During frying, fats and oils are heated to high temperatures while exposed to air and moisture, resulting in a complex series of reactions that generates a wide array of new compounds. Identification and quantification of the new compounds, however, continue to be investigated given the high number of compounds formed from each oxidisable substrate(Reference Choe and Min1–Reference Velasco, Marmesat, Dobarganes, Sahin and Sumnu3). There is general agreement that undesirable or potentially harmful compounds can result from frying, although their biological significance and the levels actually formed are far from clear(Reference Márquez-Ruiz, Dobarganes and Erickson4).
Among the different methods of cooking, i.e. frying, baking, boiling, grilling, etc., the greatest concern on the possible adverse nutritional effects has been expressed over intermittent or discontinuous frying because the highest degradation levels have been found under these conditions. Conversely, there is less opportunity for significant alteration either in commercial frying operations using continuous frying because of the high turnover with fresh oil and constant protection of the oil surface by steam water from the food, or in pan-frying, in which oils are heated only for short periods of time and rarely reused(Reference Márquez-Ruiz, Ruiz-Méndez, Velasco, Decker, Elias and McClements5). In other cooking methods, the oil is not reused and consequently its degradation is limited.
A survey of the literature shows discrepancies among nutritional studies on used frying oils and fats as experimental conditions vary widely. Besides, information on the heated oils tested is generally based exclusively on the duration of heating, temperature selected and oil used(Reference Márquez-Ruiz, Dobarganes and Erickson4). Therefore, insufficient analytical criteria are provided to establish valid relationships between the degradation compounds formed and the effects observed.
The first part of this review summarises the new compounds formed when heating oils at frying temperature with special attention to those with possible adverse effects while the second part concerns the most significant nutritional studies on used frying oils and oxidation compounds.
Formation and nature of new compounds during frying
During heating at high temperature, the fats and oils undergo the most important lipid reactions, i.e. hydrolysis, oxidation and thermal alteration, resulting in the loss of quality of the frying oil and thereby of the fried food. Hydrolysis occurs due to the moisture from the foodstuff, which involves the breakage of the ester bond, with subsequent release of fatty acids, monoacylglycerols and diacylglycerols. Because of the presence of air and exposure to high temperature, oxidation and thermal alterations take place in the unsaturated fatty acid, through chain reactions of free radicals, leading mainly to modified TAG with at least one of the three fatty acyl chains altered(Reference Dobarganes, Márquez-Ruiz and Erickson6). Table 1 lists the main groups of alteration compounds formed. Fig. 1 shows examples of simplified, representative structures of each group of compounds. All alteration compounds formed are more polar than their parent non-modified TAG, with the exception of cyclic and trans TAG, formed in minimal amounts. For this reason, the new compounds formed are known as polar compounds, and their total amount can be easily determined by adsorption chromatography(7).
Table 1 Main groups of new compounds formed during frying
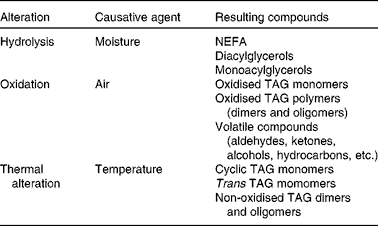

Fig. 1 Schematic representation of the main groups of alteration compounds formed during frying. A, B, C, D and E are simplified structures of hydroperoxy, hydroxy, epoxy, keto and short-chain n-oxo fatty acyl groups, respectively, in oxidised TAG.
Although general changes in the main oil constituents are known, it is not easy to foresee the rate of degradation due to the high number of variables involved in the frying process. Some of them are linked to the process itself, such as temperature, length of heating, continuous or discontinuous heating and turnover period, and others to the food subjected to frying, i.e. lipid composition, main and minor constituents, etc.; or else to the oil used such as unsaturation degree, initial quality, antioxidant content and additives. Consequently, regulations or guidelines have been established in many countries to guarantee high-quality foods. The most extended limitation establishes that the used frying oil has to be replaced when the total content of polar compounds is higher than 25 % expressed on oil weight(Reference Firestone and Erickson8).
Results obtained in many samples have shown that, at the level of used frying oil rejection for human consumption (25 % polar compounds), TAG polymers (the sum of TAG dimers and TAG oligomers) are by far the major compounds, accounting for 12–15 wt% on oil. As for oxidised TAG monomers, levels of 7–10 wt% on oil are normally found. Regarding hydrolytic compounds, variable contents are reported, but these compounds are less important quantitatively(Reference Dobarganes, Márquez-Ruiz and Erickson6, Reference Marmesat, Morales and Velasco9).
