Intake of fruit and vegetables have been associated with a reduced risk of CVD( Reference Hartley, Igbinedion and Holmes 1 , Reference Woodside, Young and McKinley 2 ). Fruits and vegetables contain various polyphenols that have been suggested to contribute to this protective effect( Reference Chong, Macdonald and Lovegrove 3 , Reference Hertog, Feskens and Kromhout 4 ).
Polyphenols constitute a large family of natural compounds widely found in plant foods. The main function of polyphenols in plants is to provide protection from various types of stress and cellular damages. Each polyphenol molecule comprises two or more phenol units. The number and structure of these phenol units make each polyphenol compound unique with regard to their bioavailability. Moreover, due to their individual bioactivities, absorption( Reference Manach, Williamson and Morand 5 , Reference Xiao and Kai 6 ), metabolism and cellular accumulation, as well as specific interaction with various signalling molecules, enzymes, and transcription factors, may vary( Reference Müller and Kersten 7 ). Therefore, it is likely that polyphenols from different fruits and berries will vary in their potential to exert effects on outcome measures in intervention studies. It has been shown that polyphenols have favourable effects on platelet aggregation( Reference Erlund, Koli and Alfthan 8 – Reference Keevil, Osman and Reed 10 ), blood pressure (BP)( Reference Erlund, Koli and Alfthan 8 , Reference Karlsen, Svendsen and Seljeflot 9 , Reference Aviram, Rosenblat and Gaitini 11 ) and blood lipid composition( Reference Eccleston, Baoru and Tahvonen 12 , Reference Gorinstein, Caspi and Libman 13 ), factors that are associated with CVD. Some studies have identified specific polyphenols with the ability to reduce BP, such as quercetin( Reference Edwards, Lyon and Litwin 14 ). However, whole foods seem to be more effective than supplements in the prevention of CVD( Reference Eilat-Adar and Goldbourt 15 ), possibly because whole foods provide a greater variety of polyphenols. In addition, reportedly combination of several different polyphenols may exert synergistic effects( Reference Liu 16 ). How polyphenols can relax vascular tone is not known; however, modulation of the balance between NO and endothelin, e.g. via improved antioxidative status, might be involved( Reference Kardum, Konic-Ristic and Savikin 17 , Reference Storniolo, Rosello-Catafau and Pinto 18 ).
It has been well established that hypertension is a strong predictor for cardiovascular morbidity and mortality( Reference Collins, Peto and MacMahon 19 , Reference MacMahon and Rodgers 20 ), but also fluctuations and variability in BP correlated with disease progression. Rothwell et al. ( Reference Rothwell, Howard and Dolan 21 ) showed that both visit-to-visit variability and maximum systolic blood pressure (SBP) are strong predictors for strokes, independent of mean SBP. In the review by Parati et al. ( Reference Parati, Ochoa and Bilo 22 ), it is reported that variability of short-term BP (within 24 h) has been closely associated with the development, progression and severity of cardiac, vascular and renal organ damages independently of mean BP.
Healthy foods taken in a liquid form can be easily added to a habitual diet. However, the effects of polyphenol-rich juices on BP have not been evaluated. Hence, we hypothesised that intake of such juices would lower BP and/or lead to a more favourable profile of risk factors for CVD in apparently healthy subjects. In the present 12-week, randomised, placebo-controlled intervention study, we tested the effect of a polyphenol-rich juice (MANA Blue) based on red grapes, cherries, chokeberries and bilberries, and a juice (Optijuice) in which MANA Blue has an added polyphenol-rich extract from blackcurrant press-residue. Following a strict procedure, three measurements of SBP and diastolic blood pressure (DBP) were recorded at each visit, and changes in (1) the first blood pressure of three measurements (BP1); (2) the mean of blood pressure measurement number two and three (BPmean); and (3) blood pressure variability (BPV), another predictor of cardiovascular incidents( Reference Rothwell, Howard and Dolan 21 , Reference Eguchi, Hoshide and Schwartz 23 ), were analysed. In addition, lipids and other blood parameters associated with CVD were determined.
