Skeletal muscles, which account for approximately 40 % of total body mass, are the largest tissue in the body and play a crucial role in locomotion and metabolism. Both skeletal muscle mass and function are regulated in response to changes in nutrition, physical activity, environment and pathological conditions. Maintenance of muscle mass and function is an important public health issue, as low muscle quality is associated with clinical problems including poor glycaemic control, limited mobility and even mortality( Reference Newman, Kupelian and Visser 1 , Reference Park, Goodpaster and Strotmeyer 2 ).
Advanced glycation end products (AGE), which are produced by a non-enzymatic reaction between glucose and proteins, are closely involved in the pathogenesis of diabetes( Reference Vlassara and Uribarri 3 , Reference Bohlender, Franke and Stein 4 ), Alzheimer disease( Reference Takeuchi and Yamagishi 5 ), cancer( Reference van Heijst, Niessen and Hoekman 6 ) and hypertension( Reference McNulty, Mahmud and Feely 7 ). Moreover, elevated AGE in blood or skin are related to low muscle mass, grip strength and walking speed in elderly and diabetic patients( Reference Dalal, Ferrucci and Sun 8 – Reference Kato, Kubo and Sugioka 11 ). Thus, a negative relationship between AGE and skeletal muscle function has been suggested.
AGE are endogenously formed but are also absorbed from exogenous sources such as foods, especially when prepared at elevated temperatures( Reference O’Brien and Morrissey 12 ). As dietary AGE are poorly absorbed (approximately 10 %)( Reference Koschinsky, He and Mitsuhashi 13 ), their potential action in human health has gone largely unnoticed. However, recent systematic reviews of randomised-controlled trials have shown that exposure to AGE-enriched diets may be responsible for the development of chronic diseases such as diabetes, CVD and chronic kidney disease through increased inflammation and oxidative stress( Reference Clarke, Dordevic and Tan 14 ), and that dietary restriction of AGE alleviates insulin resistance, oxidative stress and endothelial dysfunction( Reference Kellow and Savige 15 ). In addition, an AGE-enriched diet induced insulin resistance in mouse skeletal muscle( Reference Cassese, Esposito and Fiory 16 ). Thus, we hypothesised that dietary AGE might impair skeletal muscle function. However, whether dietary AGE affect physiological characteristics of skeletal muscle is not yet fully understood. Therefore, our aim was to evaluate the effect of long-term exposure to an AGE-enriched diet on skeletal muscle mass and contractile functions.
Myogenesis plays an important role in postnatal growth and skeletal muscle development. Sequential activation and expression of paired box 7 (Pax7) and myogenic regulatory factors including myogenic factor 5 (Myf5), myogenic differentiation (MyoD) and myogenin are essential for the initiation and progression of myogenesis( Reference Bentzinger, Wang and Rudnicki 17 ). An in vitro study revealed that AGE treatment for 48 h suppressed myogenin expression in C2C12 myotubes( Reference Chiu, Yang and Sheu 18 ). Further, an in vivo study showed that long-term intake of a high-fructose diet decreased MyoD expression in mouse skeletal muscle, which was attenuated by supplementation with pyridoxamine, an AGE formation inhibitor( Reference Mastrocola, Nigro and Chiazza 19 ). The receptor for AGE also regulates muscle differentiation through Pax7 and myogenin( Reference Riuzzi, Sorci and Sagheddu 20 ). These studies suggest that AGE impact the expressions of myogenic factors and inhibit myogenesis.
Skeletal muscle growth occurs when the rate of protein synthesis exceeds that of degradation. A central signalling pathway regulating protein synthesis is the Akt/mammalian target of rapamycin (mTOR)/70-kDa ribosomal protein S6 kinase (p70S6K) signalling pathway( Reference Miyazaki and Esser 21 ). On the other hand, protein degradation is regulated by two major systems: autophagy and ubiquitin–proteasome systems( Reference Masiero, Agatea and Mammucari 22 , Reference Bonaldo and Sandri 23 ). Previously, AGE treatment was reported to induce autophagy by suppressing the Akt/mTOR pathway in neonatal cardiomyocytes( Reference Hou, Hu and Xu 24 ). In addition, a recent study demonstrated that AGE treatment decreased the phosphorylation status of Akt at Ser473, which is an indicator of Akt activity, and increased atrogin-1, which is a key enzyme in the ubiquitin–proteasome system, in C2C12 myotubes( Reference Chiu, Yang and Sheu 18 ). These findings suggest that AGE inhibit protein synthesis as well as promote protein degradation in skeletal muscle. Therefore, our secondary aim was to evaluate the influence of dietary AGE on the regulatory systems of myogenesis and protein turnover in skeletal muscle.