From the nutritional point of view, the compounds formed through hydrolysis of TAG are likewise originated in the stage previous to the fat absorption due to the action of pancreatic lipase and hence have no relevance in this context. Similarly, the volatile oxidation compounds have no nutritional interest because, at the high oil temperature of the frying process, they are released from the oil and are not significant in the fried food. In consequence, to approach the evaluation of the possible adverse effects of heated fats and oils, attention has to be paid to non-volatile compounds coming from oxidation and thermal alterations because these remain in the oil, are absorbed by the food and subsequently ingested. Such compounds can be divided into two groups differing in molecular weight and nutritional significance.
1 Oxidised TAG monomers, characterised by the presence of extra oxygen in at least one of the fatty acyl groups of the TAG molecule (Fig. 1). This group contains different oxygenated groups, i.e. hydroperoxy (A in Fig. 1), hydroxy (B in Fig. 1), epoxy (C in Fig. 1), keto (D in Fig. 1), etc., as well as short-chain n-oxo fatty acyl groups (E in Fig. 1) as the main products(Reference Frankel10–Reference Berdeaux, Fontagné and Sémon13). Their molecular weight is similar to that of the initial or parent non-modified TAG.
2 TAG polymers (dimers and oligomers), the most specific compounds in used frying fats and oils (Fig. 1), formed initially through interaction among TAG monomers. This fraction is of great complexity because the high number of combinations of molecules increases exponentially, from dimers to trimers and higher oligomers(Reference Dobarganes, Márquez-Ruiz and Erickson6, Reference Dobarganes14). Their molecular weights are higher than those of their parent non-modified TAG, and it is very difficult to identify and quantify specific individual compounds.
It is important to remark the quantitative importance of these groups of compounds in the fried foods considering that, at low temperature during storage, foods consumed would contain rarely more than 4–5 % of oxidised TAG because of the clear detection of rancid odour at this oxidation level(Reference Dobarganes and Márquez Ruiz15). Otherwise, in the case of frying fats and oils, the upper limit of 25 % is often considerably surpassed in a significant number of oils and fats from fast food outlets reaching even values about 60 % polar compounds(Reference Saguy and Dana16, Reference Marmesat, Rodrigues and Velasco17). Hence, most of the oxidised fats in foods are expected to come from fats and oils heated at high temperature and more specifically from used frying fats and oils from discontinuous frying.
Health implications of dietary oxidised oils
Physiological and nutritional effects of frying oils have been the subject of intensive investigations since the 1950s. A detailed review of the distinct aspects studied and of the difficulties to reach thorough conclusions has been published recently(Reference Márquez-Ruiz, Dobarganes and Erickson4). The main reason for the difference in the results obtained is the composition of the oils used. On the one hand, those researchers who found high levels of toxicity used abusive heating conditions in an attempt to generate sufficient amounts of degradation products, but the level and structures of the compounds thus formed are not representative of those encountered in oils subjected to normal culinary practices. On the other hand, some researchers applied very soft conditions when heating oils disregarding that the use of good practices in the frying process is obviously safe. In this short review, only studies applying realistic experimental conditions for the preparation of used frying oils or fried foods, as well as those studies evaluating specific oxidation compounds, are discussed.
Used frying fats and oils
With a few exceptions, classical relevant papers describing works in experimental animals with used frying oils are not alarming and only slight effects on growth rate, liver enlargement and induction of detoxification enzymes involved in the defence mechanisms against in vivo lipid peroxidation have been shown(Reference Márquez-Ruiz, Dobarganes and Erickson4).
Digestibility has been generally found to decrease in used frying oils or oils heated at frying temperatures(Reference Márquez-Ruiz, Pérez-Camino and Dobarganes18–Reference Olivero-David, Paduano and Fogliano23). Specifically, it has been demonstrated that polymers are poorly hydrolysed by pancreatic lipase. These results were obtained in used frying oils from restaurants and fried food outlets collected by Food Inspection Services that contained from 7·5 to 61·4 % polar compounds. Oxidised TAG monomers were extensively hydrolysed but TAG dimers and higher oligomers gave low hydrolysis values (11–42 %)(Reference Márquez-Ruiz, Guevel and Dobarganes20). Also, it was shown that the hydrolysis of non-oxidised TAG was negatively affected by the presence of large amounts of polar compounds. These results were confirmed by true digestibility measurement through oesophageal probes(Reference González-Muñoz, Bastida and Sánchez-Muniz21).
