confers benefits upon host well-being and health’; these include dietary fibre, resistant starch and oligosaccharides(Reference Gibson, Probert and Loo8). Typical indigestible oligosaccharide prebiotics such as fructo-oligosaccharides (FOS) and galacto-oligosaccharides increase the number of beneficial organisms such as bifidobacteria(Reference Bouhnik, Flourié and Riottot9, Reference Howard, Gordon and Pace10). Thus, prebiotics may have an effect similar to that of probiotics. Recent human studies have shown that the incidence of atopic dermatitis decreases in low- and high-risk infants when fed prebiotic-supplemented formula v. placebo-supplemented formula(Reference Grüber, van Stuijvenberg and Mosca11, Reference Arslanoglu, Moro and Boehm12), although its effect is not well established because of problems such as high dropout rates. However, there have been no studies about the effect of prebiotics on immunoactive agents in breast milk when given to pregnant and lactating women. In one study of atopic dermatitis in NC/Nga mice, FOS administration in mothers has been shown to control the onset of atopic dermatitis in mouse pups(Reference Fujiwara, Takemura and Watanabe13); however, the effect of FOS administration on cytokine levels in breast milk has not been studied.
Although there have been a few studies on the effect of food products on specific cytokines in breast milk, a comprehensive analysis has not been reported. Immunoactive agents in breast milk are mostly derived from serum or mammary gland epithelial cells(Reference Palkowetz, Royer and Garofalo14), but are potentially produced by immune cells in breast milk(Reference Skansén-Saphir, Lindfors and Andersson15). Thus, a comprehensive analysis of the gene expression of human milk cells can serve as an effective method for analysing immunoactive agents in breast milk. In the present study, we carried out a comprehensive analysis of gene expression in human milk cells to characterise the effect of FOS administration in pregnant and lactating women on the production of immunoactive agents in breast milk. We also evaluated the concentrations of candidate immune-related proteins in breast milk.
Subjects and methods
Subjects and study protocol
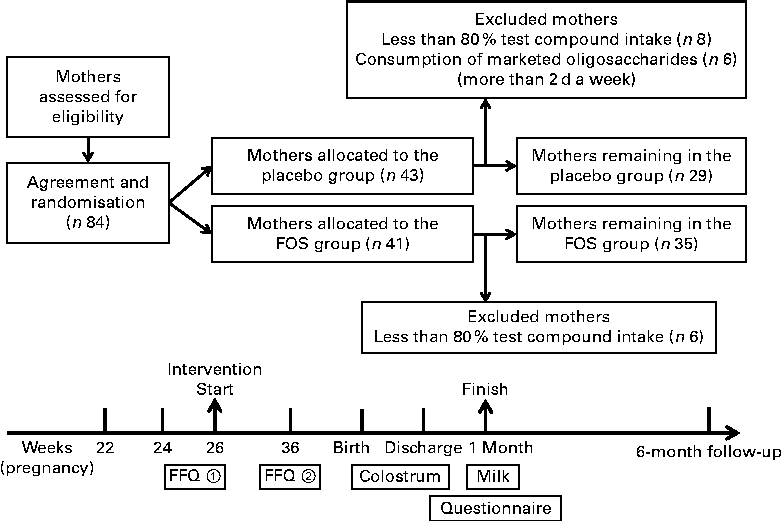
The present randomised, double-blind trial was registered in the UMIN Clinical Trial Registry (UMIN 000008142) (http://www.umin.ac.jp/). This trial was conducted at Masuda Maternity Clinic in Chiba prefecture, where healthy pregnant woman were enrolled with written informed consent. Pregnant women with complications such as pregnancy-induced hypertension were excluded. However, subjects with a history of allergic disease were not excluded. The study was conducted according to the guidelines of the Declaration of Helsinki, and all procedures were approved by the Ethics Committee of Chiba University. Subjects (eighty-four individuals) were randomly assigned to the FOS (Meioligo-P®, obtained from Meiji Food Materia Company Limited) (FOS, n 41) or sucrose (placebo, n 43) intake groups. Trial compound (4 g) was administered after breakfast and supper daily from 26 weeks of gestation to 1 month after delivery (Fig. 1).