We examined the differences in muscle mass, contractile properties and molecular responses between mice that received a diet high in AGE (H-AGE) v. one low in AGE (L-AGE) for 16 weeks. We found that N ε -(carboxymethyl)-l-lysine (CML), a well-characterised compound that serves as a marker for AGE( Reference Ikeda, Higashi and Sano 25 ), accumulated in fast-type extensor digitorum longus (EDL) muscle, and EDL muscle weight was lower in H-AGE mice compared with L-AGE mice. The contractile properties were also poorer in H-AGE mice. Furthermore, mRNA expressions of myogenic factors and phosphorylation status of mTOR/p70S6K signalling pathway were lower in EDL muscles of H-AGE mice compared with L-AGE mice.
Methods
Animals and treatment
Male ICR mice (5 weeks old) were purchased from Shimizu Breeding Laboratories (Kyoto, Japan). Mice were placed in a room maintained at 22–24°C with a 12 h light–12 h dark cycle and fed a standard diet (AIN-93G; Oriental Yeast) and water ad libitum. After 1 week of adjustment, mice were randomised to receive a diet either low in AGE (AIN-93G unbaked, n 10) or high in AGE (AIN-93G baked for 1 h at 160°C, n 10) consistent with previous studies( Reference Tan, Sourris and Harcourt 26 – Reference Forbes, Cowan and Andrikopoulos 28 ). The H-AGE diet contained five times as much AGE compared with the L-AGE diet without changes in nutrient composition( Reference Forbes, Cowan and Andrikopoulos 28 ). Both groups were followed-up for 16 weeks. Body weight and food intake were measured every week and every 2 weeks, respectively. At the end of the study period, animals were fasted for 4 h and anaesthetised intraperitoneally with mixtures of medetomidine hydrochloride (0·3 mg/kg), midazolam (4·0 mg/kg) and butorphanol (5·0 mg/kg). Under anaesthesia, soleus (SOL), EDL and plantaris (PLA) muscles, tibial, epididymal fat pads and blood samples were collected. All animal protocols were carried out in accordance with the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health (Bethesda, MD, USA) and were approved by the Kyoto University Graduate School of Human and Environmental Studies.
Grip strength test
The grip strength test was performed 5 d before the end of the 16-week study. Grip strength was measured using a force transducer (DS2-50N; IMADA) according to the published protocol (DMD_M.2.2.001). In brief, mice were allowed to rest on a horizontal mesh with their forelimbs and hindlimbs (four paws) and were then gently pulled back until their grip was broken. Three consecutive measurements were obtained within 1 min, and were averaged to determine mean grip strength. Data were normalised to body weight and expressed as N/g.
Wire-hanging test
To evaluate muscle fatigue resistance, the wire-hanging test was carried out 4 d before the end of the 16-week study consistent with the published protocol( Reference Raymackers, Debaix and Colson-Van Schoor 29 ). In brief, mice were suspended by their forelimbs from a metallic wire (2-mm thick, 50-cm long) suspended 40 cm above soft ground. As soon as the mice were suspended, the timer was started. The timer was stopped when the animal fell and re-started after it was placed again on the wire. The number of falls from the wire was recorded, and the test was stopped after ten falls or 180 s of suspension. Data were expressed as the average fall score: each animal was given a score of 10 at the beginning of the test, which was reduced by 1 after each fall. The average fall score was calculated at a given time during the test as (10n−x)/n, where n is the number of animals and x the cumulative number of falls. The longest holding time was also recorded, and the holding impulse was calculated as s×g, where s is the longest holding time and g the body weight.
