Pancreatic cancer is the eighth most common cause of cancer death worldwide, and 227 000 individuals were diagnosed with pancreatic cancer in 2008( Reference Ferlay, Shin and Bray 1 ). In the Nordic countries, pancreatic cancer contributes to 2·4 % of the cancer cases in men and 2·7 % of the cancer cases in women( Reference Center, Jemal and Ward 2 ). Pancreatic cancer is hard to detect early( Reference Parkin 3 ), and the cancer is generally advanced, and usually fatal, by the time it is diagnosed( Reference Yeo and Lowenfels 4 ). The 5-year prevalence of people living with pancreatic cancer worldwide is estimated to be 4·1 per 100 000( Reference Parkin 3 ). There is limited evidence suggesting consumption of red and processed meat, foods and beverages containing fructose and heavy alcohol drinking (more than 3 drinks/d) as risk factors of pancreatic cancer. The evidence for fruits and physical activity is too limited and inconsistent to draw any conclusion( 5 ). Other non-dietary risk factors for pancreatic cancer are more established. Approximately 25 % of cases of pancreatic cancer are attributable to tobacco smoking( 5 ), and the risk also increases with age. Factors such as body fatness and greater childhood growth have been identified as, respectively, convincing and probable causes of pancreatic cancer( 5 ). Chronic pancreatitis, insulin resistance, type 2 diabetes( Reference Yeo and Lowenfels 4 , 5 ) and infection with Helicobacter pylori have also been identified as risk factors of this cancer( Reference Yeo and Lowenfels 4 ). In addition, pancreatic cancer seems to run in some families, and some of these are caused by an inherited syndrome( 6 ). About 10 % of the pancreatic cancer cases are owing to genetic changes, indicating that most people who are diagnosed with pancreatic cancer do not have a family history of it. Potatoes are the fourth most plentiful food crop in the world after wheat, rice and maize( Reference Zaheer and Akhtar 7 ), and are a good source of fibre, carbohydrates (starches), niacin, folate, vitamin C and minerals (e.g. K, Mg and Fe)( Reference Totland, Melnes and Lundberg-Hallèn 8 , Reference McGill, Kurilich and Davignon 9 ). According to World Cancer Research Fund/American Institute of Cancer Research report (WCRF/AICR), the evidence for associations between folate, vitamin C, carbohydrates and pancreatic cancer risk is weak or inconclusive( 5 ). Generally, the research on health effects of potato consumption is limited and contradictory, especially regarding the long-term health effects of potato in diets worldwide( Reference Hagen 10 , Reference Camire, Kubow and Donnelly 11 ). Yet, some studies have found a high potato consumption to be associated with higher risk of pancreatic cancer( Reference Polesel, Talamini and Negri 12 ), colon cancer (tendency)( Reference Steinmetz and Potter 13 ) and colorectal cancer( Reference Åsli, Olsen and Braaten 14 ). Other studies have found associations between high potato consumption and risk of gastric cancer among women but not among men( Reference De Stefani, Correa and Boffetta 15 ), and risk of rectal cancer among Whites but not among African-Americans( Reference Williams, Satia and Adair 16 ). An association between potato consumption and risk of oral and pharyngeal cancer has also been observed( Reference Bravi, Bosetti and Filomeno 17 ). On the other hand, some short-term studies have implicated that potatoes contain anti-tumour agents( Reference Camire, Kubow and Donnelly 11 ). Moreover, a case–control study found that potato consumption had a protective effect on rectal cancer among women, but no associations were found for men and, in addition, no associations between potato consumption and risk of colon cancer was found( Reference Deneo-Pellegrini, Boffetta and De Stefani 18 ). A prospective study of intake of fibre from different sources and risk of colon cancer found that fibre from potatoes was inversely related to colon cancer among men; however, for women the intake of potato fibre was associated with higher risk( Reference Hansen, Skeie and Landberg 19 ). In addition, two case–control studies found potato consumption to be associated with decreased risk of bladder cancer( Reference Balbi, Larrinaga and De Stefani 20 , Reference Isa, Xie and Hu 21 ). Potatoes have a high glycaemic index (GI) and glycaemic load (GL)( Reference Ludwig 22 – Reference Wirfält, McTaggart and Pala 24 ). Even though two earlier meta-analyses concluded that there was no association between GI/GL and pancreatic cancer( Reference Aune, Chan and Vieira 25 , Reference Choi, Giovannucci and Lee 26 ), recent studies have found the opposite( Reference Hu, La Vecchia