Fig. 1 Experimental design. Trial compound (4 g) was administered twice daily from 26 weeks of gestation to 1 month after delivery. FOS, fructo-oligosaccharides.
Questionnaire evaluations and follow-up
The subjects were given diaries to record each dose of trial compound taken during the intervention period to characterise eating habits. A FFQ (Microsoft Excel Eiyou-Kun, Kenpakusha) was also given upon the initiation of enrolment (26 weeks of gestation) and 10 weeks later (36 weeks of gestation) (Fig. 1). Study compound compliance, consumption of foods similar to the study compound, parturition style and dietary habits were evaluated based on the diaries and FFQ results and medical records.
Selection of subjects
Due to ethical reasons, there was no control over the daily consumption of oligosaccharides by the study subjects. Instead, based on their diaries and FFQ results, from the placebo group, we excluded subjects who consumed marketed oligosaccharide products for more than 2 d a week. In addition, from both the groups, subjects with less than 80 % test compound intake were excluded due to insufficient intervention (Fig. 1).
Breast milk sampling
Colostrum samples were collected during hospitalisation after childbirth and breast milk samples were acquired 1 month after childbirth. The samples were collected in sterile plastic tubes and processed within 24 h. The breast milk samples were separated into whey and cellular elements by centrifugation (2500 rpm for 5 min) at room temperature. To remove fat from the whey, centrifugation was carried out at 10 000 rpm for 10 min at 4°C. The whey was used to measure the concentrations of immune-related proteins. Total RNA was extracted from the cellular fraction using the RNeasy Mini Kit (QIAGEN); samples with low RNA yields were concentrated using the RNeasy Micro Kit (QIAGEN). The quantity and quality of RNA were determined using BioSpec-nano (Shimadzu-Biotech) and the Agilent 2100 BioAnalyzer (Agilent Technologies).
Maternal blood sampling
Maternal blood samples were collected during hospitalisation after childbirth. The samples were collected in sterile plastic tubes and allowed to clot for serum analysis. Serum was separated from the samples by centrifugation (2500 rpm for 10 min) at 4°C and used to measure the concentrations of IL-27.
Comprehensive microarray analysis
Cy3-labelled complementary RNA was synthesised from 100 ng of total RNA using the Low Input Quick Amp Labeling Kit, One-Color (Agilent Technologies). After checking the quality and quantity of complementary RNA using BioSpec-nano (Shimadzu-Biotech) and the Agilent BioAnalyzer (Agilent Technologies), the complementary RNA was hybridised at 65°C for 17 h to Agilent Whole Human Genome Microarrays 4 × 44K (Design ID: 014850; Agilent Technologies). After washing, the microarrays were scanned using an Agilent DNA microarray scanner (Agilent Technologies). All procedures from labelling to scanning were carried out according to the manufacturer's protocols. The intensity value of each scanned feature was quantified using the Agilent Feature Extraction Software (Agilent Technologies), which performs background subtractions. Normalisation was carried out using Agilent GeneSpring GX version 11.0.2 (per-chip normalisation: 75 percentile shift; per-gene normalisation: none; Agilent Technologies). Data were analysed using the statistical language R (CRAN, R version 2.7.2; http://www.r-porject.org/). Gene ontology and pathway analyses were carried out using the Database for Annotation, Visualization, and Integrated Discovery (http://david.abcc.ncifcrf.gov/), National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/). High-quality RNA, such as those with optical density (OD)260/280 >1·8, were subjected to microarray analysis.
ELISA for the measurement of IL-27, soluble CD14, IgA and transforming growth factor-β1 concentrations
The Human IL-27 DuoSet ELISA kit (R&D Systems) was used to detect IL-27 in whey and serum samples. The lower limit of IL-27 detection is 156·25 pg/ml. The concentrations of IL-27 were measured according to the manufacturer's protocol in duplicate assays. The Human CD14 DuoSet ELISA kit (R&D Systems) and Human TGF-β1 DuoSet ELISA kit (R&D Systems) were also used to detect soluble CD14 and TGF-β1 in whey, respectively. The concentrations of IgA in whey were measured using an imunoturbidimetric assay.