In vitro muscle contraction
In vitro muscle contraction was analysed as described previously( Reference Toyoda, Tanaka and Ebihara 30 , Reference Tsuda, Egawa and Kitani 31 ). Isolated PLA muscles were mounted on an incubation apparatus with tension set to 0·5 g and rested in Krebs–Ringer bicarbonate buffer (117-mm NaCl, 4·7-mm KCl, 2·5-mm CaCl2, 1·2-mm KH2PO4, 1·2-mm MgSO4, 24·6-mm NaHCO3) containing 2-mm pyruvate for 30 min. All buffers were continuously bubbled with 95 % O2–5 % CO2 and maintained at 37°C. For measuring force production, muscles were stimulated using an electric stimulator (SEN-3401; Nihon Kohden) at frequencies of 5, 10, 25, 50, 75 and 100 Hz (train duration: 1 s; pulse duration: 0·1 ms; voltage: 10 V) with a 2-min rest period between contractions( Reference Head, Greenaway and Chan 32 ). Subsequently, for measuring muscle fatigability, muscles were stimulated at 100 Hz, 1-s on 1-s off, for 30 s. Force was monitored using a force transducer (TRN001; Kent Scientific) and recorded (U-228-2P-500; Pantos). Force production data were normalised to muscle weight and expressed as N/g.
Real-time RT-PCR analyses
Real-time RT-PCR analyses were performed as previously described( Reference Yasuhara, Ohno and Kojima 33 ). In brief, total muscle RNA was extracted using a miRNeasy Mini kit (Qiagen) according to the manufacturer’s protocol. RNA was reverse-transcribed to complementary DNA (cDNA) using PrimeScript RT Master Mix (Takara Bio); synthesised cDNA was then subjected to real-time RT-PCR (StepOne, Applied Biosystems) using Takara SYBR Premix Ex Taq II (Takara Bio). The relative fold change of expression was calculated by the comparative CT method using StepOne software version 2.3. To normalise the amount of total RNA present in each reaction, glyceraldehyde 3-phosphate dehydrogenase was used as an internal standard. The primer sequences are shown in Table 1.
Table 1 List of primer sequences (forward, reverse) used for real-time RT-PCR analyses
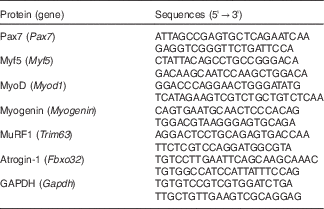
Pax7, paired box 7; Myf5, myogenic factor 5; MyoD, myogenic differentiation; MuRF1, muscle RING finger 1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Measurement of myosin heavy chain isoform composition
Analysis of myosin heavy chain (MHC) isoform composition (I, IIa, IIx and IIb) was carried out using a modification of previously described methods( Reference Talmadge and Roy 34 ). In brief, muscles were homogenised in ice-cold lysis buffer (1:40, w/v) containing 20-mm Tris·HCl (pH 7·4), 1 % Triton X, 50-mm NaCl, 250-mm sucrose, 50-mm NaF, 5-mm sodium pyrophosphate, 2-mm dithiothreitol, 4-mg/l leupeptin, 50-mg/l trypsin inhibitor, 0·1-mm benzamidine and 0·5-mm phenylmethylsulphonyl fluoride (buffer A) and centrifuged at 16 000 g for 40 min at 4°C. Extracted proteins were solubilised in Laemmli sample buffer and boiled for 5 min. The sample proteins (5 µg) were separated on SDS-polyacrylamide (7 %) gel at 120 V for 19 h in a temperature-controlled chamber at 4°C. After electrophoresis, the gels were stained with Oriole™ Fluorescent Gel Stain (Bio-Rad Laboratories). The gels were visualised using ImageCapture G3 (Liponics) and analysed using ImageJ software (National Institutes of Health). As we could not clearly distinguish between the IIa and the IIx MHC bands, this phenotype was expressed as IIa/x.
Western blot analyses
Western blot analysis was performed as described previously( Reference Ohno, Yamada and Sugiura 35 , Reference Egawa, Tsuda and Ma 36 ). Extracted samples in buffer A were solubilised in Laemmli’s sample buffer and boiled for 5 min. The samples (10 µg of protein) were separated by SDS-PAGE using 10 % polyacrylamide gel at a constant current of 40 mA/gel for 90 min. After SDS-PAGE, the proteins were transferred to polyvinylidene fluoride membranes (Hybond-P; GE Healthcare) by using trans-blot cell (Bio-Rad) at a constant voltage of 100 V for 1 h at 4°C. After the transfer, the membranes were blocked for 1 h using fish gelatin (Takara Bio). Next, the membranes were incubated overnight at 4°C with primary antibodies: Akt (9272; Cell Signaling Technology), Akt Ser473 (9271; Cell Signaling Technology), p70S6K (2708; Cell Signaling Technology), p70S6K Thr389 (9206; Cell Signaling Technology) and microtubule-associated protein light-chain 3 (LC3) (2775; Cell Signaling Technology). The membranes were washed with Tris-buffered saline with 0·1 % Tween 20 (TBS-T, pH 7·5) and then allowed to react with anti-rabbit IgG (Cell Signaling Technology) for 1 h at room temperature. After the final wash with TBS-T, protein bands were visualised using chemiluminescence (Wako). Signal density was measured using Light-Capture (AE-6971; ATTO Corporation).