and Augustin 27 , Reference Turati, Galeone and Gandini 28 ). Potatoes are usually eaten as part of a meal( Reference McGill, Kurilich and Davignon 9 ); therefore, the effect potatoes have on disease risk alone is difficult to determine. A typical Western dietary pattern with high consumption of red and processed meat, potato chips, high fat, dairy products and eggs has been associated with a higher risk of pancreatic cancer among men( Reference Bosetti, Bravi and Turati 29 , Reference Chan, Gong and Holly 30 ), although there are other studies in which this association has not been found( Reference Michaud, Skinner and Wu 31 ). Preparation methods of potatoes vary highly across the world, and this is relevant to consider when studying potato consumption and risk of disease. Boiling is assumed to be the healthiest way of preparing, as frying and roasting adds other components, such as fats and salt, to the meal. Acrylamide formation may also be a problem when potatoes are cooked at high temperatures( Reference Ludwig 22 , Reference van Bakel, Kaaks and Feskens 23 ). However, in the Scandinavian countries, boiling is the most common preparation method( 32 – 34 ). Information from 24-h dietary recalls performed as part of the European Prospective Investigation into Cancer and Nutrition (EPIC) study showed that boiling was preferred in 70–88 % (standard error of the estimates for Norway, Sweden and Denmark: 0·01) of the meals containing potatoes in the Danish, Swedish and Norwegian populations used for the current study( Reference Åsli, Olsen and Braaten 14 ). To our knowledge, no prospective study has investigated the association between potatoes as a single food, rather than potatoes being part of a dietary pattern, and the risk of pancreatic cancer. The aim of the present study was to investigate the associations between potato consumption and the risk of pancreatic cancer in a prospective cohort of women and men in the Scandinavian countries.
Methods
The HELGA study
The HELGA Study is a large population-based Scandinavian cohort consisting of 119 978 participants from The Norwegian Women and Cancer study( Reference Lund, Dumeaux and Braaten 35 ), the Northern Sweden Health and Disease Study Cohort( Reference Winkvist, Hörnell and Hallmans 36 ) and the Danish Diet, Cancer and Health Study( Reference Tjønneland, Olsen and Boll 37 ). The three cohorts are also part of the EPIC study( Reference Riboli, Hunt and Slimani 38 ). Data collection was carried out in 1998–1999 (Norway), 1992–1996 (Sweden) and in 1993–1997 (Denmark). In the Norwegian cohort, women aged 40–55 years, from all over Norway, were selected randomly and invited to participate. In the Swedish cohort, the participants consisted of men and women, aged 30, 40, 50 or 60 years, who all were invited to attend a general health screening within the northern county of Västerbotten. The Danish participants were inhabitants in the Copenhagen and Aarhus areas, aged 50–64 years, free of cancer and born in the country who were invited to participate in the Diet, Cancer, and Health study. The participants provided informed consent, and medical ethics review boards in each of the three countries approved the studies.
Dietary assessment
Semi-quantitative FFQ, filled in at baseline, were used for dietary assessment. The country-specific FFQ reflected the habitual diet during the previous year, and they have all been validated and described in detail elsewhere( Reference Hjartåker, Andersen and Lund 39 – Reference Tjønneland, Overvad and Haraldsdóttir 41 ). The Norwegian FFQ consisted of eighty-five frequency questions, the Danish consisted of 192 frequency questions and the Swedish consisted of eighty-four frequency questions. The questions on potato consumption varied between the three countries. In Norway, the participants were asked one general question on how many potatoes they ate (never/seldom, 1–4/week, 5–6/week, 1/d, 2/d, 3/d or 4+/d). No questions on preparation method were asked. In Denmark, the questionnaire contained seven different questions, where the participants chose which preparation method (boiled, baked, roasted, mashed, stewed, potato salad or French fries) they had used, and they were asked how many times during a specific period of time they ate potatoes prepared in these various ways: never, during a month (<1, 1, 2–3 times), per week (1, 2–4, 5–6 times) or per day (1, 2–3, 4–5, 6–7, 8+ times). For boiled and baked potatoes, the portion size was specified as one potato, and the participants were asked how many times during a specific period of time they ate one boiled or baked potato (e.g. 1 potato 5–6 times/week would be 5–6 potatoes/week). In Sweden, the