Statistical analysis
The experimental data were not normally distributed and therefore were evaluated by the Mann–Whitney U-test or Pearson's χ2 test using the software Dr. SPSS II 11.0 (SPSS Japan, Inc.). Differences were considered significant at P <0·05.
Results
Analysed subjects
After excluding subjects with insufficient test compound intake, the number of subjects included in the analysis was reduced. However, we found no differences between the placebo (n 29) and FOS (n 35) groups, suggesting that the comparison of these groups was appropriate. Comparison of dietary habits demonstrated no significant difference between the groups (Table 1). There was no difference in the frequency of yogurt intake between the two groups as most mothers reported eating yogurt (data not shown). There were also no significant differences among the characteristics of the mothers (Table 2). Although there were more mothers with a history of other allergic diseases in the FOS group than in the control group, this did not reach statistical significance. Subjects in both the groups consumed 8 g/d of the test compound; there were no side effects or subject withdrawals from the study (data not shown).
Table 1 Comparison of dietary habits between the groups
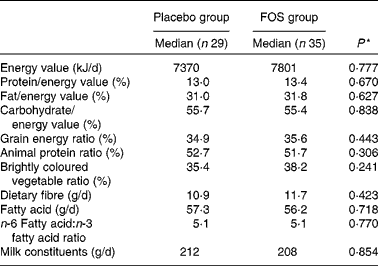
FOS, fructo-oligosaccharides.
* Mann–Whitney U-test.
Table 2 Comparison of the characteristics of subjects between the groups (Median values and number of participants)
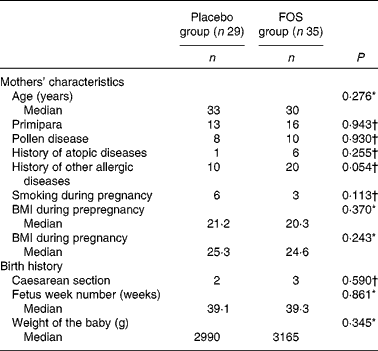
FOS, fructo-oligosaccharides.
* Mann–Whitney U-test.
† Pearson's χ2 test.
Comprehensive microarray analysis of human milk cells
Microarray of colostrum-derived samples
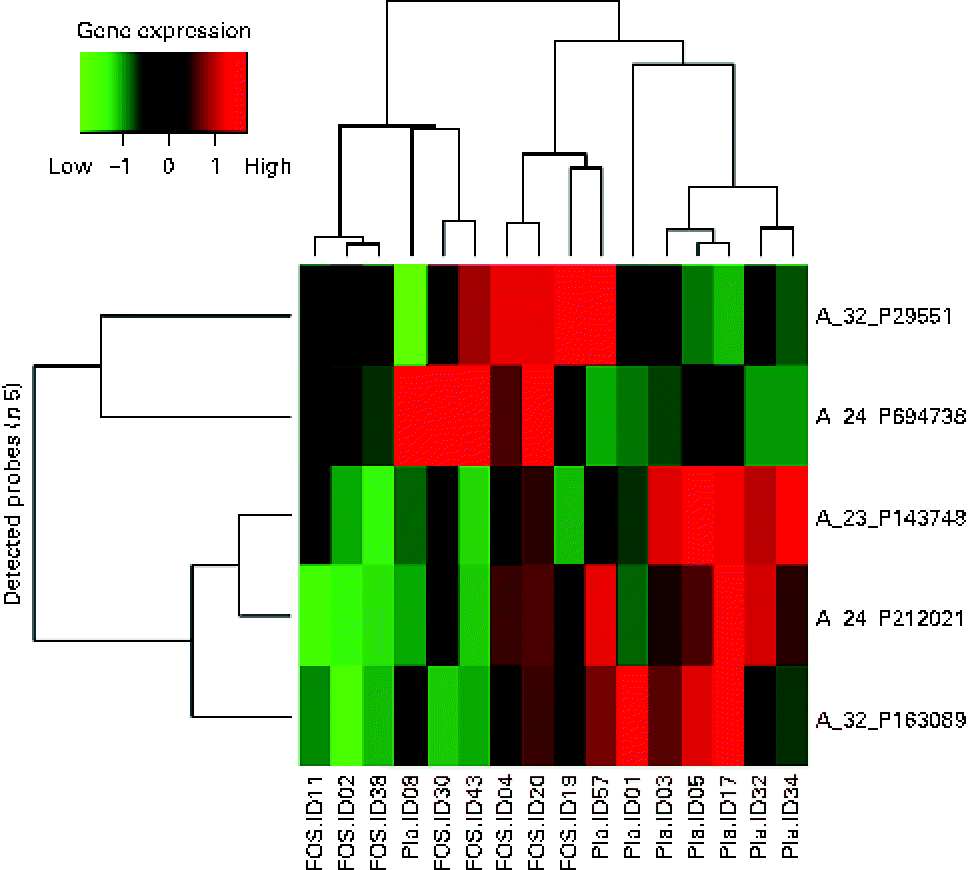
Randomly selected high-quality cellular RNA extracted from eight colostrum samples from each group were subjected to a microarray analysis and then compared. There were no significant differences in the characteristics of mothers from whom samples were collected (data not shown). Of the 41 000 probes on the microarray chip, the total number of probes extracted from the sixteen samples was 23 008. Comparison of the placebo and FOS groups yielded statistically significant differences, in which five probes exhibited a ≥ 3-fold change when a mean signal value of 1 was observed in the placebo group (P <0·05) (Table 3).