Measurement of N ε -(carboxymethyl)-l-lysine content
The muscle sample was homogenised in buffer A, and the supernatant was collected after centrifugation at 16 000 g for 40 min at 4°C. Blood samples were collected from the carotid artery into tubes containing heparin as anticoagulant, and plasma was separated by centrifugation at 8000 g for 15 min. The content of CML in muscles and plasma was measured using a CircuLex CML/N ε -(carboxymethyl) lysine ELISA Kit (Medical & Biological Laboratories) according to the manufacturer’s protocol. Protein concentration of the supernatant of muscles and plasma was determined using the Bradford technique, and the CML content in samples was expressed as µg/mg protein.
Statistical analyses
Values are expressed as means with their standard errors. Statistical significance was analysed using parametric Student’s t test or non-parametric Mann–Whitney U test (Tables 2–4 and Fig. 1) and two-way ANOVA with diet and frequencies (Fig. 2) or diet and muscles (Fig. 3–5) as main factors. In the event of significant main effects and/or interactions, post hoc Tukey–Kramer tests were performed. Differences between groups were considered statistically significant at P<0·05. The sample size was calculated by power analysis at 0·8 and a level of significance of 0·05 with a hypothesised effect size of 1·2 based on the means and variances of muscle mass data in our previous study( Reference Egawa, Goto and Ohno 37 ), which estimated the sample size to be 10. All statistical analyses were performed using Ekuseru-Toukei 2012 software (Social Survey Research Information).

Fig. 1 The grip strength test and wire-hanging test in mice fed a diet low in advanced glycation end products (L-AGE) or a diet high in AGE (H-AGE). (a) Forelimb and hindlimb (four paws) grip strength. (b) Average fall score during the wire-hanging test. (c) Longest holding time and holding impulse during the wire-hanging test. The grip strength test and wire-hanging test were carried out 5 and 4 d before the end of the 16-week study, respectively. Values are means (n 10 per group), with their standard errors. *P<0·05 v. L-AGE mice. ![]() , L-AGE;
, L-AGE; ![]() , H-AGE.
, H-AGE.

Fig. 2
In vitro force production and fatigability of plantaris muscles in L-AGE or H-AGE mice. (a) Force generation in response to electrical stimulation. (b) Percentage of force drop during fifteen contraction cycles. Isolated plantaris muscle was allowed to rest for 30 min, and the muscle was tetanically contracted at frequencies of 0, 5, 10, 25, 50, 75 and 100 Hz with a 2-min rest between contractions. A fatigue run was then carried out by stimulating the muscle at 100 Hz, 1-s on 1-s off, for 30 s. Values are means (n 10 per group), with their standard errors. *P<0·05 v. L-AGE mice. L-AGE, low in advanced glycation end products; H-AGE, high in AGE; ![]() , L-AGE;
, L-AGE; ![]() , H-AGE.
, H-AGE.

Fig. 3 Relative expressions of mRNA specific for myogenesis in L-AGE or H-AGE mice. Soleus (SOL) and extensor digitorum longus (EDL) muscles were dissected, and expressions of paired box 7 (Pax7), myogenic factor 5 (Myf5), myogenic differentiation (MyoD) and myogenin were measured using real-time RT-PCR analyses. Values are means (n 10 per group), with their standard errors. *P<0·05 v. L-AGE mice. †Significant main effect of diet (L-AGE v. H-AGE mice). L-AGE, low in advanced glycation end products; H-AGE, high in AGE; ![]() , L-AGE;
, L-AGE; ![]() , H-AGE.
, H-AGE.

Fig. 4 Relative expressions of protein specific for protein synthesis pathway in L-AGE or H-AGE mice. Soleus (SOL) and extensor digitorum longus (EDL) muscles were dissected, and expressions of Akt and phosphorylated Akt Ser473 (p-Akt), 70-kDa ribosomal protein S6 kinase (p70S6K) and phosphorylated p70S6K Thr389 (p-p70S6K) were measured using western blot analyses. Representative immunoblots are also shown. Values are means (n 10 per group), with their standard errors. †Significant main effect of diet (L-AGE v. H-AGE mice). L-AGE, low in advanced glycation end products; H-AGE, high in AGE; ![]() , L-AGE;
, L-AGE; ![]() , H-AGE.