questionnaire contained five questions where the participants chose which preparation method (boiled/baked, roasted, French fries, mashed or potato salad) they had used, and how many times during a specific period of time they ate potatoes prepared in various ways: never, sometimes during a year, per month (1–3 times), per week (1, 2–3, 4–6 times) or per day (1, 2–3, 4+). The amount of food consumed every time was assessed using pictures (a plate with meat, vegetables and potatoes) illustrating four different portion sizes the participants could choose between. In each country, frequencies of all preparation methods were combined with quantities (in g/d) and summed into a general variable on potato consumption. Both potatoes as a single food and potato as an ingredient in dishes (pots, soups and hash) were included. The dishes were broken down to the ingredients levels and the ingredients were then added to their respective food groups. As information on preparation methods was unavailable in the Norwegian cohort, and variables for specific preparation methods were not available in the standardised data set, we have focused on total potato consumption. On the basis of the high proportion of boiled potatoes consumed in Norway, Sweden and Denmark( Reference Åsli, Olsen and Braaten 14 , Reference Åsli, Braaten and Olsen 42 ), it is likely that the exposure variable in the present study mainly reflects intake of boiled potatoes. The questionnaires also contained questions on lifestyle and health. The weight and height of the Danish and Swedish participants were measured, whereas in Norway this information was self-reported.
Identification of pancreatic cancer cases
Over 95 % of pancreatic cancers are adenocarcinomas of the exocrine pancreas( 5 ). We included malignant, primary pancreatic cancer of the exocrine pancreas (carcinomas and adenocarcinomas) as defined by the International Classification of Diseases 10th revision as C25 (C25·0–C25·4 and C25·7–C25·9). Information on cancer incidence and vital status was obtained from national cancer registries, and cause of death registries.
Exclusions
We excluded 2597 participants with prevalent cancer. The preliminary number of pancreatic cancer cases was 268. We excluded 539 participants, including one case, owing to implausible reported daily energy intake (lower than 2500 kJ for both sexes, and higher than 18 000 kJ for women and 21 000 kJ for men) and another twenty-seven (no cases) owing to implausible potato intake (>1 kg/d). As we included only adenocarcinomas from the exocrine pancreas, forty-four pancreatic cancer cases were excluded because they were neuroendocrine pancreatic tumours, lymphoma, carcinoid, malignant cell and malignant tumour. One case was excluded owing to missing information on cancer morphology. Further, we excluded 2494 participants, including one case, owing to missing information on smoking. Finally, thirty-six participants with follow-up time registered as zero were excluded, as they did not contribute to follow-up. Hence, 114 240 participants (38 766 men and 75 474 women) (Norwegian cohort: 33 690, Swedish cohort: 24 305 and Danish cohort: 56 245) were included in the final analyses. Of these, 221 (121 men and 100 women) were diagnosed with pancreatic cancer during follow-up.
Statistical analysis
Participant characteristics are presented as medians and 5th–95th percentiles and frequency distributions as appropriate. We used Cox proportional hazard models with hazard ratios (HR) and 95 % CI to estimate the association between potato consumption and the risk of pancreatic cancer. Follow-up time was used as the time variable, and the participants were followed up from date of entry into the study until the date of cancer diagnosis, date of death, date of emigration or the end of follow-up (Denmark, 31 December 2007; Sweden and Norway, 31 December 2008). We present one model stratified by sex, and adjusted for age at recruitment, interaction between age and follow-up time and total energy (kJ), and one fully adjusted model, additionally adjusted for BMI, height and smoking (see below for more details). Owing to differences in the question formulation and general differences in procedures and measurements between the three cohorts, all analyses were stratified by country. Variables classified as ‘probably’ or ‘convincingly’ associated with risk of pancreatic cancer in the WCRF/AICR( 5 ) were tested as possible confounders or risk factors: BMI (weight in kg divided by height in m squared, <25 kg/m2: underweight/normal-weight, 25–29·9 kg/m2: overweight, ≥30 kg/m2: obese) and smoking status (never, former and current). We also made a finer categorisation of