Subjects and methods
Study beverages
Three different beverages were used in the present study: placebo; MANA Blue; Optijuice. Table 1 shows the nutrient and chemical characteristics of the beverages, whereas the online supplementary Table S1 shows the details and changes in content over time. MANA Blue (MANA Blue, grape, bilberry and chokeberry juice; TINE SA) is a commercially available product containing red grape (Vitis vinifera, 67·7 %), chokeberry (Aronia melanocarpa, 14·5 %), cherry (Prunus cerasium, 12 %) and bilberry (Vaccinium myrtillus, 5·8 %), while the other two drinks were specifically made by TINE SA for the present study. Optijuice was made of MANA Blue (85 %) added polyphenol-rich extract from blackcurrant press-residue (15 %), previously optimised for biological activity in vitro ( Reference Holtung, Grimmer and Aaby 24 ). Optijuice contained more total polyphenols than MANA Blue, but was lower in hydroxyciannamic acids, as this compound was lower in the blackcurrant press-residue than in MANA Blue. A placebo drink was developed with comparable amounts of energy, carbohydrates, K and colour to mimic the intervention juices. It contained Maltodextrin (6·2 g), sugar (6·2 g), KCl (280 mg), blueberry flavour (3504156, 25 mg), grape flavour (6103834, 20 mg), citric acid (0·01 mg, to pH 4) and dye (E122 and E25/azorubin/brilliant black, 5 mg), all per 100 g beverage. Subjects were provided with sufficient volume for daily intake of 500 ml for 12 weeks. The study beverages were supplied by TINE SA in identical white containers, each containing 1000 ml Optijuice, MANA Blue or placebo.
Table 1 Nutrient and chemical characteristics of beverages (per 100 g)*
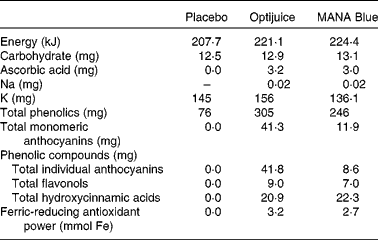
* Online supplementary Table S1 shows a more detailed list of single components as well as their change over time.
Beverage compounds
The total content of polyphenols was determined with the Folin–Ciocalteu's method and determined as gallic acid equivalents in mg/100 g of sample as described previously( Reference Holtung, Grimmer and Aaby 24 ). The pH differential absorbance method was used to determine the content of total monomeric anthocyanins, calculated as cyanidin-3-glucoside equivalents in mg/100 g of sample( Reference Holtung, Grimmer and Aaby 24 ). Individual polyphenol compounds were analysed on an Agilent 1100 series HPLC system (Agilent Technologies) equipped with a diode array detector and a MSD XCT ion trap mass spectrometer as described previously( Reference Aaby, Grimmer and Holtung 25 ). The polyphenols were quantified using cyanidin-3-glucoside, at 520 nm, for anthocyanins; rutin, at 360 nm, for flavonols; and chlorogenic acid, at 320 nm, for hydroxycinnamic acids. All the results are presented as mg/100 g of sample (online supplementary Table S1). The ferric-reducing antioxidant power was assayed as described by Benzie & Strain( Reference Benzie and Strain 26 ).
Study subjects
The volunteers were recruited by postal mail by 10 000 invitation letters to men and women, between 50 and 70 years living in Oslo, Norway, and listed in the National Population Registry, as well as by about 400 letters distributed to the lunch areas in public transport companies. The invitation letter did not ask for BP level, but for exclusion criteria including the use of regular BP-lowering medication, the presence of type 1 and 2 diabetes, smoking habit or a BMI above 35 kg/m2. About 9 % (n 921) subjects replied to the first invitation. Of these, 737 were found eligible to be invited for a screening visit. At the screening visit (n 627), additional exclusion criteria, such as allergy to grapes, cherries, blueberries/bilberries, blackcurrant or chokeberries, changes of ± 4 kg in body weight within the last 12 weeks before the start of the study, use of supplement for weight reduction, or of polyphenol-rich supplements and participation in other clinical trials or other planned activities (vacation, hospital admission, etc.), were recorded. At the same time, the volunteers' BP was screened to be within the high normal range (130/85–139/89 mmHg) or stage 1–2 hypertension (140/90–179/109 mmHg), which was the main inclusion criteria. All subjects signed a written consent to participate. During the baseline visit (n 207), subjects who did not meet the BP criteria were further excluded from the study (n 54). Subjects initiating BP-lowering medication during the study, not following the drinking regimen (at least 80 % compliance), not showing up on all visits, or incorrect BP measurements according to the procedure were also excluded from the analyses (Fig. 1).