, H-AGE.
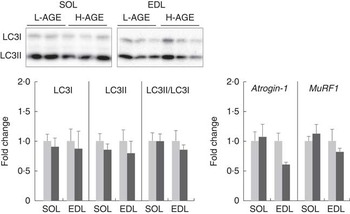
Fig. 5 Relative expressions of protein and mRNA specific for protein degradation pathway in L-AGE or H-AGE mice. Soleus (SOL) and extensor digitorum longus (EDL) muscles were dissected, and protein expressions of microtubule-associated protein light-chain 3 (LC3)-I and II were measured using western blot analyses, and mRNA expressions of atrogin-1 and muscle RING finger 1 (MuRF1) were measured using real-time RT-PCR analyses. Representative immunoblots are also shown. Values are means (n 10 per group), with their standard errors. L-AGE, low in advanced glycation end products; H-AGE, high in AGE; ![]() , L-AGE;
, L-AGE; ![]() , H-AGE.
, H-AGE.
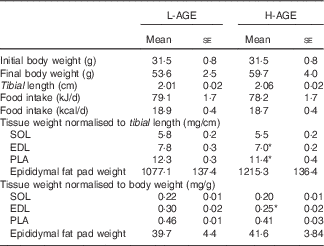
Table 2 Characteristics of low in advanced glycation end products (L-AGE)- and high in AGE (H-AGE)-fed mice (Mean values with their standard errors; n 10 per group)
SOL, soleus; EDL, extensor digitorum longus; PLA, plantaris.
*P<0·05 v. L-AGE.
Table 3 N ε -(carboxymethyl)-l-lysine content (Mean values with their standard errors; n 8–10 per group)

L-AGE, low in advanced glycation end products; H-AGE, high in advanced glycation end products; SOL, soleus; EDL, extensor digitorum longus; PLA, plantaris.
*P<0·05 v. L-AGE.
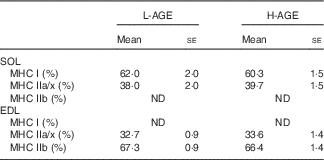
Table 4 Myosin heavy chain (MHC) isoform composition (Mean values with their standard errors; n 10 per group)
L-AGE, low in advanced glycation end products; H-AGE, high in advanced glycation end products; SOL, soleus; EDL, extensor digitorum longus.
Results
Dietary advanced glycation end products suppressed skeletal muscle growth
The characteristics of mice exposed to L-AGE and H-AGE are shown in Table 2. There were no significant differences between L-AGE and H-AGE mice in body weight, tibial length, food intake and epididymal fat pad weight. EDL muscle weight was lower in H-AGE mice compared with L-AGE mice when normalised to body weight and tibial length. PLA muscle weight was lower in H-AGE mice when normalised to tibial length and tended to be lower when normalised to body weight (P=0·096). There were no significant differences in SOL muscle weight between L-AGE and H-AGE mice.
Dietary advanced glycation end products increased N ε -(carboxymethyl)-l-lysine accumulation in skeletal muscle
To evaluate the impact of the H-AGE diet for 16 weeks, we examined accumulation of CML in skeletal muscles and plasma. H-AGE mice showed an increase in CML content in EDL muscle (Table 3). The content in SOL muscle and plasma tended to increase, but this was not significantly different.
Dietary advanced glycation end products deteriorated muscle performance in vivo
To examine the effect of the H-AGE diet on muscle performance, we assessed muscle strength and fatigue resistance in mice. The grip strength test was used to measure muscle strength. Grip strength in all four limbs in H-AGE mice was weaker than in L-AGE mice (Fig. 1(a)). To measure fatigue resistance, we carried out the wire-hanging test. The average fall score during the wire-hanging test was 3·7 at the end of the experiment in L-AGE mice, whereas the score fell faster in H-AGE mice and reached 1·4 (Fig. 1(b)). The longest holding time during the wire-hanging test was shorter by 30 % in H-AGE mice compared with L-AGE mice; holding impulse was significantly lower in H-AGE mice than in L-AGE mice (Fig. 1(c)).