smoking, dichotomising former smokers into those who quit ≤10 years ago or ≥11 years ago, and current smokers into those who smoke 1–15 cigarettes/d or ≥16 cigarettes/d. However, as this categorisation did not have any material effect on the results, we used the more robust adjustment for smoking status in the final models. Greater childhood growth measured as adult attained height and BMI at aged approximately 20 years is also a probable risk factor, and hence we adjusted for height at baseline. All continuous variables (potato consumption, total energy and height) were tested for linear associations with the outcome. Potato consumption was divided into quartiles in order to fully cover the non-linearity in pancreatic cancer risk, whereas total energy intake was applied in tertiles. Additional variables associated with potato consumption in the Norwegian cohort( Reference Åsli, Braaten and Olsen 42 ) were assessed for confounding effects: red and processed meat, vegetables, education and diabetes (self-reported). In addition, we did separate analyses by sex. As the number of cases was relatively small, stratification by diabetes was not possible, but in addition to adjust for diabetes we performed sensitivity analyses excluding diabetics. Owing to the small number of cases, we did not perform separate analyses by country, but we repeated all analyses in the Danish cohort only, as this was the largest sub-cohort containing most of the cases. In addition, we performed analyses adjusting for preparation methods in the Danish cohort. We also tested a multivariable adjusted model additionally including dietary factors (red and processed meat, alcohol, carbonated/soft/isotonic drinks and diluted syrups, and fat) that are characteristic of an obesogenic environment and the metabolic syndrome( 43 , Reference Lake and Townshend 44 ) (results not shown). As these variables did not materially change the risk estimates, they were not included in the final models. Adjustment for GI was not done, as these estimates were not available from the food composition database, but we constructed a model (data not shown) where we adjusted for intake of pasta and rice to rule out associations with other main sources of dietary carbohydrate used at dinner meals. Variables were included in the final models if they were significantly associated with pancreatic cancer, or if they influenced the HR by more than 10 %. We also adjusted for total energy intake, as this adjustment is usually appropriate to control for confounding in studies on disease and diet. In addition, as our study consisted of three sub-cohorts in different countries, this adjustment attenuated possible differences in the information obtained from the FFQ. Tests for trend were performed for all regression analyses, and quartile medians of potato consumption were used in the tests. We performed sensitivity analyses that excluded participants with a pancreatic cancer diagnosis <1 year (n 12) and 3 years (n 34) after having completed the questionnaire, owing to the possibility that preclinical symptoms affected eating habits. Proportional hazard (PH) assumptions were checked using Schoenfeld residuals, which showed sign of deviation from proportionality. By including an interaction term between age at entry and follow-up time in the models, the PH assumption was fulfilled for all variables. A χ 2 test was performed to check for heterogeneity between sexes. A restricted cubic spline regression model with four knots at fixed percentiles (5, 35, 65 and 95, as suggested by Harrell( Reference Harrell 45 )) was used to further explore dose–response effects between potato consumption and risk of pancreatic cancer. All variables were checked for multi-collinearity using variance inflation factor, and the results showed no violation of this assumption. Data were analysed using STATA version 14. Calculations showed that the sample size and number of incident pancreatic cancer cases in the HELGA study were sufficiently large to detect a HR of 1·5 for the highest quartile of potato intake compared with the lowest, with a statistical power of 85 % and significance level of 5 %.
Results
The mean follow-up time was 11·4 (95 % CI 0·3, 16·9) years. Median age of participants in the quartiles of potato consumption was 52–53 years (Table 1). The cohort consisted of more women than men (66·1 % females) (data not shown), whereas slightly more cases were men (54·8 %). The Swedish sub-cohort had the longest follow-up (mean: 13·3 years), whereas the Danish sub-cohort was the largest (49 % of the participants), provided most of the cases (68·8 %) and had, on average, the oldest participants (results not shown in table).