Fig. 1 Flow chart of study participants. BP, blood pressure.
Study ethics
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Regional Committees for Medical and Health Research Ethics, Health Region South East, Norway, and written informed consent was obtained from each subject. The study is registered at Clinicaltrials.gov (NCT01568983).
Study design
The present study was a double-blind, placebo-controlled trial, and was conducted between December 2011 and June 2012. At baseline, subjects were randomly assigned to a study group consuming 500 ml/d of (1) placebo; (2) Optijuice; or (3) MANA Blue for 12 weeks. The subjects were instructed to record the consumed beverages in a provided diary. They were also asked to refrain from other juice products (except juices made of apples and oranges), and from antioxidant supplements (such as vitamin C) before the start of the study and during the course of the study. Apart from this, the subjects were encouraged to maintain their habitual diet, physical activity and lifestyle while enrolled in the study.
All the subjects made four visits (screening, baseline, 6-week visit and 12-week visit) during the study period. On the measurement days, the subjects had been fasting from midnight the day before. For the last visit, the subjects were asked to drink the last glass of study beverage between 20.00 and 22.00 hours the night before. All the visits were between 08.00 and 10.00 hours to avoid diurnal fluctuations.
Blood pressure measurements
Fasting SBP and DBP measurements were performed blinded by a trained personnel. Totally, three measurements at 1-min intervals were recorded after 10 min of rest in a waiting room followed by another 5 min in an investigation room where the subject sat in a resting chair with the cuff mounted and the arm at the armrest. Validated oscillometric devices (Carescape V100; GE Healthcare) with suitable cuffs were used for the measurements. In the analyses, we used the first measure (BP1), the mean (BPmean) of measure number two and three and the standard deviation (SD) of all the three measurements (BPV). Normotensive and hypertensive subjects were defined as below and above a SBP of 140 mmHg, respectively.
Laboratory analyses
Fasting blood samples were collected at baseline and after 12 weeks. Venous blood samples were collected in vaccutainers and kept at room temperature or at 4°C until processing. Serum and plasma samples were obtained by centrifugation at 1500 g for 10 min at 8°C, aliquoted and frozen at − 80°C. The following analyses were performed on a Maxmat PL (Maxmat): uric acid (RM URAC0200V); creatinine (RM CREP0270V); cholesterol (RM CHOL0400V); direct LDL-cholesterol (RM LDLC0080V); direct HDL-cholesterol (RM HDLC0120V); glucose (RM GLUP0400V); TAG (RM TRIG0400V); alanine transaminase (ALAT; ALAT-GPT, RM ALAT0252V); aspartate transaminase (ASAT-GOT, RM ASAT0252V; all Maxmat procedures and products, manufacturers assay numbers in brackets); phospholipids (1001140; Spinreact); non-essential fatty acids (D07940; Dialab); total antioxidant status (NX 2332; Randox); D-roms test (MC 003; Diacron). In addition, the following haematological analyses were performed at Oslo University Hospital using standard procedures: Hb; haematocrit; platelet count; leucocyte count including a differential count; d-dimer.
Measurement of body composition
Weight, fat-free mass, fat mass, total body water and basal metabolic rate were determined using a bioimpedance analyser (Tanita TBF-300; Tanita Corporation) at the first and last visits (baseline and week 12).
Statistical analyses
We assumed a SD of the reduction of 11 mmHg, and based on an ANOVA test, we found that a total of 210 individuals would be needed to detect a difference in BP of 5 mmHg with a power of 80 % and a significance level of 0·05. After screening process, 207 subjects were eligible for the study.
Changes in BP were analysed using the ‘mixed’ command for linear mixed models in IBM SPSS (software version 16.0.1; SPSS Inc.) treating time as categorical parameter, including a random intercept in the model and the following parameterisation: β0 time+β1 treatment+β2 (time × treatment). BP estimates were based on the mixed model, and P values were generated from the SPSS test of fixed effects for the interaction term (time × treatment) from the mixed model, as is the estimated difference in change between intervention and placebo groups at different time points.