Dietary advanced glycation end products deteriorated muscle force production in vitro
We next measured force production in skeletal muscle in vitro to determine the effect of H-AGE on muscle contractile property. PLA muscle of H-AGE mice was found to produce significantly lower force at 75 and 100 Hz compared with L-AGE mice (Fig. 2(a)). There were no significant differences in the patterns of force decline between L-AGE and H-AGE mice (Fig. 2(b)).
Dietary advanced glycation end products did not affect myosin heavy chain isoform composition in skeletal muscle
As muscle fibre type composition is a determinant of muscle strength and fatigue resistance, we evaluated the effect of H-AGE on MHC isoform composition in skeletal muscles. No significant differences were observed in MHC isoform composition in either SOL or EDL muscles between L-AGE and H-AGE mice (Table 4)
Dietary advanced glycation end products decreased mRNA expressions of myogenic factors in skeletal muscle
To determine the effect of H-AGE on regulatory factors related to skeletal muscle growth, we evaluated the expression levels of mRNA involved in myogenesis. Myf5 mRNA expression level was significantly decreased in EDL muscle of H-AGE mice (Fig. 3). In addition, MyoD mRNA expression levels were lower in H-AGE mice compared with L-AGE mice. Pax7 and myogenin mRNA expression levels did not differ significantly based on an H-AGE diet.
Dietary advanced glycation end products suppressed protein synthesis signalling in skeletal muscle
To examine the effect of H-AGE on protein synthesis, we evaluated the phosphorylation status of Akt/mTOR/p70S6K signalling pathway. The phosphorylation status of Akt at Ser473 was not affected by the H-AGE diet, but the expression of phosphorylated p70S6K Thr389, an indicator of mTOR activity, was lower in H-AGE mice compared with L-AGE mice (Fig. 4).
Dietary advanced glycation end products had no effect on protein degradation pathways in skeletal muscle
To analyse the effect of H-AGE on protein degradation, we evaluated the expression levels of molecules involved in autophagy and ubiquitin–proteasome systems. The relative expression levels of LC3II/LC3I, an indicator of autophagy activity, were not affected by the H-AGE diet (Fig. 5). There were also no differences between L-AGE and H-AGE mice with regard to mRNA expressions of atrogin-1 and muscle RING finger 1 (MuRF1), another key enzyme of the ubiquitin–proteasome system (Fig. 5).
Discussion
The results of the present study revealed several novel findings about the effects of dietary AGE on skeletal muscle functions. First, mice fed an H-AGE diet for 16 weeks exhibited smaller muscle mass than L-AGE mice, which was accompanied by CML accumulation in skeletal muscle. Second, exposure to H-AGE induced skeletal muscle dysfunction, including muscle strength, fatigue resistance and in vitro muscle force production, without changes in MHC isoform composition. Third, mRNA expressions of myogenic factors, Myf5 and MyoD, in skeletal muscle were lower in H-AGE mice compared with L-AGE mice. Fourth, phosphorylation status of p70S6K Thr389 was lower in H-AGE mice than in L-AGE mice.
Recently, an epidemiological study has suggested a relationship between AGE accumulation and skeletal muscle mass in humans( Reference Kato, Kubo and Sugioka 11 ). In addition, an experimental study showed that AGE treatment in cultured muscle cells suppressed MyoD and promoted atrophy( Reference Chiu, Yang and Sheu 18 ). That study also revealed that skeletal muscle atrophy in diabetic mice was attenuated by treatment with alagebrium chloride, a breaker of AGE-based cross-links( Reference Chiu, Yang and Sheu 18 ). These observations indicate that AGE are potent factors that influence muscle mass. In the present study, H-AGE mice showed smaller muscle masses of EDL and PLA compared with L-AGE mice (Table 2), which was accompanied by elevated CML content (Table 3). This is the first report of long-term exposure to an AGE-enriched diet promoting AGE accumulation in skeletal muscles and impaired skeletal muscle growth.
AGE are also related to muscle dysfunction. It has been reported that elevated blood CML is associated with poor grip strength( Reference Dalal, Ferrucci and Sun 8 ) and slow walking speed( Reference Semba, Bandinelli and Sun 9 ). Furthermore, men with higher skin AGE levels had lower muscle strength and power( Reference Momma, Niu and Kobayashi 10 ). In the present study, we found that grip strength and wire-holding performance were poorer in H-AGE mice compared with L-AGE mice (Fig. 1). In addition, we showed that in vitro muscle force production normalised to muscle weight was lower in H-AGE mice than in L-AGE mice (Fig. 2(a)). These findings indicate that dietary AGE affect muscle contractile properties in addition to muscle mass.