Table 1 Demographic, lifestyle and dietary characteristics by potato consumption in the HELGA cohort(Medians and ranges and 5th–95th percentiles)
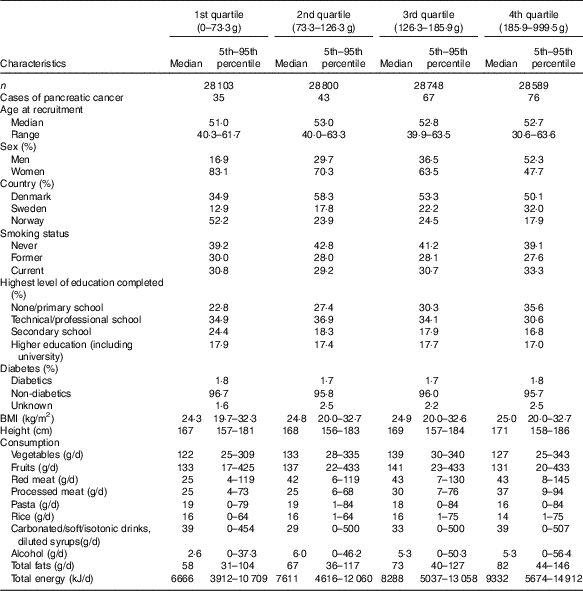
Participants in the highest quartiles of potato consumption tended to be lower educated, taller and to consume more processed meat, fat and total energy (Table 1). Consumption of red meat and alcohol were noticeably lowest in the lowest quartile, and consumption of carbonated/soft/isotonic drinks and diluted syrups was highest in the lowest and highest quartile. In addition, potato consumption was highest in the Swedish participants, and among men. Smoking showed only minor differences across the quartiles of potato consumption. Minor differences were also seen for intake of vegetables, fruits, pasta and rice. Owing to the small differences between the adjusted models (Table 2), we only present HR and CI from the adjusted model 2. Higher consumption of potatoes showed tendencies of higher risk of pancreatic cancer (HR 1·44; 95 % CI 0·93, 2·22, P for trend 0·03) when comparing the highest v. the lowest quartile of consumption in the multivariable model (Table 2). However, compared with the second quartile, both upper quartiles showed a significantly increased risk (data not shown). In the sex-specific analyses, higher consumption was associated with a higher risk of pancreatic cancer for females (HR 2·00; 95 % CI 1·07, 3·72, P for trend 0·02). No significant associations were found for men (HR 1·01; 95 % CI 0·56, 1·84). Results from the spline regression models confirmed the absence of dose–response effects between potato consumption and risk of pancreatic cancer (Fig. 1). In sensitivity analyses, excluding participants diagnosed with pancreatic cancer <1 (twelve cases) or 3 years (thirty-four cases) after completing the questionnaire did not influence the results. Analysis with a finer categorisation of smoking (including intensity and time since quitting) also did not alter the conclusions. Excluding participants with diabetes (twenty-two cases) did not influence the results. When we repeated all analyses in the Danish cohort (152 cases) only, the overall results were the same (data not shown). Repeating the sex-specific analysis or excluding participants with diabetes in the Danish cohort was not possible owing to the small number of cases.
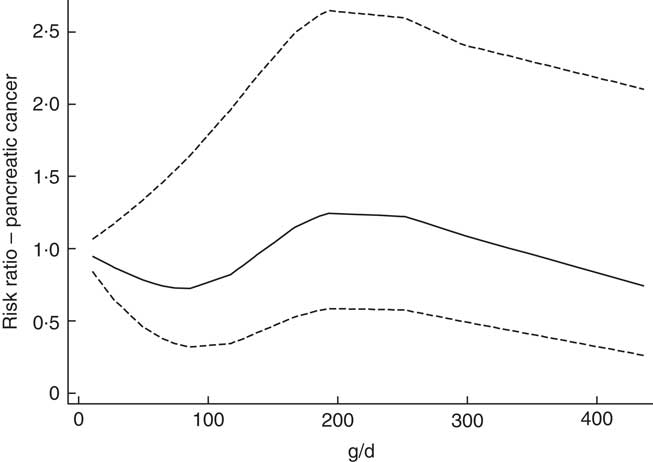
Fig. 1 Association between potato consumption (per 100 g/d) and pancreatic cancer risk. As estimated by a restricted cubic Spline regression model with four knots at fixed percentiles (5, 35, 65, 95, suggested by Harrell( Reference Harrell 45 )). Adjusted for sex, total energy (kJ), BMI, height, smoking status and stratified by country.
Table 2 RiskFootnote * for pancreatic cancer according to potato consumption in the HELGA study (n 114 240)(Hazard ratios and 95 % confidence intervals)
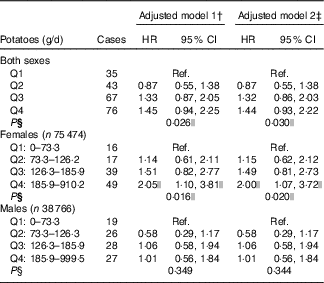
Q, quartile; Ref., referent values.
* As estimated by Cox proportional hazards regression.
† Stratified by country and sex, and adjusted for age at recruitment, interaction between age and time, and total energy (kJ).