Variability of BP was calculated as SD of the three measurements at each visit and further analysed by a mixed model as described above. The residuals of the SD showed a normal distribution. Baseline statistics summarised in Table 2 are presented as crude means with SD. Differences between groups at baseline were determined by ANOVA as were differences in the biochemical data. A comparison of first systolic blood pressure (SBP1) with mean systolic blood pressure (SBPmean) was done by paired t test. A P ≤ 0·05 was considered significant.
Table 2 Baseline characteristics of participants* (Mean values and standard deviations)
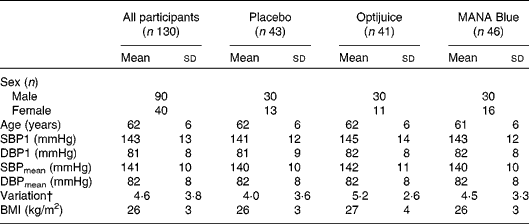
SBP1, first systolic blood pressure; DBP1, first diastolic blood pressure; SBPmean, mean of systolic blood pressure recording measures two and three; DBPmean, mean of diastolic blood pressure recording measures two and three.
* There were no statistical differences between the groups determined by ANOVA.
† Variation is the standard deviation of triplicate measurements of systolic blood pressure.
Subgroup analyses, as described above, were performed on hypertensive subjects (140–179 mmHg) and normotensive subjects (124–139 mmHg) based on SBP1 or SBPmean at baseline.
Results
Participant flow
Subjects who responded positively to the invitation letter corresponded to about 9 % of the invited cohort (n 905). Of these subjects, 737 were eligible after self-reporting and were invited for screening, and 627 persons attended the screening of BP and the interview. After the screening procedure, 420 subjects were excluded from the study because they did not fulfil the inclusion criteria or for other reasons. At baseline, another fifty-four subjects had BP below the eligibility criteria and were, therefore, not included. During the study, nineteen subjects dropped out, leaving 134 subjects who completed the intervention (Fig. 1). At the end of the study, four data sets were excluded from the analyses according to the exclusion criteria. Hence, the study group for analyses consisted of 130 subjects, with forty-three in the placebo group, forty-one in the Optijuice group and forty-six in the MANA Blue group.
Baseline characteristics of subjects
At baseline, the mean SBP1 and first diastolic blood pressure (DBP1) for all the subjects were 143 and 81 mmHg, respectively, and the corresponding mean values of SBPmean and DBPmean were 141 and 82 mmHg, respectively. Neither the BP values nor the anthropometric measures were significantly different among the three study groups (Table 2).
Effects on blood pressure in the polyphenol-rich juice groups
At baseline, we observed that in the whole study group (n 130), SBP1 was on average 2·5 mmHg higher (P< 0·001) than the SBPmean, and therefore, these two measures were analysed separately.
SBP1 was significantly reduced in both the Optijuice group and the MANA Blue intervention group at 6 weeks (P= 0·01 for both), but not after 12 weeks, compared with the placebo group (Table 3). There were no significant differences between the SBP1 time curves (P= 0·07) when analysing the (time × treatment) interaction over the full study period (12 weeks). Changes in DBP1 in the intervention groups were not different from those of the placebo group, neither for single time point nor for the complete time curve.
Table 3 Blood pressure (BP) measurements: first BP measurement in all subjects* (Mean values and 95 % confidence intervals)

Diff. placebo, estimated differences in treatment groups from placebo group; SBP1, first systolic blood pressure; DBP1, first diastolic blood pressure.
* Data shown are estimated values generated from the mixed model. P values are also taken from the mixed model.
† P value for changes from baseline to weeks 6 and 12, respectively, compared with the placebo group.
‡ P value for the overall test of no (time × treatment) effect.
§ P value for the overall test of no (time × treatment) effect using all the three treatment groups (the placebo and the two intervention groups).
∥ P value for the overall test of no (time × treatment) effect using the placebo and the pooled juice groups.
Since both intervention juices are very rich in polyphenols, we pooled the Optijuice and MANA Blue groups in the analysis to increase the statistical power. The SBP1 time curves for the pooled intervention group and placebo group were significantly different (P= 0·01). The (time × treatment) interaction revealed that after 6 weeks, SBP1 were reduced by 6·9 mmHg in the pooled group compared with the placebo (P< 0·001), while this effect was not seen after 12 weeks (Table 3). No effects were observed for DBP1.