Table 3 List of genes that exhibited a significant difference in the microarray analysis of colostrum cells (Median values and ranges)
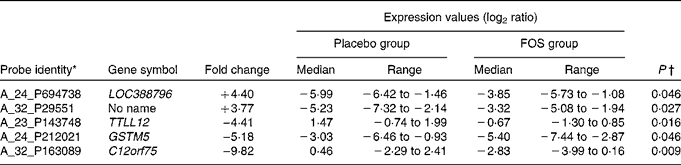
FOS, fructo-oligosaccharides; TTLL12, tubulin tyrosine ligase-like family member 12; GSTM5, glutathione S-transferase mu 5; C12orf75, chromosome 12 open reading frame 75.
* Genes in colostrum cells with ≥ 3-fold difference in expression between the FOS and placebo groups.
† Mann–Whitney U-test.
A heat map was created for the five probes that exhibited a significant difference in the microarray analysis. A hierarchical cluster analysis was carried out to classify the type of gene expression in each sample into groups: six samples on the left side and ten samples on the right side of the vertical axis, showing the clustering of the placebo and FOS groups. However, two samples from the placebo group were located on the right side, suggesting that the expression of these genes was close to that in the FOS group (Fig. 2).

Fig. 2 Heat map of colostrum samples. The heat map was based on five probes with significantly altered expression in the fructo-oligosaccharides (FOS) group. Pla, placebo.
The highest, most differentially expressed gene in the FOS group was LOC388796 (+4·40-fold; complementary DNA DKFZp686B0328 from clone DKFZp686B0328). The lowest, most differentially expressed gene was C12orf75 (–9·82-fold; chromosome 12 open reading frame 75). Glutathione S-transferase mu 5 (GSTM5) was also highly differentially expressed, with a F c value of − 5·18 (Table 3).