With regard to epidemiological studies, researchers have so far not found any direct link between used frying oils and health problems. In fact, fried foods are an important component of the Mediterranean diet, which is strongly associated with a reduced risk of cardiovascular events(Reference Estruch, Ros and Salas-Salvadó24–Reference Martínez-González and Bes-Rastrollo26). Nevertheless, a number of studies have been conducted in human subjects to unravel the possible associations between the consumption of fried foods and the incidence of prevalent diseases, mainly cancer(Reference Stott-Miller, Neuhouser and Stanford27–Reference Bosetti, Talamini and Levi30), metabolic syndrome(Reference Soriguer, Rojo-Martínez and Dobarganes31–Reference Sayon-Orea, Martínez-González and Gea35) and CHD(Reference Iqbal, Anand and Ounpuu36–Reference Guallar-Castillon, Rodríguez-Artalejo and Lopez-García38). Few studies have found an increased risk of cancer in association with consumption of deep-fried foods, specifically in prostate(Reference Stott-Miller, Neuhouser and Stanford27), breast(Reference Dai, Shu and Jin28), oral/pharyngeal(Reference Galeone, Pelucchi and Talamini29), oesophageal(Reference Galeone, Pelucchi and Talamini29) and laryngeal(Reference Bosetti, Talamini and Levi30) cancers. Nevertheless, in all these studies, the intake of frying oil was not defined and the associations reported were not attributed to degradation compounds in the frying oil but to the heterocyclic amines or polycyclic aromatic hydrocarbons formed from meat, or acrylamide formed in carbohydrate-rich foods.
Of special interest are the recent studies regarding the incidence of metabolic syndrome(Reference Soriguer, Rojo-Martínez and Dobarganes31–Reference Sayon-Orea, Martínez-González and Gea35) and the risk of CHD(Reference Iqbal, Anand and Ounpuu36–Reference Guallar-Castillon, Rodríguez-Artalejo and Lopez-García38). Variable results were obtained including association with a higher prevalence of arterial hypertension(Reference Soriguer, Rojo-Martínez and Dobarganes31), obesity(Reference Guallar-Castillón, Rodríguez-Artalejo and Lopez-García32, Reference Sayon-Orea, Bes-Rastrollo and Basterra-Gortari33), or lower HDL-cholesterol levels and a larger waist circumference(Reference Donfrancesco, Lo and Brignoli34). Also, a null association with the incidence of metabolic syndrome has been reported in the case of a moderate consumption of fried foods(Reference Sayon-Orea, Martínez-González and Gea35). With regard to the studies evaluating the effect of fried foods on the risk of CHD, either positive(Reference Iqbal, Anand and Ounpuu36) or null association(Reference Kabagambe, Baylin and Siles37, Reference Guallar-Castillon, Rodríguez-Artalejo and Lopez-García38) has been found. Concerning these studies, it is important to remark two aspects. On the one hand, most of the studies have been conducted in Mediterranean countries where olive oil, less prone to degradation than other edible oils, is commonly used for domestic frying. Therefore, research needs to be extended to other communities consuming preferentially polyunsaturated oils or solid fats. On the other hand, information provided on the oxidation of frying oils or fried foods tested was too scarce. Hence, one important variable contributing to the differences between the results obtained in different studies could be the level of oxidation compounds present in the diet. In fact, a significant association between consumption of fried foods and some of the components of metabolic syndrome was found when the amounts of oxidation compounds in the diet increased. In this respect, two examples are worthy to comment. First, in Soriguer et al.’s(Reference Soriguer, Rojo-Martínez and Dobarganes31) study, oil samples were taken from the kitchens of a random subset of 538 participants and 10 % of the oils collected contained over 20 % polar compounds. A strong association was found between the consumption of such oils and the risk of hypertension, even after inclusion in the models of variables influencing hypertension, such as age, sex and obesity. Also, Sayon-Orea et al. (Reference Sayon-Orea, Martínez-González and Gea35), in the SUN cohort study, classified 8289 participants in three groups according to their frequency of fried food consumption and found that those participants who consumed fried foods >4 times/week had a higher risk to develop two out of five components of metabolic syndrome: central adiposity and high blood pressure, compared with those who consumed ≤ 2 times/week.