We did not observe any significant differences between the groups when time curves for SBPmean or DBPmean were investigated (online supplementary Table S2), neither for all the three groups separated nor if the two juice groups were pooled.
Larger effect of polyphenol-rich juice on blood pressure in hypertensive subjects compared with normotensive subjects
Sub-analyses of the interventions on hypertensive subjects (SBP in the range of 140–179 mmHg) based on SBP1 at baseline showed that the SBP1 time curves were not significantly different for the treatment groups (Table 4). In the pooled juice group, however, the SBP1 time curve was significantly different from the placebo group (P= 0·05). This difference is explained by a significantly higher reduction in the pooled group after both 6 weeks (P= 0·03) and 12 weeks (P= 0·04) than the placebo group. DBP1 was not affected by the juice interventions (data not shown).
Table 4 Changes in first blood pressure in hypertensive and normotensive subjects* (Mean values and 95 % confidence intervals)

Diff. placebo, estimated differences in treatment groups from placebo; SBP1, first systolic blood pressure; hypertensive subjects, subjects with SBP1 in the range of 140–179 mmHg at baseline; normotensive subjects, subjects with SBP1 below 140 mmHg at baseline.
* Data shown are estimated values generated from the mixed model. P values are also taken from the mixed model.
† P value for changes from baseline to weeks 6 and 12, respectively, compared with the placebo group.
‡ P value for the overall test of no (time × treatment)-effect, using.
§ All the three treatment groups (the placebo and the two intervention groups), and using.
∥ The placebo and the pooled juice groups.
Changes of BP in normotensive subjects (range of 124–139 mmHg based on SBP1 at baseline) after the intervention are presented in Table 4. In the pooled analysis of Optijuice and MANA Blue groups, we observed significant differences for the SBP1 time curve compared with the placebo group (P= 0·02). However, this significant difference seems to be due to a net increase in SBP1 in the placebo group after 6 weeks (5·5 mmHg) rather than a reduction in the juice groups. No effects were seen for DBP1 (data not shown).
No effects of the interventions in hypertensive or normotensive subjects, based on SBPmean at baseline, were observed in the SBPmean measures (online supplementary Table S3) or DBPmean measures (data not shown).
Effects of polyphenol-rich juice on standard deviation as a measure of the variance of three blood pressure measurements
BP variance is a relevant measure in CVD development( Reference Parati, Ochoa and Bilo 22 ). We observed that the SD of the three measurements of SBP at each visit was reduced in the pooled juice group by 1·4 mmHg (6 weeks) and 1·7 mmHg (12 weeks). Compared with the placebo group, this gave a significant reduction (P= 0·03; Table 5). The reduction was more pronounced in hypertensive subjects (2·03 mmHg at 6 weeks, 2·83 mmHg at 12 weeks; P= 0·01). In normotensive subjects, a significant difference between placebo and pooled groups was not observed (Table 5).
Table 5 Blood pressure variance* (Standard deviations and 95 % confidence intervals)
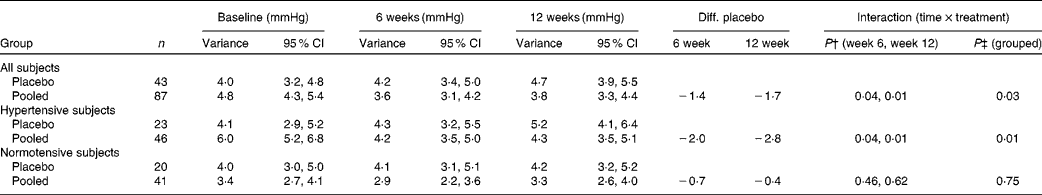
Diff. placebo, difference in the intervention group from the placebo group; hypertensive subjects, mean value of systolic blood pressure (SBP) triplicate above 140 mmHg; normotensive subjects, mean value of SBP triplicate below 140 mmHg.
* Data shown are estimated values of standard deviation, the variance, of triplicate SBP measurements and Diff. placebo generated from the mixed model. P values are also taken from the mixed model.
† P value for changes from baseline to weeks 6 and 12, respectively, compared with the placebo group.
‡ P value for the overall test of no (time × treatment) effect.