Microarray of 1-month breast milk-derived samples
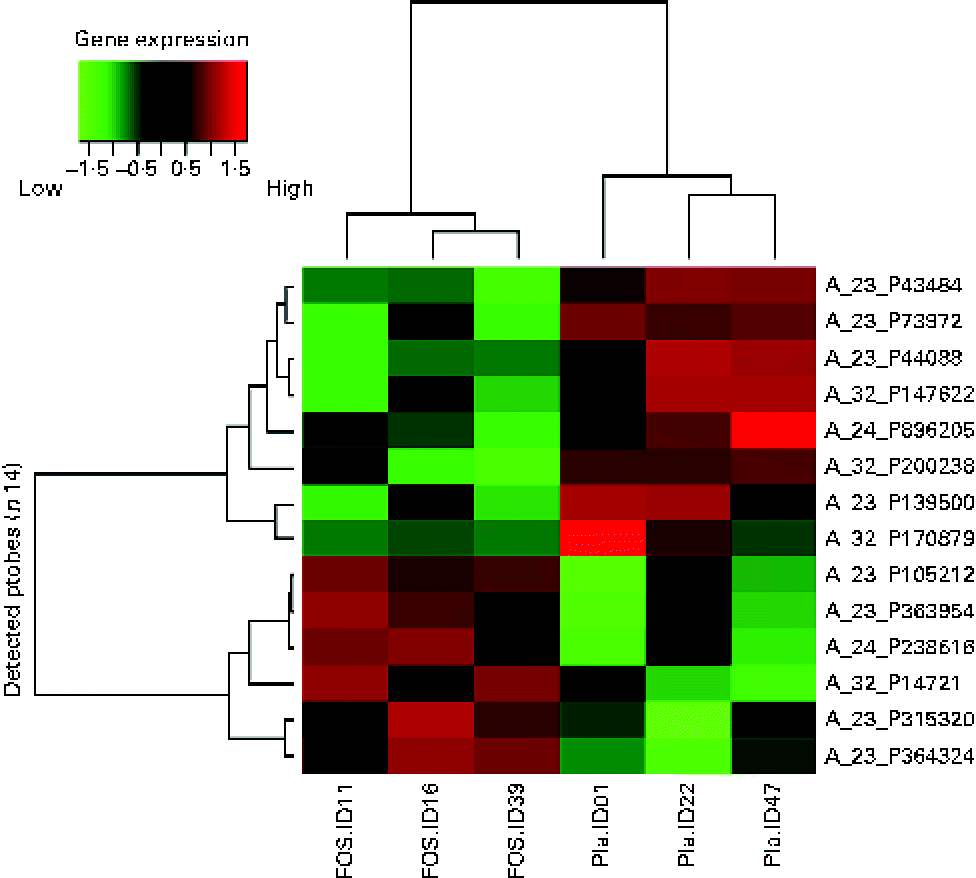
Randomly selected high-quality cellular RNA extracted from three breast milk samples from each group were subjected to a microarray analysis and then compared. There was no significant difference in the characteristics of the collected samples (data not shown). As shown in the subject identity in Figs. 2 and 3, the colostrum and 1-month breast milk samples were not collected from the same subjects. Similar to the microarray analysis of colostrum samples, the number of probes extracted from these six samples was 19 354. Significant differences were found, with fourteen probes exhibiting a difference of more than 3-fold (P <0·05); these probes were organised in a heat map. A hierarchical cluster analysis was used to classify gene expression in each sample into groups: three samples on the left side and three samples on the right side of the vertical axis, showing a clustering of the placebo and FOS groups (Fig. 3).

Fig. 3 Heat map of 1-month milk samples. The heat map was based on fourteen probes with significantly altered expression in the fructo-oligosaccharides (FOS) group. Pla, placebo.
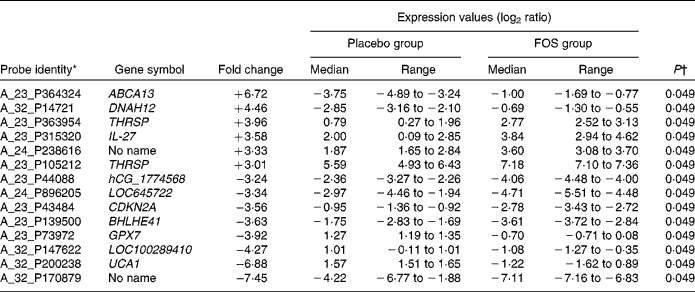
Among the genes with high signal values in the FOS group, ABCA13 (ATP-binding cassette, subfamily A (ABC1), member 13) was the most differentially expressed (+6·72-fold), followed by DNAH12 (+4·46-fold; dynein, axonemal, heavy chain 12, transcript variant 1), THRSP (+3·96-fold; thyroid hormone responsive (SPOT14 homologue, rat)), IL-27 (+3·58-fold) and THRSP (+3·01-fold). Genes in the FOS group with low signal values included UCA1 (–6·88-fold; urothelial cancer associated 1), LOC100289410 (–4·27-fold; similar to hCG1774568), GPX7 (–3·92-fold; glutathione peroxidase 7), BHLHE41 (–3·63-fold; basic helix-loop-helix family, member e41), CDKN2A (–3·56-fold; cyclin-dependent kinase inhibitor 2A), LOC645722 (–3·34-fold; similar to hCG2037003) and hCG_1774568 (–3·24-fold; complementary DNA clone IMAGE:2959727) (Table 4).