In this regard, some reports have shown that myofibrillar proteins are subject to glycation modification. Glycation of actomyosin increased with ageing in mice and rats( Reference Watanabe, Ogasawara and Suzuki 38 ). Glycation of myosin altered filament formation( Reference Katayama, Haga and Saeki 39 ), structure and ATPase activity( Reference Syrovy and Hodny 40 – Reference Ramamurthy, Hook and Jones 42 ). AGE-modified actin is also accumulated with ageing( Reference Snow, Fugere and Thompson 43 ). Therefore, the modification of muscle contractile proteins such as myosin and actin is a possible mechanism inducing the loss of muscle contractile properties.
Muscle strength is also affected by muscle fibre type composition( Reference Pette and Staron 44 ). The fibre types based on MHC isoforms essentially reflect muscle contractile properties( Reference Harridge, Bottinelli and Canepari 45 ). In the present study, MHC isoform composition did not differ between L-AGE and H-AGE mice (Table 4), indicating that dietary AGE-induced muscle dysfunction is not attributed to the alteration of muscle fibre type composition.
In the present study, dietary AGE affected muscle mass more in fast-type muscles, EDL and PLA, than in slow-type muscles, SOL (Table 2). This indicates that the susceptibility to AGE might be dependent on fibre type. Indeed, CML accumulated in EDL muscle but not in SOL muscle during the 16-week treatment period (Table 3). In addition, previous reports have shown that AGE accumulation in diabetic rat skeletal muscle was greater in fast-type fibre( Reference Snow and Thompson 46 ). These findings suggest that dietary AGE promote AGE accumulation preferentially in fast-type fibres of whole-body muscle, and thereby induce decreased muscle performance. However, some reports have shown that the presence of AGE did not differ between fibre types in diabetic( Reference Snow, Lynner and Nielsen 47 ) or ageing rats( Reference Ramamurthy and Larsson 48 ). Thus, additional studies are required to elucidate any dependency on fibre type for AGE influence.
Postnatal skeletal muscle growth is regulated by the sequential expressions of myogenic factors such as Pax7, Myf5, MyoD and myogenin. Pax7 is the specific marker for quiescent and activated muscle satellite cells, which are the resident skeletal muscle stem cells( Reference Seale, Sabourin and Girgis-Gabardo 49 , Reference Hawke and Garry 50 ). Myf5 is expressed during embryonic development and integrates various developmental signalling pathways to initiate myogenesis( Reference Francetic and Li 51 ). MyoD is considered a master control gene for MyoD( Reference Perry and Rudnick 52 ); myogenin participates in later stages of myogenesis and is directly associated with the differentiation process that leads to myotube formation and maturation( Reference Andres and Walsh 53 , Reference Mak, To and Kong 54 ). In the present study, Myf5 mRNA expression level in EDL muscle was lower in H-AGE mice than in L-AGE mice (Fig. 3). Furthermore, MyoD mRNA expression level was suppressed in H-AGE mice compared with L-AGE mice. These findings suggest that dietary AGE inhibit muscle formation during a relatively early stage of myogenesis, resulting in muscle growth deterioration.
MyoD is also a crucial modulator of muscle fibre type. MyoD binds to an enhancer box (E-box) element in the MHC promoter and induces its transcription( Reference Wheeler, Snyder and Patterson 55 ). In particular, MyoD expression is high in fast-type muscle and is involved in the induction of fast-type fibre phenotype( Reference Hughes, Koishi and Rudnicki 56 ). Over-expression of MyoD has been shown to up-regulate the expression of fast-type MHC( Reference Ekmark, Rana and Stewart 57 ). Conversely, MyoD-knock-out mice showed a shift towards slower fibre phenotype in fast-type muscles( Reference Hughes, Koishi and Rudnicki 56 , Reference Macharia, Otto and Valasek 58 ). These observations raise the possibility that the down-regulation of MyoD mRNA expression in H-AGE mice induced fibre-type transition towards a slower muscle phenotype. However, dietary AGE did not affect MHC isoform composition in the present study (Table 4). In this respect, some studies have reported inconsistent shifts in MHC with MyoD expressions( Reference Caiozzo, Wu and Baker 59 , Reference Vissing, Andersen and Harridge 60 ). Thus, the alteration of MyoD expression may not always be linked to the shift in MHC isoform distributions.