‡ Additionally adjusted for BMI, height and smoking.
§ Test for trend, based on median potato consumption in each quartile.
|| Values are significant.
Discussion
In this study, we found that participants reporting higher consumption of potatoes showed tendencies at higher risk of pancreatic cancer. In the sex-specific analyses, we found that higher consumption of potatoes was associated with a higher risk of pancreatic cancer for women only. When we further explored the associations by spline regression, the absence of dose–response effects was confirmed. The strengths of this study include its prospective design, which minimises the influence of recall bias. In addition, the high consumption of particularly boiled potatoes in these countries gave us an opportunity to study a population with high potato consumption, where it is not likely that, for example, acrylamide or fat will influence the results. Nevertheless, potatoes are not eaten isolated, but as part of a meal. Further, owing to the use of cancer registries and population registries in the Scandinavian countries, the case coverage was excellent and loss to follow-up was minimal. Another strength of the study is that we, in addition to using standard Cox proportional hazard models, repeated all analyses with spline regression to further explore a possible dose–response effect between high potato consumption and pancreatic cancer risk. It has been discussed that there are other regression methods that could be better suited for analysis than the standard categorical analysis we used at first( Reference Greenland 46 ), and one of them is spline regression as standard categorical analysis does not make efficient use of within-category information. As the standard categorical analysis showed relatively inconsistent and non-linear results, with high potato consumption being associated with higher risk of pancreatic cancer in some subgroups, it was important to explore this further. As the results from the spline regression supported the results from the Cox regression, and there was no apparent biological evidence to support the results, we cannot, on the basis of our results, conclude that there is an association between potato consumption and pancreatic cancer. One limitation of the study was that even though the study had a large sample size, the number of cases was relatively small, as expected with a relatively rare cancer type. This restricted our possibility to perform subgroup and stratified analyses, as this affected the statistical power. We tried solving this by, for instance, excluding those with diabetes instead of performing separate analysis on diabetics. The results remained unchanged. Adjustments for diabetes did not have any material effect on the results either. We applied a finer categorisation for smoking status, which did not have any effect on the results. Nevertheless, the study will probably suffer from some residual confounding. Our results were based on data from self-administered questionnaires, which is a potential source of measurement error. However, the food data were standardised by common standardisation guidelines. The questions on potato consumption varied across the three countries, and the questionnaire in the Norwegian cohort did not have any questions on preparation method. Therefore, total potato consumption was summed up into a general variable in g/d. Ideally, questions on preparation method should have been included; however, information from 24-h dietary recalls performed as part of the EPIC study showed that boiling was the most common preparation method in meals containing potatoes in the Danish, Swedish and Norwegian populations included in the current study( Reference Åsli, Olsen and Braaten 14 ). Adjustment for GI was not done, as these estimates were not available from the food composition database. In addition, information on H. pylori, insulin resistance and chronic pancreatitis, which have been associated with pancreatic cancer, was not available. In addition, it is important to keep in mind that the results of the present study were based on only one measurement, and dietary changes could have occurred during the follow-up period. As potatoes are usually eaten as part of a meal, it is also important to keep in mind that the effect potatoes have on disease risk alone is difficult to determine. Foods that are part of a meal interact with other dietary components and nutrients, and thus the impact of potato consumption on disease risk may depend on which other foods they are grouped with in a dietary pattern( Reference McGill, Kurilich and Davignon 9 ). These issues are also present regarding GI, as the postprandial glycaemic response is influenced by several factors in a meal, such as the source and amount of carbohydrates and the type and amount of fibre present( Reference McGill, Kurilich and Davignon 9 ). In addition, the ingredients or other foods eaten together with potatoes will influence the GI and the postprandial glycaemic response. The variety of potatoes and the preparation method will also play a part( Reference McGill, Kurilich and Davignon 9 ). The research on pancreatic cancer and GI is inconsistent; two earlier meta-analyses concluded that there was no association( Reference Aune, Chan and Vieira 25 , Reference Choi, Giovannucci and Lee 26 ), whereas two recent studies found that diets with high GI and GL increased the risk of pancreatic