Biomarker analyses
Blood samples for haematological and biochemical analyses were collected at baseline and at the end of the study, at week 12. The mean baseline values were within the normal range for all markers (data not shown). The results showed that only ALAT was significantly different in the three groups during the time course (P< 0·001), on average − 0·7, − 8·9 and 1·2 units/l in the placebo, Optijuice and MANA Blue study groups, respectively. In the Optijuice group, two data sets were above normal range at baseline and reduced over 50 % by the end of the study. These data sets were considered out of range and were removed before analyses not to create a false positive reduction in the Optijuice group. At baseline, the average values for ALAT were 25·8, 26·8, 24·8 units/l for placebo, Optijuice and MANA Blue, respectively. At the end of the study, the average values for ALAT were 25·2, 17·9 and 26·0 units/l for placebo, Optijuice and MANA Blue, respectively.
Anthropometric analyses
Body composition and weight were determined at the first and last visits (baseline and week 12). There were no significant differences in weight or body composition (data not shown).
Discussion
Previous epidemiological studies and some intervention studies have suggested a role for polyphenols in BP reduction( Reference Erlund, Koli and Alfthan 8 , Reference Karlsen, Svendsen and Seljeflot 9 , Reference Aviram, Rosenblat and Gaitini 11 , Reference Naruszewicz, Łaniewska and Millo 27 ). The present study, which is the first placebo-controlled intervention study on the effects of berry juice on BP, strongly indicates that polyphenol-rich berry juice alone can reduce BP and short-time BP variation. We analysed changes of the first of three BP measurements (BP1), the mean of the two following measurements (BPmean), as well as the BPV to evaluate the effect of the polyphenol-rich juices on BP.
The present results demonstrated that BP1 was significantly reduced in the pooled polyphenol-rich juice group compared with the placebo group. It is well known that the first recording in repeated BP measurements is usually higher than the next two recordings( Reference Sabater-Hernández, Sánchez-Villegas and Lacampa 28 ), as observed in the present study. This may be regarded as a ‘white coat effect’( Reference Sabater-Hernández, Sánchez-Villegas and Lacampa 28 ), i.e. an observed increased BP taken at a doctor's office compared with BP measured at home or with ambulatory BP. In many studies, this measurement has, therefore, been excluded from the analyses. Probably, BP1 is more sensitive to stress and sympathetic activation, similar to the elevated BP observed during mental or acute stress tests( Reference Flaa, Eide and Kjeldsen 29 – Reference Rostrup, Mundal and Kjeldsen 31 ). The association between stress-related elevated BP and CVD is well established( Reference Rozanski, Blumenthal and Kaplan 32 ). The present results suggest that a possible mechanism of the beneficial effects of fruits and berries on CVD could be through reduction of the elevated BP during stressful situations and not necessarily on the resting BP, which, in the present study, was not significantly changed during the intervention period.
Further, we observed that the BPV, determined by the SD of the three measurements at each visit, was reduced by the polyphenol-rich intervention. Akita et al. ( Reference Akita, Kuwahara and Itoh 33 ) showed that cacao liquor polyphenols reduced BPV in rabbits. Hodgson et al. showed that black tea lowered the rate of BPV in human subjects( Reference Hodgson, Croft and Woodman 34 ), although they were not able to detect the same effects by specific vitamin or grapeseed interventions( Reference Hodgson, Croft and Woodman 35 ). The present study is the first to show a reduction in BPV in a clinical placebo-controlled intervention trial. The reduction in BPV may probably reduce the risk of CVD( Reference Parati, Ochoa and Bilo 22 ) as both visit-to-visit and ambulatory BPV are predictors of cardiovascular incidents( Reference Rothwell, Howard and Dolan 21 , Reference Eguchi, Hoshide and Schwartz 23 ). Possible mechanisms behind these findings may be that high BPV leads to stress on the vessel wall, which may, again, result in damage and initiation of CVD. We defined BPV as the SD of the three SBP measurements at each visit. Other studies have used SD of ambulatory or visit-to-visit BP measurements( Reference Parati, Ochoa and Bilo 22 ), or even the slope of SBP from beat to beat( Reference Mancia, Parati and Castiglioni 36 ). We suggest that the variation in three SBP measurements over a time period of 3–4 min may also reflect a relevant pathophysiological condition similar to BPV determined by other methods.