In addition to myogenesis, protein synthesis and subsequent accumulation of protein are necessary for skeletal muscle growth. A previous study indicated that AGE induced myotube atrophy via inhibition of the Akt pathway( Reference Chiu, Yang and Sheu 18 ). In the present study, the phosphorylation status of p70S6K Thr389 was lower in EDL muscle of H-AGE mice compared with L-AGE mice, although no change was observed in Akt Ser473 phosphorylation (Fig. 4). These results suggest that dietary AGE affect the mTOR/p70S6K signalling pathway independently of Akt in skeletal muscle. On the other hand, AGE may possibly modulate protein degradation as noted by previous studies, which have shown that AGE treatment in cultured skeletal and cardiac muscles activated autophagy and ubiquitin–proteasome pathways( Reference Chiu, Yang and Sheu 18 , Reference Hou, Hu and Xu 24 ). However, no influences on the ratio of LC3II:LC3I and mRNA expressions of atrogin-1 and MuRF1 were observed (Fig. 5), which suggests that dietary AGE do not affect protein degradation. Therefore, it is suggested that dietary AGE deteriorate skeletal muscle growth by inhibiting protein synthesis.
AGE are generally found in several foods prepared at high temperatures( Reference O’Brien and Morrissey 12 ). Therefore, animal models exposed to heat-treated foods are often used to evaluate the influence of AGE in vivo ( Reference Cassese, Esposito and Fiory 16 , Reference Tan, Sourris and Harcourt 26 – Reference Forbes, Cowan and Andrikopoulos 28 , Reference Coughlan, Yap and Tong 61 – Reference Patel, Baker and Liu 65 ). However, a limitation in using these models is that the heat applied may produce unexpected products in diets. From this perspective, our study cannot exclude the possibility that products other than AGE may affect muscle dysfunction. Therefore, further investigations using inhibitors of AGE accumulation should be conducted to understand the direct links between AGE and muscle functions.
In conclusion, in the present study, we showed that exposure to an AGE-enriched diet for 16 weeks promoted CML accumulation in skeletal muscle. Furthermore, we observed impairment in skeletal muscle functions including skeletal muscle mass, muscle strength and fatigue resistance in H-AGE mice compared with L-AGE mice. AGE-enriched diet-associated skeletal muscle dysfunction might be due to a failure of postnatal myogenesis and protein synthesis. The present study shows a possibility that long-term exposure to an AGE-enriched diet impairs postnatal growth and skeletal muscle development.
Acknowledgements
We thank all the members of the Laboratory of Sports and Exercise Medicine, Kyoto University and the Laboratory of Physiology, Toyohashi SOZO University for their help. We also thank Kyoto University Research Center for Low Temperature and Materials Sciences for instrumental support.
This study was supported in part by JSPS KAKENHI grant numbers 26560371 (T. E.), 16K12942 (Y. O.), 16K16450 (S. Y.), 16K13022 (K. G.), 26350818 (K. G.) and 15K01711 (T. H.); JSPS Fellows (A. G., 14J00286); the Ministry of Agriculture, Forestry and Fisheries; Integration Research for Agriculture and Interdisciplinary Fields (funding agency: Bio-oriented Technology Research Advancement Institution, NARO) (T. H., 14532022); the Council for Science, Technology and Innovation; and SIP (funding agency: NARO) (T. H., 14533567). Additional research grants were provided by the Nakatomi Foundation (T. E.); the All Japan Coffee Association (T. E. and K. G.); the Vascular Disease Research Foundation (T. H.); the Naito Foundation (K. G.); the Descente Sports Foundation (K. G.); and the Graduate School of Health Sciences, Toyohashi Sozo University (K. G.).
T. E., K. G. and T. H. were involved in the conception and design of the study; T. E., S. T. and A. G. performed experiments; T. E., A. G. and S. Y. analysed the data; T. E., Y. O., S. Y., K. G. and T. H. interpreted the results of experiments; T. E. prepared the figures; T. E., S. Y. and K. G. drafted the manuscript; T. E., A. G., Y. O, K. G. and T. H. edited and revised the manuscript; T. E., S. T., A. G., Y. O., S. Y., K. G. and T. H. approved the final version of the manuscript.
The authors have no conflicts of interest to declare.