cancer( Reference Hu, La Vecchia and Augustin 27 , Reference Turati, Galeone and Gandini 28 ). As mentioned, adjustment for GI was not possible in the present study, but we constructed a model (data not shown) where we adjusted for intake of pasta and rice to rule out associations with other main sources of dietary carbohydrate used at dinner meals, and the results were still the same. Adjusting for preparation methods (boiled, baked, fried, roasted, mashed, potato salad and stewed) in the Danish cohort did not change the original results (results not shown). Participants in the highest quartile of potato consumption showed some characteristics that are known to be associated with pancreatic cancer, like they tended to have a higher BMI. The participants in the highest quartile were also slightly taller. Regarding dietary factors, they consumed more processed meat, and had a generally higher intake of total fat and total daily energy intake. The evidence regarding these dietary factors in association with pancreatic cancer is limited( 5 ); however, these factors are characteristics of an obesogenic environment and the metabolic syndrome( 43 , Reference Lake and Townshend 44 ). The metabolic syndrome is a cluster of risk factors related to risk for stroke, heart disease, diabetes type 2, overweight, obesity and insulin resistance( 43 ), and the incidence of this syndrome increases with age( Reference Kuk and Ardern 47 ). However, multivariable adjusted models including these variables did not materially change the risk estimated, and they were not included in the final models. The main reason for not including them was the limited number of pancreatic cancer cases in our study sample, and we aimed to retain power in our statistical analyses. The metabolic syndrome has become more common owing to increasing obesity rates among adults( 43 ). In addition, several studies have found the metabolic syndrome to be associated with a higher risk of pancreatic cancer( Reference Esposito, Chiodini and Colao 48 , Reference Rosato, Tavani and Bosetti 49 ). The key component of the metabolic syndrome in pancreatic carcinogenesis was suggested to be diabetes( Reference Rosato, Tavani and Bosetti 49 ). As mentioned, exclusion of participants with diabetes (twenty-two cases) did not influence the results in the present study. Even though there is a lack of research regarding long-term cancer-related health effects of potatoes( Reference Camire, Kubow and Donnelly 11 ), there are several studies that have found an association between potato consumption and cancer: in particular, one case–control study found that a high potato consumption was associated with a higher risk of pancreatic cancer( Reference Polesel, Talamini and Negri 12 ). It is also interesting that some studies on dietary patterns, rather than studies on potatoes per se, show that a western dietary pattern that includes potatoes has been associated with a higher risk of pancreatic cancer among men( Reference Bosetti, Bravi and Turati 29 , Reference Chan, Gong and Holly 30 ). However, these findings are not consistent( Reference Michaud, Skinner and Wu 31 ). In addition, some studies have found potato consumption to be associated with a higher risk of other cancers of the digestive system, such as rectal cancer( Reference Williams, Satia and Adair 16 ), colon cancer (tendencies)( Reference Steinmetz and Potter 13 ) and colorectal cancer( Reference Åsli, Olsen and Braaten 14 ). Other studies have shown contradictory or inconclusive results regarding gastric, colon and rectal cancer( Reference De Stefani, Correa and Boffetta 15 , Reference Deneo-Pellegrini, Boffetta and De Stefani 18 , Reference Hansen, Skeie and Landberg 19 ).
Conclusion
In this study, we cannot conclude that there is an association between potato consumption and risk of pancreatic cancer. However, potatoes are a staple food in many countries, and there are generally few and inconsistent studies within this field. In addition, the inconsistent findings in the present study emphasise the need for more and larger studies investigating the association between potato consumption and pancreatic cancer, and potatoes and health in general.
Acknowledgements
The authors thank Katja Boll for assistance with data preparation. In addition, the authors thank pathologist Lill Tove Busund for assisting with cancer information.
This work was supported by the Norwegian ExtraFoundation for Health and Rehabilitation through EXTRA funds. The Norwegian ExtraFoundation for Health and Rehabilitation had no role in the design, analysis or writing of this article.
L. A. Å., G. S., A. O., E. L. and T. B. designed the research; L. A. Å. analysed data and wrote the paper; T. B. supervised the statistical analyses, performed supporting analyses, commented on the manuscript and has contributed in organising data in the Norwegian Women and Cancer Study; A. O. supervised and commented on the analyses and the manuscript and has contributed in organising data in the Danish Diet, Cancer and Health Study; E. L., Principal Investigator of NOWAC, and A. T., K. O., L. M. N. and F. R. contributed in designing the local cohort studies and commented on the manuscript; G. S. supervised the statistical analyses, commented and supervised the drafting of the manuscript and has contributed in organising data in the Norwegian Women and Cancer Study. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.