We were surprised to observe that the reduction in SBP1 was most evident in the intervention group after 6 weeks (6·4 mmHg, pooled group) while only a 0·8 mmHg further reduction was detected between weeks 6 and 12. This time course could reflect the reduction of anthocyanins we observed in both juices over time. However, we did not observe any differences in the effect on SBP1 between the Optijuice and the MANA Blue groups at neither 6 nor 12 weeks, although the Optijuice contained five times more anthocyanins at both time points (41·8–20·3 mg/100 g for Optijuice and 8·6–4·1 mg/100 g for MANA Blue). That is, if the concentration in MANA Blue at starting point (8·6 mg/100 g) was sufficient for the observed effect in the six first weeks, there has to be other reasons than the decrease in anthocyanin concentration for the lack of further reduction in SBP1 in the Optijuice group, still containing 20·3 mg/100 g. Therefore, we assume that even the lowest concentration of anthocyanins in the present juices were sufficient to exert the observed effects.
For the placebo group, the SBP1 time curve had a different shape; here, there was no reduction in the first 6 weeks while the most evident reduction occurred between weeks 6 and 12. This could be explained, in part, by seasonal variations( Reference Hozawa, Kuriyama and Shimazu 37 ) or other reasons for natural fluctuation, which also the intervention group would be susceptible to. These results underline the great importance of including placebo groups in intervention studies to obtain reliable results.
It is of particular interest to reduce and control BP in subjects with SBP/DBP ≥ 140/90 mmHg. We, therefore, performed a sub-analysis to examine the effect of intervention in hypertensive and normotensive subjects, both for BP1 and BPmean. We observed that subjects with SBP1/DPB1 ≥ 140/90 mmHg showed a significant reduction in SBP1 (7·3 and 6·8 mmHg after 6 and 12 weeks, respectively; P= 0·05) when combining the two polyphenol juice groups compared with the placebo group. This is in accordance with other studies showing that intervention with fruits and berries has the strongest effect on a higher starting BP( Reference Erlund, Koli and Alfthan 8 , Reference Karlsen, Svendsen and Seljeflot 9 ).
To date, there are few clinical trials supporting the notion that fruit and berries, through their polyphenol content, are potential BP-lowering foods( Reference Erlund, Koli and Alfthan 8 , Reference Karlsen, Svendsen and Seljeflot 9 , Reference Naruszewicz, Łaniewska and Millo 27 , Reference Stowe 38 ), although this has long been suggested by epidemiological studies( Reference Hertog, Feskens and Kromhout 4 ). The mechanism behind the effects of polyphenol-rich food has not been identified and the research of which polyphenols that are most important for the biological effects is quite scarce. Therefore, we believe that it is important to include a variety of polyphenol-rich fruits and berries in interventions with the purpose of studying beneficial effects of polyphenols. In line with this, we included a combination of grape, cherries, bilberries, chokeberries and blackcurrant in the intervention juices. Since peels and seeds in fruits and berries are enriched with polyphenols, a large amount of the valuable polyphenols are often lost in the press-residue instead of in the juice( Reference Sandell, Laaksonen and Järvinen 39 ). Therefore, an extract from blackcurrant press-residue, previously optimised for biological activity( Reference Holtung, Grimmer and Aaby 24 ), was introduced in one of the juice groups.
Both juices had high levels of total polyphenols and ferric-reducing antioxidant power, both measures of antioxidant capacity or reducing properties (Table 1). The amounts of total polyphenols and ferric-reducing antioxidant power in Optijuice, which contained the blackcurrant peel extract, were about 20 % higher than in MANA Blue juice. The concentrations of flavonols were also somewhat higher (28 %) in Optijuice, while the concentrations of total hydroxycinnamic acids were equal in the two juices, explained by the low content of hydroxyciannamic acids in blackcurrant. The main difference between the juices was the higher content of anthocyanins, the major polyphenol compounds in the juices, where Optijuice had about 5-fold higher concentration than MANA Blue. In addition, the composition of anthocyanins differed, Optijuice, naturally being especially rich in anthocyanins from blackcurrants (i.e. glucosides and rutinosides of delphinidin and cyanidin (online supplementary Table S1). Despite these differences, we did not observe any differences on the effect on BP between these juices. In the present study, it was, therefore, not possible to reveal any effects of dose or content of polyphenols. We, therefore, chose to pool the two groups to increase the statistical power in several of the analyses.