Table 4 List of genes that exhibited a significant difference in the microarray analysis of 1-month milk cells

FOS, fructo-oligosaccharides; ABCA13, ATP-binding cassette, subfamily A (ABC1), member 13; DNAH12, dynein, axonemal, heavy chain 12; THRSP, thyroid hormone responsive (SPOT14 homologue, rat); CDKN2A, cyclin-dependent kinase inhibitor 2A; BHLHE41, basic helix-loop-helix family, member e41; GPX7, glutathione peroxidase 7; UCA1, urothelial cancer associated 1.
* Genes in cells in milk 1 month after delivery with ≥ 3-fold difference in expression between the FOS and placebo groups.
† Mann–Whitney U-test.
Concentrations of IL-27, soluble CD14, IgA and transforming growth factor-β1 in whey
Based on the microarray analysis results, samples obtained from the FOS group with high gene expression levels were scanned for cytokine IL-27, and then the protein concentrations of IL-27 in colostrum and breast milk samples were measured. The concentrations of IL-27 in colostrum samples were significantly higher in the FOS group (n 35; range: < 0·156–46·6 ng/ml; median: 2·4 ng/ml) than in the placebo group (n 29; range: < 0·156–95·8 ng/ml; median: 0·2 ng/ml) (P =0·027, Fig. 4(a)). Comparison of 1-month breast milk samples revealed that the FOS group had significantly higher concentrations of IL-27 (n 35; range: < 0·156–32·6 ng/ml; median: 1·7 ng/ml) than the placebo group (n 29; range: < 0·156–23·4 ng/ml; median: 0·2 ng/ml) (P =0·040, Fig. 4(b)). Conversely, there was no significant difference between the FOS and placebo groups with regard to the concentrations of soluble CD14 (median: 54·7 v. 56·8 μg/ml; range: 21·3–91·1 v. 29·8–126·2 μg/ml; P =0·36), IgA (median: 1·07 v. 0·76 mg/ml; range: 0·03–0·33 v. 0·03–0·45 mg/ml; P =0·91) and TGF-β1 (median: 680 v. 753 pg/ml; range: 204–1700 v. 270–2777 pg/ml; P =0·14) in colostrum samples. These results refer to the same sixty-four subjects.
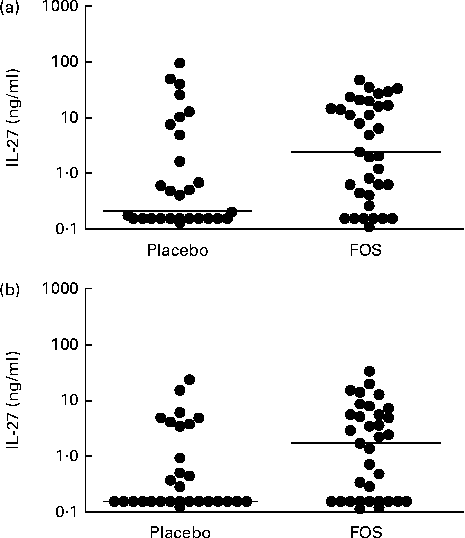
Fig. 4 Comparison of the concentrations of IL-27 in (a) colostrum samples and (b) 1-month milk samples obtained from the placebo and fructo-oligosaccharide (FOS) groups. The Mann–Whitney U-test was used to analyse differences between the experimental groups (bar, median) ((a) P= 0·027; (b) P= 0·040).
Serum concentrations of IL-27
The serum concentrations of IL-27 tended to be higher in the FOS group than in the placebo group (median: 5·18 v. 1·69 ng/ml; range: 0·73–40·19 v. 0·33–65·74 ng/ml), but this difference was not statistically significant (P =0·084).
Discussion
In the present study, it has been demonstrated for the first time that the consumption of FOS by pregnant and lactating women increases the expression of various molecules in cells present in breast milk (five in colostrum samples and fourteen in 1-month breast milk samples). The function of most of these genes has not been well elucidated, except that of the genes related to oxidative stress (GSTM5 and GPX7). Thus, differentially expressed genes were not directly associated with immune response; however, enhanced expression of the immunoregulatory cytokine IL-27 was observed at the transcript and protein levels.