Paradoxically, feeding experiments in animals have consistently demonstrated that thermally oxidised oils improves the blood lipid profile, i.e. a reduction in TAG and cholesterol levels in plasma and VLDL, attributed to the activation of hepatic PPARα(Reference Chao, Chao and Lin39–Reference Ringseis, Gutgesell and Dathe42). Even more, it has also been postulated that PPARα activation in the vasculature would inhibit pro-atherogenic events such as monocyte recruitment and smooth muscle cells proliferation and migration(Reference Kammerer, Ringseis and Eder43). The authors suggest that some of the multiple components found in thermally oxidised oils may exhibit potent regulatory activity on lipid metabolism(Reference Ringseis and Eder44). Nevertheless, thermally oxidised oils also cause oxidative stress in animals(Reference Izaki, Yoshikawa and Uchiyama45–Reference Olivero-David, Bastida and Schultz49) probably due to the depletion of antioxidants such as tocopherols in serum and tissues. Hence, the possible atheroprotective effect due to activation of PPARα is probably compromised by the simultaneous induction of intense oxidative stress(Reference Ringseis, Gutgesell and Dathe42).
Model systems and specific oxidation compounds
A major handicap for researchers focused on individual compounds formed during frying is that few specific compounds have been quantified so far. Alternatively, some investigators make use of model compounds, usually methyl linoleate or linoleic acid, subjected to thermal oxidation under controlled conditions. In this section, the research undergone in relation to the most important groups of compounds, i.e. oxidised TAG monomers and TAG polymers (Fig. 1), is considered separately. Following enzymatic hydrolysis by pancreatic lipase, TAG yield NEFA that are ultimately absorbed. As examples, Fig. 2 represents the hydroxyl fatty acid released from an oxidised TAG monomer and the epoxy fatty acid and dimeric fatty acid released from an oxidised TAG dimer.
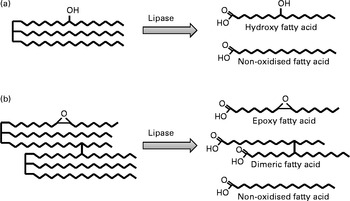
Fig. 2 Schematic representation of fatty acids released from simplified structures of oxidised TAG monomer (a) and oxidised TAG dimer (b) by pancreatic lipase hydrolysis.
Oxidised TAG monomers
Given that the synthesis and analysis of hydroperoxides, the primary oxidation compounds, are well resolved, many studies on their physiological effects have been carried out. Although their occurrence in used frying oils is limited due to their instability at high temperatures, the main effects attributed to hydroperoxides are worth commenting. TAG monohydroperoxides appear to be hydrolysed by pancreatic lipase at similar degree as its parent TAG(Reference Miyashita, Takagi and Frankel50). Nonetheless, previous reactions in the stomach seem to play an important role(Reference Kanner and Lapidot51). Thus, linoleic acid hydroperoxides administered intragastrically was converted into hydroxyls, epoxyketones, hexanal and 9-oxononanoic acid, time-dependently(Reference Kanazawa and Ashida52, Reference Kanazawa and Ashida53). Other authors have reported that gastrointestinal glutathione peroxidase plays an important role in the mucosal transport and in the conversion of hydroperoxides to more stable hydroxy or aldehydic compounds(Reference Aw54–Reference Aw57). Therefore, dietary hydroperoxides will be largely lost before absorption. In fact, hydroperoxides found in the atherosclerotic process(Reference Chisolm and Steinberg58–Reference Stocker and Keany61) are most likely formed endogenously under particular dietary circumstances involving impaired antioxidant status(Reference Duthie, Wahle and James62). With regard to studies focused on colorectal cancer, tumour-promoting effects(Reference Bull, Nigro and Marnett63, Reference Kanazawa, Sawa and Akaike64) and complex metabolic effects of chronic exposure to subtoxic levels have been reported in rats(Reference Tsunada, Iwakiri and Noda65, Reference Tsunada, Iwakiri and Fujimoto66) and human cells(Reference Wang, Gotoh and Jennings67, Reference Jurek, Udilova and Jozkowicz68). However, other authors have stressed the fact that, with regard to the effect of dietary lipids, dietary components other than oxidised lipids may be responsible for such adverse effects, such as the consumption of red meat and the metabolic activity of the microflora in the colon(Reference Yang and Schaich69). Thus, a Hb–Fe-rich diet was found to lead to an increased incidence of colon cancer in rats, attributable to the generation of peroxyl radicals from dietary or membrane lipids of intestinal epithelial cells(Reference Sawa, Akaike and Kida70).