In the present study, subjects were instructed to refrain from other juice products, from antioxidant supplements and otherwise encouraged to maintain their habitual diet, physical activity and lifestyle during the study. Our main intention with the present study was to investigate the effect of intake of 500 ml polyphenol-rich juice per d in an open randomised controlled trial with free-living subjects without any other constrains. Other polyphenol-rich beverages such as coffee, tea and wine have shown beneficiary effects on the risk factors of CVD, although not unambiguous on BP. A normal intake of these beverages or other polyphenol-rich foods may have affected BP in the present study, both by itself and by synergy with the study juices. However, since the present study was placebo controlled, we suggest that the effects in the study are caused by the study juices and not by lifestyle or intake of other polyphenol-rich foods.
Biochemical markers associated with polyphenol intake as well as BP changes were analysed. Of all the biochemical markers analysed, only ALAT, a liver damage marker, was significantly reduced in only the Optijuice groups, containing blackcurrant. The protective effect of polyphenols, in general( Reference Laurent, Chabi and Fouret 40 ), and blackcurrant, in particular( Reference Szachowicz-Petelska, Dobrzynska and Skrzydlewska 41 ), on the liver has previously been suggested. The average values of all biochemical markers tested in the study population were within normal range. In general, it is not desired to alter normal blood values by food intervention. We were, therefore, not surprised that the study juices did not lead to other changes in the biochemical markers tested in the present study.
Conclusions
In the present study, the polyphenol-rich juice significantly reduced SBP1 in a group of middle-aged individuals. The reduction was more pronounced in hypertensive than in normotensive subjects. Further, we found that the juice also reduced BPV.
Our results suggest that a possible mechanism of the beneficial effects of fruits and berries for CVD protection could be through reduction of the stress-sensitive BP and not necessarily reduction of the resting BP. If future studies can confirm these findings, we suggest that such juice may be beneficial for subjects with high BP and may contribute to postpone introduction of hypertensive drugs.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114515000562
Acknowledgements
The authors thank the volunteers who participated in the present study. The authors acknowledge Findus, Lier, Norway, for producing the blackcurrant press-residue extract and TINE SA for providing the study beverages. Mona Ringstad, Nofima, is acknowledged for performing the analysis of ascorbic acid, total polyphenols and monomeric antocyanins. Kari Holte, Anne Randi Enget (Department of Nutrition), Hege Hardersen and Anette Brantzæg (external support) provided highly appreciated and valuable contribution to the study.
The present study was supported by TINE SA and The Research Council of Norway (project 186902/I10). The investigators conducted the study, were responsible for data retrieval and management, and performed the data analyses and wrote the article. The contractual agreement between the University of Oslo and TINE SA allowed the sponsor to review and comment on the article; however, the investigators remained solely responsible for its content and the decision to submit the results for publication. Hence, TINE SA had no role in the design and analyses of the study or in the writing of this article.
R. B. has an interest in AS Vitas, Oslo, Norway. The other authors declare no competing financial interests.
The authors' contributions are as follows: T. E. T. was involved in the study design, recruitment of subjects, test sampling from subjects, analyses and interpretation of the data, statistical analyses, and drafting and finalising the manuscript; L. H. was involved in the study design, recruitment of subjects, test sampling from subjects, analyses and interpretation of the data, statistical analyses, and revision of the manuscript; S. K. B. was involved in the study design, statistical analyses, interpretation of the data and revision of the manuscript; K. A. was involved in the study design, interpretation of the data and revision of the manuscript; M. T. was involved in the statistical analyses, interpretation of the data and revision of the manuscript; S. Å. W. was involved in the recruitment of subjects, test sampling from subject, analyses of blood samples and revision of the manuscript; I. P. was involved in the study design, test sampling from subjects, interpretation of the data and revision of the manuscript; A. S. K. was involved in the study design and in the revision of the manuscript; K. R. was involved in the study design, medical advice, interpretation of the data and revision of the manuscript; P. O. I. was involved in the study design, medical advise, interpretation of the data and revision of the manuscript; R. B. was involved in the study design, interpretation of the data and revision of the manuscript.