IL-27 is produced by activated dendritic cells and macrophages(Reference Jankowski, Kopiński and Goc16). The present study did not analyse the human milk cell phenotype, but because many macrophages are found in breast milk(Reference Xanthou17), it is thought that macrophages are the main IL-27-producing cells in human milk.
The measurement of IL-27 concentrations in colostrum and maternal blood samples after delivery revealed no correlation (data not shown), suggesting that IL-27 in breast milk does not derive from serum, but is mainly produced by macrophages in breast milk. The protein concentrations of IL-27 were high in colostrum and 1-month breast milk samples obtained from the FOS group, whereas the microarray analysis of human milk cells revealed a significant difference in IL-27 expression only in 1-month breast milk samples. The reason for this discrepancy is unclear; however, one possible reason is that the colostrum and mature milk samples used for the microarray analysis were different, as only high-quality RNA was subjected to this analysis. Moreover, there is a high chance of inter-individual variations in the macrophage population of colostrum. The composition of colostrum varies widely between individuals, and there have been reports of large differences in cell type and proportion(Reference Xanthou17–Reference Hawkes, Bryan and Gibson19). In contrast, 1-month breast milk production is more stable and subject to less inter-individual variation.
IL-27, together with IL-12, IL-23 and IL-35, belongs to the family of cytokines associated with T-cell differentiation(Reference Yoshida, Nakaya and Miyazaki20, Reference Stumhofer and Hunter21). IL-27 induces the differentiation of T helper (Th)1 cells, regulates the induction of Th17 cells, increases the production of IL-10(Reference Stumhofer, Silver and Laurence22, Reference Fitzgerald, Zhang and El-Behi23), and induces type 1 regulatory T cells (Tr1)(Reference Awasthi, Carrier and Peron24, Reference Pot, Apetoh and Awasthi25). In the absence of IL-27, the Th2 response is increased, and because the Th17 and Th2 immune responses are controlled by the administration of IL-27, a strong involvement in allergy and inflammation control is likely. In the immune system of the digestive tract, IL-27 is responsible for maintaining epithelial barrier function and is involved in anti-inflammatory and antibacterial activities(Reference Diegelmann, Olszak and Göke26). Enteritis has been shown to resolve in experimental mice given parenteral IL-27(Reference Sasaoka, Ito and Yamashita27). Whether the oral administration of IL-27 is resistant to digestion is not well understood; therefore, it is important to analyse the biological activity of breast milk IL-27 in the infant digestive tract.
Some previous reports have indicated that the concentrations of soluble CD14, IgA, and TGF-β are increased in breast milk(Reference Dunstan, Roper and Mitoulas6, Reference Prescott, Wickens and Westcott7). In contrast to the demonstrated effects of probiotics, no significant difference in colostrum TGF-β, total IgA and soluble CD14 concentrations between the FOS and control groups was observed in the present study. These data suggest that prebiotics may act differently when compared with probiotics.
In conclusion, the present study demonstrated the effect of the consumption of FOS by pregnant and lactating women on gene expression in cells present in breast milk, particularly the enhanced expression of the immunoregulatory cytokine IL-27. Furthermore, the increased concentration of IL-27 in whey suggests a significant biological effect. Future studies should investigate the correlation between IL-27 concentration in breast milk and allergic disorders in infants and the effect of the consumption of prebiotics by pregnant and lactating women on the prevention of allergic disorders.
Acknowledgements
The authors are extremely grateful to the pregnant women for their participation. They also thank Aoki for her technical assistance.
The present study was partly supported by a grant of Program for Fostering Regional Innovation from the MEXT (Ministry of Education, Culture, Sports, Science and Technology). MEXT had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions were as follows: N. S., K. N., M. Y., O. O., Y. N., S. I., K. M., S. S. and Y. K. designed the study; T. K., Y. I., T. N., Y. M., Y. I., T. A., K. C. and K. M. conducted the experiments; T. K., K. N., Y. N. and N. S. analysed the data and wrote the paper.
None of the authors has any conflicts of interest.