With respect to major oxidised compounds in frying oils, high absorption of dietary hydroxy and epoxy fatty acids incorporated in TAG (B and C, respectively, in Fig. 1) was also reported in human subjects, through an excellent approach based on labelled fatty acids included in TAG(Reference Wilson, Fernie and Scrimgeour71, Reference Wilson, Lyall and Smyth72). Later, 13-hydroxylinoleic acid was found to reduce cholesterol content in a macrophage cell line, probably by stimulating apoA-I-dependent cholesterol efflux in a PPAR-dependent manner(Reference Kammerer, Ringseis and Biemann73). Quite in contrast, cytotoxic effects of monoepoxy linoleate or leukotoxin and its corresponding diol, leukotoxindiol, have been reported(Reference Mitchell, Moran and Grant74–Reference Slim, Hammock and Toborek77). However, physiological levels of epoxides in human subjects and significance of dietary epoxides are unknown.
TAG polymers
Polymerisation reactions are accelerated by the high temperatures used in frying but the identification of specific polymeric structures formed is very difficult. Consequently, there are no relevant studies on their effects on metabolic pathways with the exception of those concerning their absorption and digestibility.
Dimeric fatty acids are normally described as non-polar or non-oxidised and polar or oxidised according to the absence or presence, respectively, of one or more oxygenated function either in the dimeric linkage or in the fatty acyl chain. Studies on non-polar dimeric fatty acids (simplified structure in Fig. 2(B)) indicated very low lymph recoveries as values about 1 % were found(Reference Perkins and Taubold78, Reference Combe, Constantin and Entressangles79). Furthermore, experiments with rats fed labelled non-polar dimers showed recoveries of about 3 % radioactivity in urine and CO2 while approximately 80 % of the radioactivity was recovered in the gastrointestinal tract and faeces after 48 h(Reference Strauss, Piater and Sterner80). In contrast, polar dimeric and polymeric fatty acids were comparatively better absorbed(Reference Combe, Constantin and Entressangles79, Reference Márquez-Ruiz and Dobarganes81). This could be in part due to depolymerisation reactions occurring under the strongly acidic conditions in the stomach, as suggested by the presence of non-altered labelled fatty acids in faeces, which were absent in diets(Reference Márquez-Ruiz and Dobarganes81).
Even though complexity of dimers and polymers is a major handicap for nutritional studies, two key points support further analytical and nutritional research. First, TAG polymers constitute the major fraction in used frying oils and fats, and second, the low absorption of the dimeric and polymeric fatty acids released does not necessarily means lack of health risk. In fact, it involves increased levels of non-digested, non-absorbed lipids throughout the gastrointestinal tract that might potentially affect epithelial cells and microflora metabolism. In connection with this subject, it has been reported that both unabsorbed lipids and bile acids secreted in response to a high fat intake might injure the intestinal mucose by their detergent activity, and metabolites of bile acids formed by intestinal bacteria (secondary bile acids) act as tumour promoters(Reference Vonk, Kalivianakis and Minich82). Also, an interesting aspect is the potential contribution of intestinal flora to the production of mutagens from the oxidation of faecal lipids and the effect of vitamin E as a chemopreventive agent(Reference Campbell, Stone and Whaley83).
Table 2 includes the main studies discussed in this short review focused on used frying/thermally oxidised oils, fried products and on model compounds used in feeding studies.
Table 2 Summary of the main studies discussed in this review on used frying/thermally oxidised oils, fried products and on model compounds tested in feeding experiments

Conclusions
From the literature reviewed, there is general agreement that a moderate consumption of used frying oils under normal culinary practices is safe, but it is also evident that some compounds formed can impair their nutritional value or be potentially harmful. Therefore, of primary importance is the improvement of the quality control of used frying oils, particularly in the case of fried foods prepared through discontinuous frying process in which used frying oils can reach degradation levels much higher than that established for human consumption.
Overall, the nutritional studies reported on the effects of used frying oils and fats lack the analytical data necessary to establish valid relationships between the alteration compounds present and the effects evaluated on molecular targets, metabolic pathways and chronic diseases. In order to avoid confounding and alarming results, a crucial research assignment is the development of methodologies directed to define the chemical structure and actual levels of the multitude of non-volatile compounds generated from frying and present in the diet. Nowadays, there is no information on the intake and fate of oxidised compounds, which would be essential to guarantee that dietary oxidised lipids are not responsible for any of the adverse physiological effects claimed.
Finally, a specific aspect that remains largely to be explored is the fate of poorly absorbed compounds present in used frying oils, in terms of their interactions with gastrointestinal mucosa and fluids, and with microflora metabolism.
Acknowledgements
The present study was funded by the Spanish Project AGL2010-18307.
The authors have no conflicts of interest.






